Abstract
High Intensity Focused Ultrasound (HIFU) capably bridges the disciplines of surgery, oncology and biomedical engineering science. It provides the precision associated with a surgical tool whilst remaining a truly non-invasive technique. Oxford has been a centre for both clinical and preclinical research in HIFU over the last twenty years. Research into this technology in the UK has a longer history, with much of the early research being carried out by Professor Gail ter Haar and her team at the Institute of Cancer Research at Sutton in Surrey. A broad range of potential applications have been explored extending from tissue ablation to novel drug delivery. This review presents Oxford’s clinical studies and applications for the development of this non-invasive therapy. This includes treatment of solid abdominal tumours comprising those of the liver, kidney, uterus, pancreas, pelvis and prostate. It also briefly introduces preclinical and translational works that are currently being undertaken at the Institute of Biomedical Engineering, University of Oxford. The safety, wide tolerability and effectiveness of this technology is comprehensively demonstrated across these studies. These results can facilitate the incorporation of HIFU as a key clinical management strategy.
Introduction
High Intensity Focused Ultrasound (HIFU) is a minimally invasive therapeutic technique that uses non-ionising ultrasound waves to cause tissue necrosis. A shift in focus to find a reliable alternative to open surgery has highlighted other available methods including transarterial chemo-embolisation (TACE), percutaneous ethanol injection (PEI), and energy-based ablative techniques such as radiofrequency, microwave, cryoablation and HIFU. Unlike its minimally invasive counterparts, HIFU provides the only truly non-invasive method. Its unique ability to perform selective tissue necrosis in a well-defined area from a distant source is central to its attraction. HIFU is becoming more recognised across the world as a tool for tumour ablation and other applications. The scope of this technique, however, goes beyond the treatment of cancers to chronic pain management, various benign conditions, and haemostasis. HIFU has been used at the Churchill Hospital Oxford for treatment of solid abdominal tumours including those of the liver, kidney, uterus, pancreas, pelvis and prostate. By bridging the disciplines of surgery, cancer medicine and biomedical engineering science, this technology promises to play a significant role in the future of surgery.
HIFU presents several benefits as a minimally invasive therapeutic tool. The unique principles of HIFU, which confer these benefits, have been discussed elsewhere [Citation1,Citation2]. As with any novel treatment, its advantages and limitations must be judged against the background of its therapeutic alternatives. Surgery, a widely used treatment for solid malignancies, may not be suitable for the patient for various reasons. This includes a high surgical risk or treatment considered in a palliative setting. In such cases, HIFU may be favourable as it is less debilitating, and can even provide effective pain relief. There is also a faster recovery time when compared with traditional surgery. Seeding is a risk posed by surgery and other invasive methods, but dissemination of cancer cells has not been seen in HIFU [Citation3–7].
Other conventional therapies for solid malignancies include radiotherapy and systemic chemotherapy, both of which lead to host immune suppression. However, HIFU does not cause this. On the contrary, studies have found that HIFU may directly enhance cancer specific immunity post-treatment [Citation3]. A proposed mechanism for this is enhanced T cell immunity [Citation8,Citation9]. Further to this, a study conducted using a mouse model suggested that in situ tumour ablation provided an antigen source for the generation of a specific anti-tumour response [Citation10]. Radiotherapy as a treatment modality is usually limited by a safe level of maximum exposures. In contrast, HIFU, often administered in a single session, has no maximum dose limit, and is hence repeatable in a refractive case [Citation3]. This is due to the lack of ionising radiation. HIFU also bears the advantage of being tissue-independent in terms of its effectivity, unlike radiotherapy and chemotherapy; even in mitotically inactive tissue, coagulative necrosis is achieved [Citation11].
