Abstract
Background
Radiofrequency (RF)-assisted devices are widely used for hemostasis during liver resection. This study compared the use of dual switching (DS) versus single switching (SS) control modes for RF-based liver resections in a pig model.
Methods
The RF-based system comprised a 200-W generator and three electrodes with 4-cm tips arranged in a linear configuration using an adaptor. Eight Lanyu pigs were used to assess ablation outcomes with electrode spacing of 2 or 3 cm, and ablation durations of 1.5, 2 or 3 min. All combinations were tested in DS and SS modes. Procedures were performed on left lateral, caudal and right anterior liver lobes, and after which transections were performed using a scalpel. Blood loss, complete ablation rate and ablation speed were compared.
Results
DS mode was shown to induce significantly less blood loss than SS mode when the electrode spacing was set at 2 cm and the ablation duration was 2 min or 3 min (p=.010 and .012, respectively). Extended ablation duration and narrow electrode spacing tended to induce less blood loss, regardless of operating mode. Bloodless resection was achieved using DS mode with electrode spacing of 2 cm and ablation duration of 2–3 min. The highest rate of complete ablation (11.3 cm2/min) was achieved using DS mode with electrode spacing of 2 cm and ablation duration of 1.5 min.
Conclusion
RF-based hepatic resection using DS mode is safe and feasible, resulting in less blood loss than SS mode with a higher rate of complete ablation (i.e., superior ablation efficiency).
Introduction
The fact that the liver is a soft visceral organ with complex vascular anatomy and abundant blood flow, makes liver resection a high-risk procedure. Recent advances in medical care and surgical techniques have lowered the risk of liver resection; however, the procedure is still associated with mortality of roughly 5% and complication rate of 40% [Citation1,Citation2]. Worldwide, a steady increase in the incidence of hepatocellular carcinoma is increasing the need for liver resection [Citation3,Citation4]. Liver resection is also indicated in some cases of metastatic colorectal cancer [Citation5]. In all such cases, the control of bleeding during liver resection is an issue of importance.
The conventional approach to liver resection involves the use of the clamp-crush or finger fracture technique, both of which are associated with considerable intraoperative bleeding during parenchymal transection [Citation6]. Intraoperative bleeding increases the likelihood that transfusion will be required, which in turn increases the increased risk of complications and tumor recurrence [Citation7,Citation8]. Numerous devices are used to reduce bleeding during liver resection, such as ultrasonic surgical aspirators, ultrasonic dissection devices, bipolar electrosurgical devices, microwave assisted surgical devices [Citation9–11] and radiofrequency (RF) based devices. Nonetheless, the clinical benefits of these devices have yet to be proven [Citation12].
An RF-based liver resection method was introduced by Habib in 2002, and has become known as ‘the bloodless hepatectomy technique’ [Citation13]. The perpendicular insertion of the RF-based device into the liver creates a parallel line of ablation [Citation14,Citation15], thereby minimizing blood loss [Citation13] and reducing the likelihood that blood transfusion will be required during surgery [Citation16]. RF-based techniques initially used a single electrode for coagulation; however, the advent of switching controllers has made it possible to use three electrodes simultaneously to increase the speed of parenchymal coagulation during linear ablation. Development of the dual switching (DS) control mode has further improved the efficiency of radiofrequency ablation (RFA). In DS mode, synchronous parallel RF energy is alternated between a pair of electrodes. In conventional single switching (SS) control mode, RF energy is applied to one of the electrodes, and switching between electrode tips is based on changes in tissue impedance. Researchers have reported that the ablation duration in DS mode shorter and the ablation area is larger when compared to SS mode [Citation17–20]. Nonetheless, researchers have yet to systemically demonstrate that DS mode is superior to SS mode for RF-based liver resection.
Our primary objective in the current study was to confirm the safety and feasibility of DS mode RFA for liver resection. We also compared the efficiency of DS and SS modes using an in vivo porcine liver model. Finally, we sought to determine the optimal electrode interval and ablation duration in terms of efficiency.
Materials and methods
The animal use protocol in this study was reviewed and approved by the Institutional Animal Care and Use Committee of the Institute, and all reporting has been conducted in strict adherence to ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments) [Citation21]. A total of 42 liver resections were performed on the left lateral, caudal and right anterior liver lobes of 14 Lanyu pigs using an RF-based device. The RF system comprised a 200-W generator (VIVA Multi RF; STARmed, Goyang, South Korea), and three electrodes with 4-cm tips configured using a linear adaptor (). Ablation involved the insertion of electrodes (Octopus; STARmed, Goyang, South Korea) aligned linearly along a pre-determined resection plane (). Transection of the liver parenchyma was performed using a scalpel following the completion of ablation ().
Figure 1. (a, b) Three electrodes with 4-cm tips were arranged in a linear manner using a linear adaptor.
