?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To compare safety and efficacy of combined therapy with conventional transarterial chemoembolization (cTACE)+microwave ablation (MWA) versus only TACE or MWA for treatment of hepatocellular carcinoma (HCC) >3–<5 cm.
Methods
This randomized controlled trial (NCT04721470) screened 278 patients with HCC >3–<5 cm. Patients were randomized into three groups: 90 underwent TACE (Group 1); 95 underwent MWA (Group 2); and 93 underwent combined therapy (Group 3). Patients were followed-up with contrast-enhanced CT or MRI. Images were evaluated and compared for treatment response and adverse events based on modified response evaluation criteria in solid tumor. Serum alpha-fetoprotein (AFP) concentration was measured at baseline and during every follow-up visit.
Results
Final analysis included 265 patients (154 men, 111 women; mean age = 54.5 ± 11.8 years; range = 38–76 years). Complete response was achieved by 86.5% of patients who received combined therapy compared with 54.8% with only TACE and 56.5% with only MWA (p = 0.0002). The recurrence rate after 12 months was significantly lower in Group 3 (22.47%) than Groups 1 (60.7%) and 2 (51.1%) (p = 0.0001). The overall survival rate (three years after therapy) was significantly higher in Group 3 (69.6%) than Groups 1 (54.7%) and 2 (54.3%) (p = 0.02). The mean progression-free survival was significantly higher in Group 3 than groups 1 and 2 (p < 0.001). A decrease in AFP concentration was seen in 75%, 63%, and 48% patients of Group 3, 2, and 1, respectively.
Conclusions
Combined therapy with cTACE + MWA is safe, well-tolerated, and more effective than TACE or MWA alone for treatment of HCC >3–<5 cm.
Introduction
Hepatocellular carcinoma (HCC) is an extremely aggressive malignant tumor and one of the leading causes of cancer death [Citation1,Citation2]. Surgical resection is still considered the best treatment option for patients with early-stage solitary HCC. However, not all patients are candidates for surgical resection because most are diagnosed in intermediate or advanced stage of cancer and have poor liver function due to hepatitis, cirrhosis, and alcohol-related liver disease. Liver transplantation often provides a therapeutic alternative but the scarcity of organ donors restricts its application [Citation3–5].
Transarterial chemoembolization (TACE) has been recognized in recent years as the first line therapy in patients with surgically unresectable HCC [Citation6,Citation7]. However, the long-term effects of TACE are unsatisfactory, and complete responses are seldom seen after a single session of TACE [Citation8–10]. Therefore, new therapeutic methods are being investigated to maximize the clinical efficacy of TACE and to offer a good prognosis for patients with HCC. One such strategy is combined therapy using TACE followed by microwave ablation (MWA). As a thermal in situ destruction therapy, MWA has been demonstrated to be an efficient and safe treatment modality [Citation11,Citation12].
In the clinical context of treatment algorithms for HCC, knowledge regarding comparative efficacy of the different therapeutic modalities is lacking; the existing studies have a retrospective design and relatively small sample size. Hence, this prospective study was conducted to compare the safety and efficacy of combined therapy (TACE + MWA) versus TACE or MWA alone for the management of HCC >3–< 5 cm.
Patients and methods
Ethical statement
The institutional review board of Zagazig University Hospital approved the study protocol (approval no. 6615; approved Dec 18, 2016) and all participants signed written informed consent. The study was registered on ClinicalTrials.gov (NCT04721470). The research was conducted following the ethical standards of the Declaration of Helsinki.