HIFU has an excellent side effect profile. There are no surgical scars, there is a lower infection risk and the normal surrounding tissue is largely undisturbed. Modern technology allows for real-time image monitoring with HIFU. There are occasional adverse events that occur with treatment and although serious side effects are rare, occasional skin heating can lead to skin burns [Citation3,Citation12]. These, however, are usually superficial. Transient fever and pain have also been reported [Citation1,Citation3]. Transrectal devices for the treatment of prostate cancer occasionally lead to rectal wall injury, which has in turn caused recto-urethral fistulae [Citation3]. However, this complication has not been described in the current generation of commercially available clinical devices. Safety features have been implemented as well as an improved device design including rectal wall cooling. Another risk to consider is visceral perforation if there are tumours in close proximity to the bowel; ultrasound is also unable to propagate well through air. Hence, bowel and lung cancers, or tumours in their close vicinity, are unable to be a target.
HIFU is sensitive to organ movement so this must be controlled during the treatment procedure (2). One of the major limiting factors of HIFU is the treatment time which is often dependent on the volume of blood supply of the targeted tumour as well as the tumour volume. The combination of long treatment times and the need for immobility generally lend HIFU to be conducted under anaesthesia, although sedation is possible in some situations such as uterine fibroids [Citation3]. Even whilst taking the limitations into account, the consensus across various studies have found HIFU to be a safe and promising surgical tool.
Clinical applications of HIFU
HIFU has been a research focus in the UK for many years, mainly through the work of Professor Gail ter Haar and her team at the Institute of Cancer Research in Sutton Surrey [Citation13]. For the last 20 years, Oxford has been involved in both clinical and preclinical studies for the development of HIFU therapy. Since 2002, Oxford has established a dedicated therapeutic ultrasound unit that was founded by Professor David Cranston on the site of the Churchill Hospital, one of the main research sites for Oxford medicine. This was the first clinical HIFU centre in the UK dedicated to clinical studies and applications. Initially housed in a separate outbuilding in 2002 to enable CE-marking of the first extracorporeal focused ultrasound device in Europe, the HIFU unit became an integral part of the newly-built £125m Oxford Cancer Centre in 2009. The Oxford University Hospital was the first in the West to have the clinical Model-JC HIFU machine, made in Chongqing China. Following four successful trials that took place between 2002 and 2007 at Oxford, regulatory approval for the use of HIFU technology in Europe was given to Chongqing HAIFUTM Medical Technology Company. They obtained a CE mark for their products.
Dedicated pre-clinical focused ultrasound facilities are situated in the Biomedical Ultrasonics, Biotherapy and Biopharmaceuticals Laboratory (BUBBL) founded by Professor Constantin Coussios in 2004. Following successful fundraising and construction of the Institute of Biomedical Engineering, located on the Churchill Hospital site in 2008, BUBBL was permanently relocated in state-of-the art research facilities constructed for and dedicated to therapeutic ultrasound research, a 3-minute walk from the clinical therapeutic ultrasound unit.
Early Oxford trials
Liver
Some of the most prevalent malignancies worldwide are found in the liver, both primary and secondary tumours [Citation14]. Hepatic metastases are a leading cause of death in cancer patients, with 20–30% of those diagnosed with colorectal cancer already having liver metastases at the time of diagnosis. Surgery is the principal treatment for hepatic tumours but hepatic resection is only possible in 10–25% of cases [Citation14,Citation15]. A current alternative is combination chemotherapy, but the outcome is poor with a median survival of only 12 months [Citation15]. HIFU is the only entirely non-invasive local therapy to be proposed to date for the treatment of hepatic malignancies.