Figure 2. Electrodes were inserted into the pre-determined resection plane in a linear arrangement to conduct ablation.
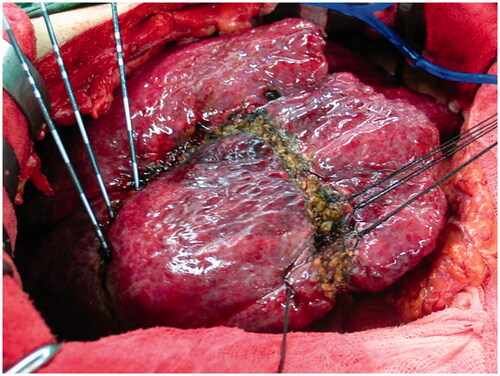
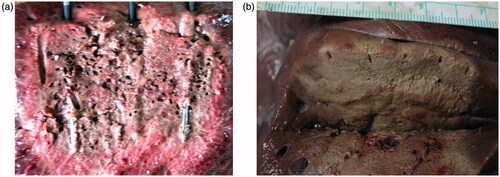
Figure 3. (a, b) After the completion of ablation, transection of the liver parenchyma was performed using a scalpel.
In the current study, SS control mode involved the delivery of RF energy (maximum 200 W) to one of the three electrodes. If the system detected an increase in impedance of >50 Ω above the baseline value, then the current was automatically switched to another electrode. If an increase in impedance was not detected, then power switching was performed at approximately 30 s. In the event that impedance reached 300 Ω above the baseline, then no power was delivered to that particular electrode for at least 15 s. DS control mode involved the synchronous parallel delivery of RF energy to a pair of the three electrodes. As in SS mode, current was automatically switched to a different electrode pair within 30 s. In DS mode, RF energy was limited to 240 W (120 + 120 W) for a period of one minute, which was then increased to 330 W (165 + 165 W) until tissue impedance rose to 170% of the baseline value. If the impedance of either electrode exceeded 170% of the baseline value, then the current was immediately switched to another pair of electrodes. If the impedance of either electrode failed to exceed 170% of the baseline value, then the maximum power (400 W; 200 + 200 W) was automatically switched to another electrode at approximately 30 s. Basically, current switching between electrode pairs depended on changes in tissue impedance during RF energy delivery.
Based on our previous experience using RF apparatus, we tested two electrode intervals (2 or 3 cm) and three ablation durations (1.5, 2 or 3 min) and their effects on ablation. Combining two electrode intervals with three ablation durations resulted in six configurations, all of which were assessed in DS control and SS control modes for a total of 12 experiment groups (groups A–L) ().
Table 1. Experimental groups and mode (DS or SS), electrode interval (2 cm or 3 cm) and ablation time (1.5, 2 or 3 min).
Following liver resection using a scalpel, the specimen underwent careful examination to record the ablation area (length and width constituting the resection plane), and the depth of ablation (constituting the bloodless cutting zone). Complete ablation was defined as an ablation zone exceeding 1 cm in depth without any un-ablated regions.
Ablation performance in DS mode and SS mode was assessed using all six configurations in terms of perioperative hemodynamic parameters, including the volume of intraoperative blood loss, the rate of complete ablation, and ablation duration.
Statistical analysis
Data were presented as mean ± standard deviation. Continuous variables were compared using Student’s t test. All statistical analyses were performed using SPSS version 18 for Windows (SPSS Inc., Chicago, IL). A value of p<.05 was considered statistically significant.
Results
Safety and intraoperative blood loss
A total of 42 RFA-assisted hepatectomies were performed, including 24 in DS mode and 18 in SS mode. Our decision to perform a larger number of resections in the DS group (4 vs. 3) rather than an equal number in the two groups was based on the principle of the 3Rs (Replacement, Reduction and Refinement), which stipulates that researchers adopt methods that provide comparable levels of information from fewer animals. By the time, we completed three resections in the SS groups (after the DS group), we had obtained sufficient information. The vital signs and hemodynamic parameters of all pigs remain stable throughout the procedures and no pigs died during surgery. Perioperative ablation data are listed in . The mean perioperative blood loss values in DS mode vs. SS mode were as follows: Group A vs. group G, 5.5 ± 6.4 vs. 58.3 ± 31.7 mL (p=.086); B vs. H, 29.3 ± 31.6 mL (p=.01); C vs. I, 0.0 vs. 27 ± 33.9 mL (p=.012); D vs. J, 67.8 ± 17.4 vs. 94 ± 23.1 mL (p=.54); E vs. K, 22.8 ± 21.4 vs. 58.3 ± 51.1 mL (p=.079); F vs. L, 22.5 ± 21.0 vs. 51.7 ± 41.6 mL (p=.182). Overall, blood loss was less severe under narrow electrode spacing (2 cm) than under wider spacing (3 cm), regardless of ablation duration. Blood loss was less severe under longer ablation durations, regardless of operating mode (DS or SS) and electrode spacing (2 cm or 3 cm). Blood loss was less severe under DS mode than under SS mode, when using electrode spacing of 2 cm and ablation duration of 2 or 3 min (p=.010 and .012, respectively). Bloodless resection was achieved only in DS mode with electrode spacing of 2 cm and ablation duration of 2–3 min.