Study design and population
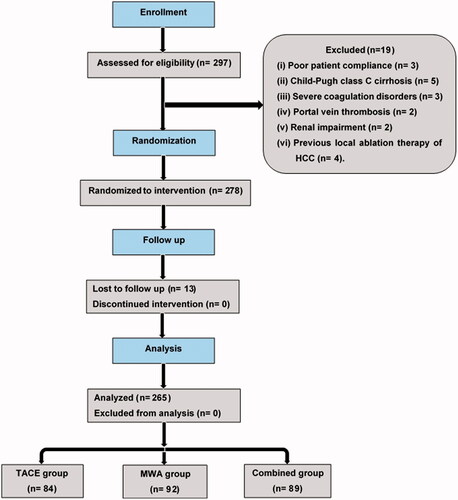
A randomized controlled trial conducted between January 2017 and May 2020. Total 297 patients were screened for study enrollment. Inclusion criteria were: (1) solitary HCC >3–<5 cm; (2) absence of extra-hepatic metastases; (3) absence of a history of encephalopathy or refractory ascites; and (4) Child–Pugh class A or B cirrhosis. Exclusion criteria were: (1) poor patient compliance (3 patients); (2) Child–Pugh class C cirrhosis (5 patients); (3) severe coagulation disorders (3 patients); (4) portal vein thrombosis (2 patients); (5) renal impairment (2 patients); and (6) previous local ablation therapy of HCC (4 patients), which excluded 19 patients. A final cohort of 278 patients was obtained. Study participants were randomized into 3 groups based on treatment modality: 90 patients underwent TACE (Group 1); 95 patients underwent MWA (Group 2); and 93 patients underwent combined therapy (Group 3). Patients were randomized to one of the three groups by one of the authors using serially numbered containers. The flowchart of the inclusion process for the study population is illustrated in .
Sample size calculation
The epidemiological information package (EPI INFO) version 3.5.3 was used to calculate the minimum required sample size for each group at a confidence interval (CI) of 95%, a power of 90% to decrease the type II error, and a drop-out of 10%. The sample size calculation was based on previously published study on combined therapy [Citation13], which reported a response rate of 81.5% in the combined group and 55.4% in the TACE group. Consequently, the sample size calculation revealed that 73 patients per group were needed to detect this effect.
Transarterial chemoembolization
The TACE procedure was performed using an angiography unit (Cath Lab system, Artis Zee Ceiling VC21C, Siemens). Under local anesthesia, the femoral puncture was performed using a 6-Fr introducer sheath, and a 4- or 5-Fr catheter (Cobra or Simmons II, Imager, Boston Scientific, Natick, MA, USA) was introduced over a 0.035 inch hydrophilic guidewire (Terumo, Tokyo, Japan) to catheterize the hepatic artery, superior mesenteric artery, and any suspected parasitic artery supplying the tumor. Selective injection of doxorubicin 50 mg (dissolved in 5 ml saline) mixed with ethiodized oil (Lipiodol, Guerbet) was performed mostly through a 2.7-Fr microcatheter (Progreat, Terumo, Tokyo, Japan). The doxorubicin solution was mixed with the ethiodized oil using a 3-way stopcock in 1:2 ratio. The embolic materials, such as Gelatin sponge (Gelfoam Upjohn Company, Kalamazoo, MI, USA) or polyvinyl alcohol particles (45–250 µm) (Contour SE, Boston Scientific), were injected to occlude the tumor feeding vessels completely.
Microwave ablation
The procedure was ultrasound-guided using a Hitashi EUB-5500 system with a 3.5–5 MHz probe. MWA was achieved using an HSAMICA® microwave generator (HS Hospital service S.P.A. Roma, Italy) called AMICA GEM machine. A single needle was inserted at the distal part of the center of the lesion using the freehand technique under ultrasound guidance. Then, the needle was moved to the middle part of lesion to ensure complete ablation. A comprehensive protocol supplied by the MWA generator supplier (AMICA), including the time and power of ablation, was established based on the tumor size for each patient. The ablation hyperechoic region was tracked during the therapy using grayscale sonography to determine the endpoint of treatment. The antenna was slowly removed, 1 cm at a time, after tumor ablation, and the MW emission continued at 20 W until the antenna was pulled to just below the entrance to the skin. This technique allows needle track cauterization to prevent tumor seeding and to minimize post-ablation bleeding.
Combined therapy
For administration of the combined therapy, cTACE was first performed, followed by MWA after 15 days. The same team of interventional radiologists, with >12 years of experience, performed both TACE and MWA.