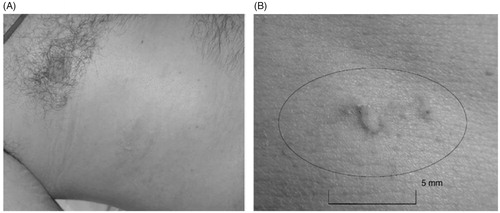
At Oxford, two prospective non-randomised phase II studies were conducted to evaluate HIFU effectiveness with liver and kidney tumour ablation. From November 2002 to May 2005, 30 patients, who had not responded to conventional therapies, were recruited. 22 patients had liver metastases and all the 8 patients with kidney tumours had renal cell carcinoma [Citation16]. The 22 patients recruited for the liver part of the study were subdivided into two groups: those being followed up with radiological imaging (15 patients); and those undergoing further surgery (7 patients). The patients all initially attended a single session of HIFU treatment and were followed up for radiological assessment on day 12 by MRI. An example of this imaging can be found in . Those recruited into the surgical trial underwent surgical resection of the tumours, enabling this group to have both radiological and histological assessment. Overall, evidence of ablation (either radiological or histological) was seen in 28 patients (93%) [Citation15]. Of the 29 patients who had radiological evaluation on day 12 post-HIFU, 27 had zones of ablation on the MRI. Accuracy was assessed as ‘good’ in 24 patients (89%). Specifically looking at the liver patients, ablation was seen in an astounding 100% of the patients, both radiologically or histologically assessed. Following treatment, adverse effects were mild and short-lived. Transient pain was reported in 25 patients (81%), superficial skin toxicity in 12 patients (39%) and one moderate skin burn was reported. An example of Grade 1 skin toxicity following intercostal HIFU treatment for liver metastasis can be seen in . These results demonstrated that HIFU was not only effective and accurate, but also a safe treatment method for liver tumours.
Figure 1. MRI scans demonstrating secondary colorectal carcinoma within the right lobe of the liver. Left: An MRI scan of a patient before HIFU treatment. Right: An MRI scan after HIFU treatment showing lack of contrast uptake (arrow).
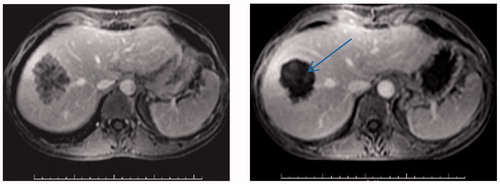
Figure 2. Photographs of Grade 1 skin toxicity following HIFU treatment for liver metastasis. (B) This is a magnified version of photo A, showing the lesion on a measured scale. This image is taken from Illing et al. 2005 (16).
A further study was designed as a part of these trials to test the ability of MRI to predict zones of necrosis following HIFU treatment [Citation15,Citation17]. Six patients with liver tumours were recruited in which tumour resection was conducted post-HIFU treatment. This allowed for histological analysis alongside the imaging results. Contrast-enhanced MRI successfully predicted complete ablation in three cases. In one case, the region of ablation on MRI seemed smaller than predicted at the time of HIFU, but histology showed complete ablation. The final two cases showed incomplete ablation. Furthermore, the estimated intra-operative ablation through B-mode ultrasound showed a clear correlation with the post-operative MRI estimates. In order to evaluate the intra-operative and MRI assessments of ablation, a slope of best fit was determined using linear regression. Results showed a slope of linear regression of 1.23 (95% CI = 0.68–1.77, p < 0.0001), indicating a clear correlation. HIFU-ablated tissue demonstrated coagulative necrosis as well as heat-fixation. However, the heat-fixed cells appeared normal under haematoxylin and eosin staining. Hence, this staining method is not a reliable indicator for HIFU-induced tissue death.
These studies have demonstrated that HIFU is a reliable treatment method for liver tumours within the western population. This is of particular importance as the mean UK population has a significantly higher body fat content in the abdomen when compared to the mean Chinese population, where the device was originally tested. Moreover, intra-operative assessment of treatment provides an accurate measure of the zone of ablation and correlates well with MRI follow-up [Citation15].
Kidney
The kidney is another solid organ which has been a target for HIFU therapy. The mainstay for treating localised renal cell carcinoma is surgery, but this has an associated morbidity. Nephron-sparing surgery is becoming increasingly attractive following a growing incidence of small renal cancers. Improved abdominal imaging, combined with an increase in occurrence, has led to this increased incidence [Citation18]. HIFU allows for ‘trackless’ ablation of kidney tumours. The phase II study mentioned in the liver section above, conducted at Oxford, evaluated the safety and feasibility of treating renal tumours; the extracorporeal Model-JC Tumour Therapy System (Chongqing HAIFUTM, China) was used [Citation16]. Eight patients with renal tumours were recruited. Under general anaesthesia, the patients underwent a single therapeutic HIFU session. To assess the response, MRI was conducted 12 days post-HIFU. These patients were then subdivided into two groups: those undergoing further surgical resection (five patients); and those being followed up with imaging alone (three patients). The former group allowed for both radiological and histological assessment. Results showed that discrete zones of ablation were found radiologically in 67% post-HIFU exposure. Although this data is still promising, it was not as successful as the results from the liver studies. A key difference between these organs is the layer of perinephric fat present surrounding the kidneys. This presents a fundamental barrier to HIFU treatment.