Table 2. Perioperative ablation data and experimental outcomes.
Completeness of ablation
In DS mode (regardless of ablation duration), electrode spacing of 2 cm resulted in 100% ablation, whereas electrode spacing of 3 cm resulted in 33% ablation. In SS mode (regardless of ablation duration), electrode spacing of 2 cm resulted in 55.6% ablation, whereas electrode spacing of 3 cm resulted in 22.2% ablation. With an electrode spacing of 3 cm, the ablation rate was proportional to the ablation duration; however, the extent of ablation at 3 min was only 50% in DS mode and 33% in SS mode.
These findings suggest that a complete ablation plane (100%) in liver parenchyma can only be achieved in DS mode with an electrode spacing of 2 cm and ablation duration of no less than 1.5 min.
Ablation efficiency in DS mode and mean delivered energy
The mean ablation rates in DS mode with electrode spacing of 2 cm varied as a function of ablation duration, as follows: 1.5 min (11.3 cm2/min), 2 (9.6 cm2/min) and 3 min (7.0 cm2/min). Regardless of the parameter combination, more energy was delivered in DS mode (1.01 kcal/min) than in SS mode (0.72 kcal/min).
Discussion
Our results demonstrated that using DS mode for RF-based hepatic resection is safe and feasible. We also determined that DS mode is more efficient than SS mode. The settings that achieved complete ablation in the most efficient manner were as follows: DS mode; electrode spacing = 2 cm; ablation duration = 1.5 min. Overall, DS mode was associated with superior results in terms of blood loss, complete ablation rate and ablation efficiency.
This is the first experimental study to demonstrate the safety and efficacy of DS mode for RF-based liver resection. Regardless of operating mode, the ablation rate was proportional to ablation duration and inversely proportional to electrode spacing. Blood loss was inversely proportional to ablation duration and proportional to electrode spacing. A narrow electrode spacing of 2 cm was superior to wide spacing in terms of achieving complete ablation with minimal blood loss. Complete ablation was achieved in DS mode with an electrode spacing of 2 cm and ablation duration of no less than 1.5 min.
Our extensive experience in treating patients with liver cancer using RF ablation devices convinced us that it is possible to construct a bloodless resection plane comprising multiple linear ablation zones. Percutaneous ablation was initially performed using RF-based devices. RF-based devices are easier to use than other surgical devices commonly used for liver resection, and can be operated by a single operator (i.e., without the help of a skilled assistant). These devices are particularly beneficial when treating patients with a high risk of intraoperative bleeding, such as those with cirrhotic liver, portal hypertension and coagulopathy. RF-based hepatic resection can reduce blood loss and provide a greater ablative margin at the resection plane, on which the complete ablation of liver parenchyma in the resection plane depends. A larger treatment margin is associated with more complete ablation and better hemostasis. Incomplete ablation can lead to tissue necrosis, resulting in surface bleeding and residual viable tumor cells along the cutting planes. Our results revealed that complete ablation could only be achieved in DS mode with electrode spacing of 2 cm. Note, however, that those results were convincing, with even the shortest ablation duration of 1.5 min achieving 100% complete ablation in all four cases. In DS mode with electrode spacing of 2 cm, even the longest ablation duration (3 min) resulted in only 50% ablation.
The superior ablation efficiency of DS mode can be attributed to the delivery of more energy by each electrode in a given period of time. The mean energy delivered by each electrode was as follows: 2 cm electrode spacing (1.013 kcal/min in DS mode and 0.707 kcal/min in SS mode) and 3 cm electrode spacing (1.012 kcal/min in DS mode and 0.731 kcal/min in SS mode). Overall, DS mode delivered 35% more energy per electrode per minute. A number of methods have been proposed to increase the efficiency of energy delivery in RFA, include the use of multiple electrodes, multi-tine electrodes and saline-perfusion electrodes [Citation22–24]. Saline-perfusion electrodes have been shown to improve the efficiency of RF energy delivery by increasing electrical conductance; however, it is limited by heterogeneous distribution within the target tissue, which results in inconsistent ablation [Citation25,Citation26]. The use of multiple electrodes is regarded as a more reliable approach to improving the efficiency of energy delivery [Citation27–32].