Follow-up
Contrast-enhanced computed tomography (CECT) or dynamic magnetic resonance imaging (MRI) and blood tests, including liver function tests, complete blood count, and assessment of serum alpha-fetoprotein (AFP) concentrations were conducted during follow-up at one month, six months, one year, and up to three years after therapy. All follow-up images were consensually reviewed by three consultant radiologists with >10 years of experience in diagnosis and interventional treatment of HCC. Images were evaluated for treatment response and adverse events (AEs). The AEs were recorded and classified following the guidelines of the Society of Interventional Radiology [Citation14].
Treatment response
Tumor response was evaluated by CECT or dynamic MRI based on the modified response evaluation criteria in solid tumor (m-RECIST) established by the American Association for the Study of Liver Diseases [Citation15], i.e., complete response (CECT displayed no signs of intra-lesion contrast enhancement in the arterial phase; CR), partial response (CECT showed areas of enhancement within the limits of the lesions in the arterial phase; PR), stable disease (SD), and progressive disease (any evidence of tumor progression was present or if new lesion formation was identified; PD). The recurrence rate, mortality rate, overall survival rate, and progression-free survival (PFS) were also measured in relation to management lines.
Serum AFP evaluation
The serum AFP concentration was measured at baseline and during every follow-up visit. The AFP variation rate (ΔAFP) was defined as the percentage of change between the AFP concentration at baseline and the postprocedure AFP concentration after 1–2 months as shown in the following equation:
When ΔAFP was greater than the cutoff value (ΔAFP [%] >cutoff value), it was considered to be the response to the treatment procedure, whereas AFP non-response was defined as ΔAFP less than the AFP cutoff value (ΔAFP [%] <cutoff value) [Citation16].
Statistical analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (version 26, IBM, Armonk, NY, USA). Categorical variables were represented as numbers and percentages. Continuous data were expressed as mean ± standard deviation. The differences among treatment groups were analyzed using the Student’s t-test and analysis of variance test for numerical variables and the chi-squared test for qualitative data. Kaplan–Meier survival was used to estimate the overall survival rate and median month of survival. A receiver operating characteristic curve was used to detect the most appropriate cutoff value for AFP. Statistical significance was defined as p < 0.05.
Results
Patients
This study initially enrolled 278 patients with HCC >3–< 5 cm. Of these 278 patients, 13 were lost to follow-up (6, 3, and 4 in the TACE, MWA, and combined group, respectively) and were excluded from the final analysis. Hence, the final analysis included 265 patients (154 male, 111 female; mean age 54.5 ± 11.8 years; range 38–76 years). Data of the study participants are summarized in . The distribution among groups was: 84 patients underwent TACE (52 male, 32 female; mean age 51.3 ± 9.2; range 41–75 years); 92 patients underwent MWA (50 male, 42 female; mean age 53.8 ± 10.3; range 38–72 years); and 89 patients underwent combined therapy (52 male, 37 female; mean age 52.1 ± 9.5; range 48–76 years). No significant difference (p > 0.05) was noted among the groups regarding age, sex, body mass index, causes of liver disease, Child–Pugh classification, AFP concentration, and tumor size.
Table 1. Patients’ data.
Postoperative AEs and complications
Postprocedural minor AEs – nausea, vomiting, abdominal pain, and low-grade fever – occurred in 24.7%, 47.6%, and 38% patients of the combined, TACE, and MWA group, respectively. The combined therapy was well-tolerated, only one patient showed severe hepatic dysfunction. In the TACE group, three patients showed severe hepatic dysfunction; in the MWA group, two patients showed tumor seeding ().
Table 2. Postoperative adverse events and complications.
Treatment response
Tumor response was compared among the groups after one month (). Of the patients who received combined therapy, 86.5%, 3.3%, 5.6%, and 4.55% achieved CR, PR, SD, and PD, respectively, compared with 54.8%, 32.1%, 6%, and 7.1% for the TACE group, and 56.5%, 27.2%, 6.5%, and 9.8%, for the MWA group, respectively (p = 0.0002).