A further study was conducted at Oxford, which investigated HIFU for managing small renal tumours over a three year period [Citation18]. Seventeen patients were recruited with radiologically suspicious, non-metastatic renal tumours of an average size of 2.5 cm. These patients were at a higher risk for surgery or had declined surgery. Two patients were not able to undergo treatment as there was intervening bowel. The other patients were treated with extra-corporeal HIFU, again using the Model-JC system (Chongqing HAIFUTM, China), under general anaesthesia. All the patients only required one overnight hospital stay. Follow-up was conducted at 12 days post-HIFU and then every six months for a mean of 36 months. Of the fifteen patients that underwent HIFU, ten achieved a stable lesion with minimal morbidity; they were managed by HIFU alone. Four of the patients had imaging which suggested incomplete ablation and therefore went on to have other treatment. A further study was conducted where renal tumours less than 4 cm were treated via HIFU followed by partial nephrectomies [Citation14,Citation19]. These surgically resected specimens were then examined histologically. This study brought similar results as the previous. The success of the ablation relied on other factors including tumour position in relation to the ribs, as well as subcutaneous and perinephric fat.
Some patients with renal tumours have been treated in Oxford outside of trials. One case report describes a patient who developed a renal cell cancer in a donor allograft, detected eight years after the transplant [Citation20]. Renal cell carcinomas have an increased incidence after transplantation, although this is usually in the patient’s native kidney. The patient wanted nephron sparing treatment rather than a graft nephrectomy. Therefore, they were given HIFU treatment followed by a partial nephrectomy. The HIFU treatment was successful meaning that the patient was able to remain dialysis independent. Oxford is the only centre in England with an extracorporeal HIFU unit in which renal tumours are treated.
Transplanted kidneys have their perinephric fat removed which is advantageous for HIFU application. HIFU treatment is usually limited by heat deposition in perinephric fat, which has a de-focusing effect. To explore this limiting factor in renal applications, a study characterised the acoustic properties of fresh, unfixed human perinephric fat [Citation21]. The attenuation coefficient and the speed of sound through perinephric fat from patients undergoing cancer surgery was measured. No measurements of this kind with HIFU in human tissue had been previously published. Patients undergoing surgical resection due to renal cancer were recruited. The mean attenuation was 11.9 Np/m/MHz. This is significantly higher than human fat attenuation from other locations. Human perinephric fat is 2–4 cm in thickness as an average, with values higher in obese patients. With these values, the driving signal intensity would be reduced 3–62% compared with a hypothetical patient without perinephric fat. This has a significant effect on treatment delivery as increased energies would need to be used, increasing the side effect profile. Furthermore, the extent of spatial shift in focal position cause by the fat tissue at various positions in the HIFU field was quantified. Freshly excised porcine subcutaneous fat was used, which has a similar macroscopic and microscopic structure to human fat. 100 mm of fat lead to a de-focusing effect of approximately 1 mm in both transverse directions. This is approximately the same width as a full HIFU beam off target. Hence, this study shows the significant effect perinephric fat might have on the intensity and accuracy of HIFU treatment.
Prostate
HIFU has been shown to be a very promising approach for prostate conditions, especially prostate cancer. More than 100 sites across Europe use trans-rectal HIFU therapy [Citation2]. Currently, Sonablate®500 (Focus Surgery, Indianapolis, IN, USA), an American transrectal machine, is being used in Oxford for HIFU treatment of prostate cancer. The success of this can have a significant impact on the key treatment modalities used for prostate cancer. Whole gland therapy with transrectal US-guidance has an unwanted side effect profile including acute urinary symptoms; transurethral resection of the prostate (TURP) is therefore sometimes required prior or post-HIFU. However, HIFU is a favourable method for focal ablation. This minimises the effects of whole gland ablation including sexual, urinary and bowel side effects.