This study has a number of limitations that should be considered in the interpretation of our results. The small number of procedures we performed limited the statistical power. Differences in the anatomy and parenchymal structure of pig livers from those of human livers limit the extrapolation of results to humans. We did not evaluate the rate of abscess formation or survival benefits, and we did not use imaging methods (e.g., computed tomography and magnetic resonance imaging), which are commonly used to evaluate ablation efficacy and check for thermal injury to hepatic vessels and bile ducts.
Conclusion
Our study using an in vivo pig model demonstrated the safety and feasibility of RF-based hepatic resection in DS mode. Overall, DS mode was associated with a higher rate of complete ablation than SS mode and superior ablation efficiency. The highest ablation efficiency was achieved in DS mode with electrode spacing of 2 cm and ablation duration of 1.5 min.
Disclosure statement
All authors have no conflicts of interest or financial ties to disclose.
References
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406.
- Reissfelder C, Rahbari NN, Koch M, et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. Br J Surg. 2011;98(6):836–844.
- Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E86.
- Akgul O, Cetinkaya E, Ersoz S, et al. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20(20):6113–6122.
- Lin TY. A simplified technique for hepatic resection: the crush method. Ann Surg. 1974;180(3):285–290.
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860–869.
- Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115(3):303–309.
- Jin T, Liu X, Dai C, et al. Beneficial impact of microwave ablation-assisted laparoscopic hepatectomy in cirrhotic hepatocellular carcinoma patients: a propensity score matching analysis. Int J Hyperthermia. 2019;36(1):530–537.
- Rao Z, Ling W, Dai X, et al. Precoagulation with microwave ablation for hepatic parenchymal transection during liver partial resection. Int J Hyperthermia. 2019;36(1):146–150.
- Dimitri M, Staderini F, Brancadoro M, et al. A new microwave applicator for laparoscopic and robotic liver resection. Int J Hyperthermia. 2019;36(1):75–86.
- Poon RT. Current techniques of liver transection. HPB (Oxford). 2007;9(3):166–173.
- Navarra G, Spalding D, Zacharoulis D, et al. Bloodless hepatectomy technique. HPB (Oxford). 2002;4(2):95–97.
- Pai M, Jiao LR, Khorsandi S, et al. Liver resection with bipolar radiofrequency device: Habib 4X. HPB (Oxford). 2008;10(4):256–260.
- Hiroishi K, Eguchi J, Baba T, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45(4):451–458.
- Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236(5):560–563.
- Lee FT Jr., Haemmerich D, Wright AS, et al. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14(11):1437–1442.
- Yoon JH, Lee JM, Han JK, et al. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol. 2013;14(3):403–411.
- Yoon JH, Lee JM, Hwang EJ, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014;15(2):235–244.
- Choi TW, Lee JM, Lee DH, et al. Percutaneous dual-switching monopolar radiofrequency ablation using a separable clustered electrode: a preliminary study. Korean J Radiol. 2017;18(5):799–808.
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412.
- Ni Y, Mulier S, Miao Y, et al. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30(4):381–400.
- Mulier S, Miao Y, Mulier P, et al. Electrodes and multiple electrode systems for radio frequency ablation: a proposal for updated terminology. Adv Exp Med Biol. 2006;574:57–73.
- Lee JM, Han JK, Lee JY, et al. Hepatic radiofrequency ablation using multiple probes: ex vivo and in vivo comparative studies of monopolar versus multipolar modes. Korean J Radiol. 2006;7(2):106–117.
- Lee JM, Han JK, Kim SH, et al. Comparison of wet radiofrequency ablation with dry radiofrequency ablation and radiofrequency ablation using hypertonic saline preinjection: ex vivo bovine liver. Korean J Radiol. 2004;5(4):258–265.
- Lee JM, Han JK, Chang JM, et al. Radiofrequency ablation of the porcine liver in vivo: increased coagulation with an internally cooled perfusion electrode. Acad Radiol. 2006;13(3):343–352.
- Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation: simultaneous production of separate zones of coagulation in an in vivo porcine liver model. J Vasc Interv Radiol. 2005;16(12):1727–1735.
- Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology. 2006;241(1):116–124.
- Lee JM, Han JK, Kim SH, et al. Bipolar radiofrequency ablation using wet-cooled electrodes: an in vitro experimental study in bovine liver. AJR Am J Roentgenol. 2005;184(2):391–397.
- Appelbaum L, Sosna J, Pearson R, et al. Algorithm optimization for multitined radiofrequency ablation: comparative study in ex vivo and in vivo bovine liver. Radiology. 2010;254(2):430–440.
- Baldwin K, Katz SC, Rubin A, et al. Bipolar radiofrequency ablation of liver tumors: technical experience and interval follow-up in 22 patients with 33 ablations. J Surg Oncol. 2012;106(7):905–910.
- Clasen S, Rempp H, Schmidt D, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol. 2012;81(1):111–113.