Table 3. Follow-up and treatment response.
Follow-up
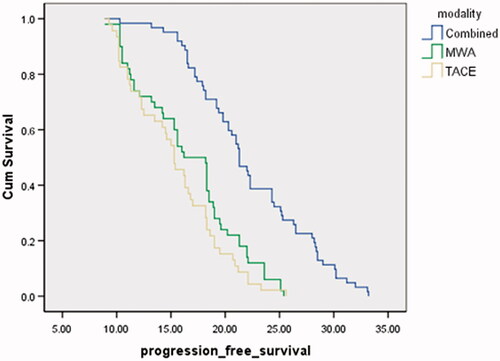
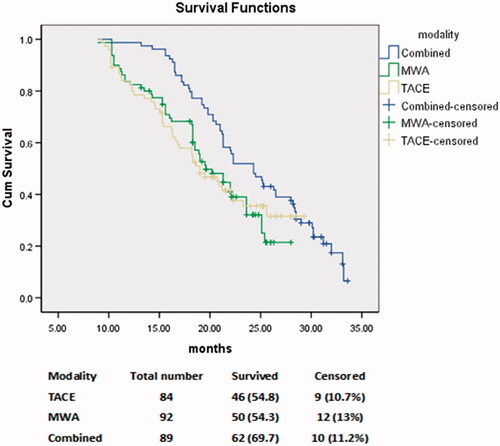
All patients were followed up to three years after therapy (). The recurrence rate after 12 months was significantly lower in the combined group (20/89, 22.47%) than the TACE group (51/84, 60.7%) and the MWA group (47/92, 51.1%) (p = 0.0001). The overall mortality rate was significantly lower in the combined group (19.1%) than the TACE group (34.5%) and the MWA group (32%) (p = 0.02). The overall survival rate was significantly higher in the combined group (62/89, 69.6%) than the TACE group (46/84, 54.8%) and the MWA group (50/92, 54.3%) (p = 0.02) (). The median survival time was significantly higher in the combined group (24 months) than the TACE group (19 months) and the MWA group (21 months) (p = 0.02). The mean PFS was significantly higher in the combined group (22.3 months) than the TACE group (15.4 month) and the MWA group (16.7 months) (p < 0.001) ().
Figure 2. Comparison of the survival function among the three groups: transarterial chemoembolization (TACE), microwave ablation (MWA), and combined TACE + MWA. The overall survival rate was significantly higher in the combined group (69.6%) than the TACE group (54.8%) and the MWA group (54.3%) (p = 0.02).
Serum AFP evaluation
summarizes the result of the AFP evaluation among the three groups. The best cutoff value for the ΔAFP for predicting survival was 45.5%. This cutoff value resulted in a sensitivity of 84%, 70.8%, and 65.5% and a specificity of 88%, 80%, and 83% in the combined group, TACE group, and MWA group, respectively ().
Figure 4. Receiver operating characteristic (ROC) curves show the sensitivity and specificity of serum alpha-fetoprotein concentration response among the three treatment groups: (a) transarterial chemoembolization (TACE), (b) microwave ablation (MWA), and (c) combined TACE + MWA.
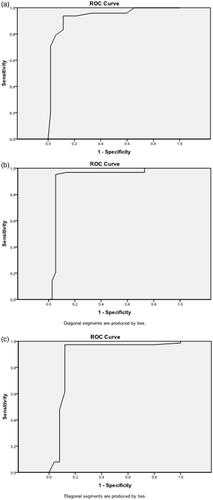
Table 4. AFP evaluation among the three groups.
Representative cases of our study are illustrated in ().