An ongoing multi-centre prospective development study including the Churchill Hospital, the INDEX study, is being conducted to evaluate focal therapy using HIFU [Citation22]. 140 men with low or intermediate risk prostate cancer were recruited into the trial. The risk profile was determined using the following specifications: PSA < 15, Gleason ≤ 7, ≤ T2cN0M0. Characterisation of the prostate was then performed using a combination of multi-parametric MRI and template prostate mapping biopsies. Following HIFU focal therapy, the effect of treatment was assessed with biopsies of the area, followed by biopsies at 36 months. An MRI was also performed between 1 and 3 weeks to ensure appropriate delivery of HIFU treatment. The primary outcome is the absence of clinically significant disease from the 36-month post-treatment biopsy and secondary outcomes cover the genito-urinary symptoms.
Furthermore, a large prospective, multicentre open-label feasibility study was conducted across five NHS hospitals in England which would compare management with HIFU and radical prostatectomy [Citation23]. Eighty-two men were recruited in total, with 41 in each management subgroup. This feasibility study successfully showed that randomisation is possible when recruiting patients for partial ablation with HIFU and radical prostatectomy. This study will be utilised as a platform to create a future randomised control trial (RCT) which can compare clinical and cost-effectiveness, as well as quality of life outcomes. Hence, high quality evidence can be collected to support partial ablation as an alternative management strategy within the NHS.
Prostate cancer management is dependent on the stage of the tumour, there is no universal gold standard treatment [Citation1]. This is unlike benign prostate hypertrophy (BPH) where TURP has shown excellent results. Studies have not yet shown HIFU to surpass the efficacy of TURP in benign disease. This may be due to lower treated volumes in a given time. Another reason may be because tissue is physically removed in TURP whereas the necrotic tissue must be phagocytosed with HIFU treatment. Therefore, unless a larger volume of tissue can be treated in a similar time, HIFU does not yet show promise to produce results comparable to TURP in treating BPH.
Uterine fibroids
HIFU has become a standard of care for fibroid ablation in other countries [Citation24]. 20–25% of women of childbearing age have uterine fibroids [Citation25]. Symptomatic fibroids can considerably decrease quality of life with symptoms including menorrhagia, pelvic pain or increased urinary frequency. Conventional therapy includes hysterectomy, myomectomy or hormone therapy. The first study on the NHS population looking at ultrasound-guided HIFU ablation for symptomatic uterine fibroids was conducted at Oxford [Citation24]. This was a single-centre study at Oxford University Hospitals. 22 patients were referred with symptomatic fibroids; these patients had all declined standard surgical or radiological intervention. 12 out of the 22 patients underwent a single session of US-guided HIFU ablation with a total of 14 fibroids ablated. They all received a 2-year MRI follow up and uterine fibroid symptom severity scores were used to monitor success of the treatment. There were no reported severe side effects, however a 2nd degree burn was present over a surgical scar where a patient previously had a caesarean section. Results showed that the mean volume reduction rates of treated fibroids were 49.3% ± 23.7% at 12 months (p < 0.05). Moreover, the mean symptom severity scores improved significantly from 56.5 ± 29.1 at baseline to 33.4 ± 23.3 (p < 0.01) at 3 months, 45 ± 35.4 (p < 0.05) after one year and finally 40.6 ± 32.7 at two years post-treatment. This study demonstrates the clinical efficacy of US-guided HIFU treatment of uterine fibroids with a low risk of complications.
An advantage brought by HIFU for symptomatic fibroids is its repeatability; if symptoms were to recur the procedure can be repeated without a limit unlike radiotherapy. Based on Oxford clinical results, in combination with published articles worldwide, the National Institute of Health and Care Excellence (NICE) promulgated the first clinical guidance of ultrasound-guided high intensity transcutaneous focused ultrasound for symptomatic uterine fibroids in 2019. This has promoted HIFU fibroid treatment from clinical study to the clinical practice setting in Oxford, and more fibroid patients will benefit from this non-invasive treatment in the future.