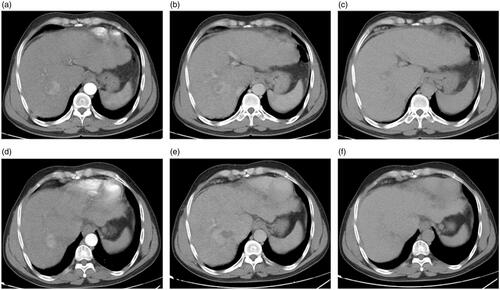
Figure 5. A 65-year-old man with a history of antiviral treatment for hepatitis C virus. Contrast-enhanced MRI before treatment: (a) arterial, (b) portal, and (c) delayed phases show a single 4.9-cm focal lesion at segment VI with HCC criteria (arterial enhancement and delayed washout). The patient underwent TACE and then MWA after two weeks. Follow up contrast-enhanced CT 24 months after the procedure: (d) arterial, (e) portal, and (f) delayed phases show complete response. The effect of MWA is seen in the center of the lesion, and the effect of TACE is seen in the lesion periphery.
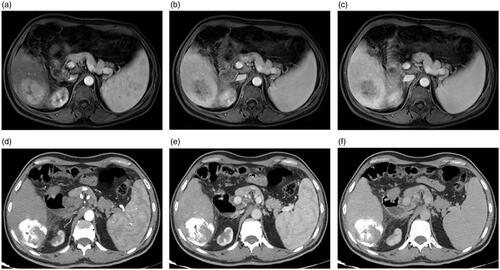
Figure 6. A 60-year-old man with a history of antiviral treatment for hepatitis C virus. Contrast-enhanced CT before treatment: (a) pre-contrast, (b) arterial, and (c) delayed phases show a single 4.8-cm focal lesion at segment VI with HCC criteria (arterial enhancement and delayed washout). TACE was performed and results of follow up contrast-enhanced CT after 12 months are shown: (d) pre-contrast, (e) arterial, and (f) delayed phases show complete response with no significant enhancement or washout. Follow-up contrast-enhanced CT after 24 months are shown: (g) pre-contrast, (h) arterial, and (i) delayed phases show complete response with a significant decrease in the size.
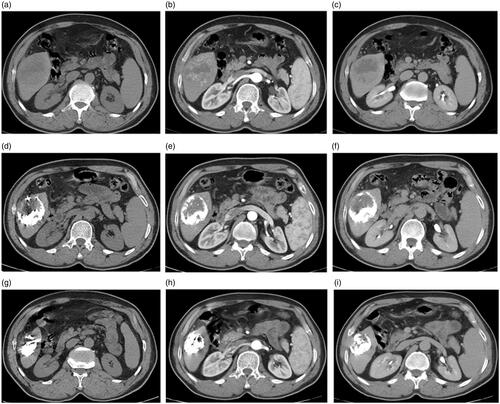
Figure 7. A 54-year-old man with a history of antiviral treatment for hepatitis C virus. Contrast-enhanced CT before treatment: (a) arterial, (b) portal, and (c) delayed phases show a single 4.4 cm focal lesion at segment VII with HCC criteria (arterial enhancement and delayed washout). MWA was performed and results of follow up contrast-enhanced CT after 12 months are shown: (d) arterial, (e) portal, and (f) delayed phases show tumor recurrence with significant enhancement at the arterial phase and characteristic washout at the delayed phases.
Discussion
The main objective of any therapeutic modality for HCC is to be safe and effective with a prolonged survival period and a low recurrence rate. For cases wherein various treatment procedures are available, the selection of the most valuable treatment for the patient may be a challenge, and comparison between the different treatment procedures for HCC can help to determine the best choice. This study examined three therapeutic approaches – combined therapy (TACE + MWA), only TACE, and only MWA – for the treatment of HCC >3–<5 cm. Although the efficacies and the outcomes of these procedures have been extensively investigated [Citation13,Citation17–22], to date, the comparison among them has not been researched in depth. Moreover, few clinical research reports in the literature have addressed the treatment of patients with HCC >3–<5 cm. In this current trial, the goal was to combine both TACE and MWA to achieve the best benefit from each technique and to overcome the disadvantages of either treatment alone.