Pelvic chordoma
In China, HIFU therapy for soft tissue sarcoma is also an established treatment method [Citation1]. A chordoma is a form of low-grade soft tissue sarcoma, which arises from the remnants of the notochord. This tumour subtype is rare, with a prevalence of less than one case per million per annum. The critical problem with treating this tumour type is the location as it is adjacent to many critical pelvic structures. Hence the mainstay treatment option, curative surgical resection, is not always feasible. Another key problem is delayed presentation as the symptoms are slowly progressive and non-specific during the early stages of the disease. Symptoms include bladder and bowel dysfunction, sacral and lower limb pain, as well as pelvic masses. Alongside surgery, adjuvant radiotherapy is given. Radiotherapy has a maximum dose and so repeated cycles are not always possible. HIFU is able to overcome some of these challenges and has been shown to successfully treat sacral chordomas in four patients at the Churchill Hospital, Oxford [Citation11,Citation26].
The suitability of the patients was determined from a combination of the patient’s MRI pelvis and the clinical data. Thirteen patients were referred of which four were suitable and received treatment. Any previous surgical intervention or radiotherapy, if conducted more than 12 months prior, did not contraindicate HIFU treatment. Patients 2, 3 and 4 all had treatment under general anaesthesia but patient 1 was only given sedation, which allowed them to verbally feedback during the procedure. This helped prevent sciatic nerve injury but ensured the patient remained comfortable. Only 3 of the patients were followed up as patient 4 lived abroad and was not able to attend follow-up due to reduced mobility. In patients 1 and 2, a reduction in tumour volume was seen 6 months post-HIFU. Patient 3 only had reports; the original imaging scans were not able to be seen. However, the reports showed a stable reduction of tumour volume. illustrates MRI scans pre- and post-HIFU treatment in patient 1, with successful tissue necrosis after treatment. The side effect profile following treatment was very promising. The most commonly experienced symptom was mild discomfort, resolved by analgesia overnight. Patient 2 developed tissue oedema at the target site and numbness over the buttock, right leg and perineum. They had to intermittently self-catheterise but they were able to return to normal bladder habits one month post-treatment. Patient 3 developed a superficial blister and experienced leg weakness which improved within one month.
Figure 3. MRI scans in an axial plane demonstrating the sacral chordoma in patient 1. (A) Pre-HIFU treatment MRI scan. (B) HIFU induced necrosis highlighted by the arrow in a post-HIFU treatment MRI scan. Images taken from Gilles et al. [Citation11].
![Figure 3. MRI scans in an axial plane demonstrating the sacral chordoma in patient 1. (A) Pre-HIFU treatment MRI scan. (B) HIFU induced necrosis highlighted by the arrow in a post-HIFU treatment MRI scan. Images taken from Gilles et al. [Citation11].](/cms/asset/9f3d1d94-00b0-4e17-8076-7d102c21dfdd/ihyt_a_1899311_f0003_b.jpg)
This case series demonstrated both the safety and feasibility of successful ablation of chordoma [Citation11]. Although there are some risks named above, when placed against its alternative – debulking surgery – it still is positively comparable. Also, surgery may involve pelvic exenteration. In order to further investigate the effectiveness of HIFU in sacral bone tumours, a national trial was established: phase IIb trial of clinical efficacy, South East Scotland REC 02 Ref12/SS/0144, ISCRTN 91527768. The primary end points of the trial include 5-year survival, pain and of quality-of-life measures. The volume of tumour ablation as well as the number and level of adverse events were chosen as secondary end points. This further research will help determine whether this non-invasive treatment gives rise to improved patient outcomes.
Pancreas
Pancreatic cancer is a devastating disease with a poor prognosis and is predicted to become the second most common cause of death in the Western world within the next decade. In the UK, there were 9408 newly diagnosed cases of pancreatic cancer in 2013, with 8847 associated deaths in 2014. After diagnosis, only 20% of patients are alive after 1 year, 3% alive after 5 years and <1% survive after 10 years. Life expectancy for metastatic cancer is 5–6 months and 10–12 months for locally advanced cancer.