The present study showed that combined therapy provided notably better results than TACE or MWA alone in the treatment of HCC >3–<5 cm. After receiving combined therapy, 86.5% of patients achieved CR compared with 54.8% and 56.5% who received TACE and MWA alone, respectively. Moreover, the response rate in the study sample was also significantly higher for combined therapy than for TACE or MWA alone (p = 0.0002). These results are not surprising because: MWA works directly on the center of the lesion, whereas TACE has more therapeutic effects on the greater vascularized periphery of the lesion; therefore, the combination may reasonably be expected to boost the lesion response [Citation18,Citation23]. Such findings are close to those of previously published studies [Citation4,Citation12,Citation13,Citation19,Citation24–26].
Regarding the timing for administration of combined therapy, TACE was performed first, followed by MWA after 15 days, to avoid challenging the liver and creating any liver dysfunction. Similarly, Chen et al. [Citation19] stated that patients typically undergo MWA 1–2 weeks after TACE because patients need time to recover from TACE-induced post-embolization syndrome and clear the ethiodized oil from the normal liver parenchyma to demonstrate the tumor clearly on imaging. In contrast, Si et al. [Citation17], in a study on the treatment of large HCC lesions >5 cm, used MWA immediately followed by TACE and reported limited complications.
Regarding survival and recurrence rates, the present study found a significant survival gain for combined therapy. The recurrence rate after 12 months was significantly lower in the combined group than TACE and MWA groups (p = 0.0001). The overall survival rate was considerably higher in the combined group than TACE and MWA groups (p = 0.02). The mean PFS was significantly higher in the combined group than TACE and MWA groups (p < 0.001). These results are concurrent with the previous studies [Citation4,Citation12,Citation13,Citation22,Citation4,Citation27,Citation28], which showed improved overall and recurrence-free survival and significantly longer time of tumor progression in patients treated with TACE + MWA compared with TACE alone. In contrast, Chen et al. [Citation19] reported that the combined group (TACE + MWA) had a better time of tumor progression (p = 0.001); however, no significant difference in overall survival (p = 0.317) was observed. Nevertheless, these studies reported the outcomes for combined therapies on the tumors with variable size ranges and stages (based on Barcelona Clinic Liver Cancer guidelines) and uneven tumor burden. The present study focused mainly on tumor size >3–<5 cm.
Regarding the safety of the procedure, in keeping with the literature [Citation4,Citation16,Citation22,Citation25,Citation28,Citation29], the current study demonstrated that the combined therapy was well-tolerated and did not significantly increase the risk of AEs compared with TACE and MWA alone. Although minor AEs were commonly seen in the combined therapy group, they were transient with prompt recovery. In general, the combined therapy is a relatively safe procedure for HCC >3–<5 cm with acceptable AEs.
Serum AFP concentration is a well-established tumor marker for HCC screening and diagnosis, and the degree of AFP seems to be related to the prognosis of patients with HCC [Citation30]. The findings of this study are consistent with the results of previous studies [Citation31,Citation32], which have reported AFP concentration as an important prognostic factor for patients with HCC undergoing different locoregional treatment modalities. In the present study, a decrease in the AFP concentration was seen in 75%, 63%, and 48% of patients who underwent combined therapy, MWA alone, and TACE alone, respectively. The study found that the best cutoff value of the ΔAFP for survival prediction was 45.5%. This cutoff value resulted in a sensitivity of 84%, 70.8%, and 65.5% and a specificity of 88%, 80%, and 83% in the combined group, TACE group, and MWA group, respectively.
The present study has many strong points because it was performed as a broad prospective study with a longer follow-up period to escape the selection bias of a retrospective study. This research further extends the literature by offering insights into the potential benefits of combined therapy in the treatment of HCC >3–<5 cm. Moreover, this study has established a basis for future research to evaluate the efficacy of this combined therapy. Nevertheless, some limitations must be addressed. First, all procedures were performed at a single institution. Second, bias arising from both the physicians’ experiences and the patient population is possible. Third, the higher costs of the combined therapy must be considered.
Conclusion
In conclusion, this study confirmed that the combined therapy is safe, well-tolerated, and more effective than TACE or MWA alone for the treatment of HCC >3–<5 cm. Based on these findings, combined therapy is likely to be a promising therapeutic option for HCC >3–< 5 cm. Future multicenter prospective studies with more patients are warranted to confirm the benefits of this combined therapy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- Chinn SB, Lee FT Jr, Kennedy GD, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. Am J Roentgenol. 2001;176:789–795.
- Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. Am J Roentgenol. 2002;179:93–101.
- Mostafa EM, Ganguli S, Faintuch S, et al. Optimal strategies for combining transcatheter arterial chemoembolization and radiofrequency ablation in rabbit VX2 hepatic tumors. J Vasc Interv Radiol. 2008;19:1740–1748.
- Smolock AR, Cristescu MM, Hinshaw A, et al. Combination transarterial chemoembolization and microwave ablation improves local tumor control for 3- to 5-cm hepatocellular carcinoma when compared with transarterial chemoembolization alone. Abdom Radiol (NY). 2018;43:2497–2504.
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–143.
- European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma" [J Hepatol 69 (2018) 182-236]. J Hepatol. 2019;70:817.
- Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244:151–156.
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307.
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339–344.
- Yang WZ, Jiang N, Huang N, et al. Combined therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation for small hepatocellular carcinoma. WJG. 2009;15:748.
- Seki T, Tamai T, Nakagawa T, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245–1251.
- Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization‐percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456–463.
- Zheng L, Li HL, Guo CY, et al. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018;19:237–246.
- Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28:1432–1437.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol. 2012;56:S75–S87.
- Si ZM, Wang GZ, Qian S, et al. Combination therapies in the management of large (≥ 5 cm) hepatocellular carcinoma: microwave ablation immediately followed by transarterial chemoembolization. J Vasc Interv Radiol. 2016;27:1577–1583.
- Yi Y, Zhang Y, Wei Q, et al. Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone. Chin J Cancer Res. 2014;26:112–118.
- Chen QF, Jia ZY, Yang ZQ, Fan WL, et al. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization–microwave ablation therapy for hepatocellular carcinoma tumors≤ 5 cm: a propensity analysis at a single center. Cardiovasc Intervent Radiol. 2017;40:1748–1755.
- Yuan W, Yang MJ, Xu J, et al. Radiofrequency ablation combined with transarterial chemoembolization for specially located small hepatocellular carcinoma. Technol Cancer Res Treat. 2018;17:1533033818788529.
- Abdelaziz AO, Abdelmaksoud AH, Nabeel MM, et al. Transarterial chemoembolization combined with either radiofrequency or microwave ablation in management of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2017;18:189–194.
- Zhang R, Shen L, Zhao L, et al. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:219–224.
- Poggi G, Montagna B, Di CP, et al. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res . 2013;33:1221–1227.
- Li W, Man W, Guo H, et al. Clinical study of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of advanced hepatocellular carcinoma. J Can Res Ther. 2016;12:217.
- Ginsburg M, Zivin SP, Wroblewski K, et al. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26:330–341.
- Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849.
- Liu C, Liang P, Liu F, et al. MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia. 2011;27:654–662.
- Li Z, Jiao D, Han X, et al. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided microwave ablation in the treatment of small hepatocellular carcinoma. Cancer Imaging. 2020;20:13
- Hu H, Chen GF, Yuan W, et al. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. 2018;34:1351–1358.
- Kohles N, Nagel D, Jüngst D, et al. Prognostic relevance of oncological serum biomarkers in liver cancer patients undergoing transarterial chemoembolization therapy. Tumour Biol. 2012;33:33–40.
- Tsai MC, Wang JH, Hung CH, et al. Favorable alpha-fetoprotein decrease as a prognostic surrogate in patients with hepatocellular carcinoma after radiofrequency ablation . J Gastroenterol Hepatol. 2010;25:605–612.
- Liu L, Zhao Y, Jia J, et al. The prognostic value of alpha-fetoprotein response for advanced-stage hepatocellular carcinoma treated with sorafenib combined with transarterial chemoembolization. Sci Rep. 2016;6:19851–19859.