Surgery gives the best chance of a cure, but only 15–20% patients are suitable for surgery. Pancreatic cancer often afflicts the elderly and frail and many patients, even with operable cancers, are not fit for major surgery. Palliative chemotherapy with newer regimens has increased the median survival from 6 months to 11 months but long-term survival beyond 3 years is still rare. Moreover, many patients do not tolerate this intensive chemotherapy. Radiotherapy has not yet shown definite benefit.
HIFU has been largely reported as a palliation option to treat patients with advanced pancreatic cancer. In Oxford, a multi-centre clinical trial is being carried out to investigate the feasibility, safety and efficacy of HIFU for patients with pancreatic cancer. Patients with locally advanced cancers or who are not suitable for surgery, without metastatic disease, are recruited into the trial. They have chemotherapy for 3 months as per current standard of care, followed by HIFU treatment. Patient care is then transferred back to the treating oncologist once patients recover from HIFU and have no evidence of ongoing complications. All treated patients are followed up for two years. The primary outcome measure is progression free survival from HIFU. The secondary outcome measures included overall survival, local progression rates, complications related to HIFU and quality of life. Immune monitoring following HIFU includes peripheral blood regulatory and cytotoxic cell profiles. Up to now, two patients have successfully received HIFU treatment without any side effects. Due to the Covid-19 pandemic, the trial was suspended for 6 months in 2020 but is now open to recruiting patients.
Breast
HIFU for breast cancer may be a suitable treatment modality in combination with other therapies [Citation14]. It may lead to improved cosmetic alongside oncologic outcomes by allowing conservation of mammary shape. Furthermore, breast tissue is ideally placed superficially allowing a clear path for the HIFU beam [Citation27]. In China, HIFU is well-established in breast cancer treatment. 106 patients have been treated in multicentre studies using the Chongqing HIFU system [Citation1]. At Oxford, a pilot study looking at breast cancer has been funded and approved by the local ethic committee. The results from these studies will hopefully bring similar effectiveness as those carried out in China.
Brain
Historically, HIFU studies were tested for Parkinson’s disease. William Fry et al. produced lesions deep in the brains of cats and monkeys [Citation1,Citation28]. However, progress was restricted by practical and technological limitations. Challenges brought forward include aberration caused by the skull when a HIFU beam is applied. A craniotomy was required in order to obtain an acoustic window to the brain and drug soon superseded this method [Citation14]. HIFU can now be put through intact skull via time reversal therapy which overcomes the aforementioned limitation [Citation1]. Therefore, it is possible to treat brain tumours where focal ablation will be beneficial.
In Oxford we have investigated neurostimulatory responses in healthy volunteers to establish and further investigate the mechanistic basis of FUS neurostimulation. We are presently developing an approach for drug delivery that relies on highly targeted therapeutics delivered alongside an unfocused low-intensity ultrasound field in the brain.
Ultrasound-mediated drug delivery
A novel application has been investigated to explore the feasibility of ultrasound-mediated anti-tumour drug delivery. This work is beyond ablation, which uses a lower ultrasound energy for treatment. A pioneering trial of Oxford clinical therapeutic ultrasound research has been the exploration of ultrasound as a tool for drug delivery. This uses both thermal mechanisms for mild-hyperthermia triggered drug delivery from thermosensitive liposomes, and cavitational mechanisms for enhanced delivery and distribution of unmodified small-molecule and antibody therapeutics. The first-in-human trial of ultrasonically triggered drug delivery in oncology, TARDOX, was completed in 2018 and published in Lancet Oncology [Citation29]. Two further studies, one to treat pancreatic tumours with thermosensitive liposomal doxorubicin and one to treat metastatic colorectal tumours in the liver by cavitation-enhanced antibody delivery, are currently starting.
The aim of the TARDOX trial [Citation29–32] was to assess the safety and feasibility of delivering doxorubicin to solid tumours via thermosensitive liposomes. This was a phase I trial conducted at Churchill Hospital. The patient group included those with solid tumours of any histological subtype, which were unresectable and unable to ablate. Ten patients were enrolled. Each patient received an intravenous infusion of lyso-thermosensitive liposomal doxorubicin. Following this, they were exposed to focused-US for a single target liver tumour. Biopsies were obtained pre- and post-procedure to ascertain the doxorubicin concentration intratumourally. Results showed an average increase of a factor of 3.7 from 2.34 μg/g after drug infusion compared with 8.56 μg/g post-focused US. The primary end-point was chosen to be at least a doubling of doxorubicin concentration in a minimum of half the patients. This was successfully met as two to ten times increases in concentrations were seen in seven patients. The side effects reported include transient neutropenia in five patients as well as confusion in one patient. This study has demonstrated that the extracorporeal focused US method can enhance the delivery and distribution of anti-cancer drugs by causing an increased intratumoural drug concentration. Along a similar line of thought, focused US has been proposed as a vehicle for delivering targeted gene therapy [Citation14]. The US would lead to induced cavitation of DNA-laden microbubble contrast agents.
Future directions
Oxford HIFU clinical experience has only demonstrated the beginnings of its clinical potential. Many further studies need to be conducted in order to explore the broad range of possibilities for its applications. Tissue ablation has been the main focus as a target so far. However extracorporeal devices have been established as targeted drug delivery adjuncts. Under the direction of Professor Constantin Coussios, Oxford has built on its expertise for the generation, mapping and control of cavitation activity. The technology is directed at a wide range of novel applications of heat and cavitation-mediated drug delivery, immune-stimulation, transdermal vaccination, sonodynamic therapy and bone healing, as well as a focusing regime whereby focused ultrasound causes no measurable irreversible changes to tissue. US-mediated cavitation serves as a local stimulatory or transport mechanism that can mediate long-term, and ideally systemic, therapeutic effects in the absence or presence of co-administered therapeutics.
As well as the expanding clinical target for HIFU, the future of this method has the potential to further benefit from advances in the technology. This could offset some of its present limitations. The speed of the procedure is partially dependent upon the size of the target. With larger areas that need to be ablated, the time required may present as a significant limiting factor. Hence, exploring a technique to increase the speed of tissue destruction will be of great benefit. There are various methods postulated to achieve this. The acoustic field can be modified using phase array transducers or the tissue itself can be modified. An example of the latter is by altering vascular perfusion by introducing small gas bubbles which locally increases the rate of temperature rise. By honing the technology and broadening the clinical targets, HIFU will be able to realise its full potential as a surgical tool.
Conclusions
The current body of data is promising and indicates that HIFU is a technology that will bring immense utility to the medical world. Oxford has explored various targets for HIFU, both benign and malignant. These studies have been pivotal to the incorporation of HIFU as a surgical management strategy in Europe. Over 190 trial patients have been successfully treated in Oxford with minimal morbidity and no mortality. The studies all bring forward three key attributes of HIFU: its safety, wide tolerability and effectiveness. Larger clinical trials will further elucidate this utility and define its emergent role. This role is ever-expanding by using it in conjunction with other treatment modalities; ongoing clinical research is in place to enhance current technology. With these advancements, it has the potential to successfully fill the gap for patient cohorts where the current standard treatment option is unfeasible. HIFU can assuredly provide the truly non-invasive targeted treatment option that has long been sought after by clinicians.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kennedy JE, Ter Haar GR, Cranston D. High intensity focused ultrasound: surgery of the future? Br J Radiol. 2003;76:590–599.
- Izadifar Z, Chapman D, Babyn P. An introduction to high intensity focused ultrasound: systematic review on principles, devices, and clinical applications. J Clin Med. 2020; 9:460.
- Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327.
- Kennedy JE, Wu F, Ter Haar GR, Gleeson F V., Phillips RR, Middleton MR, Cranston D. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931–935.
- Kennedy JE, Ter Haar GR, Wu F, Gleeson F V., Roberts ISD, Middleton MR, Cranston D. Contrast-enhanced ultrasound assessment of tissue response to high-intensity focused ultrasound. Ultrasound Med Biol. 2004;30:851–854.
- ter Haar G, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperth. 2007; 23:89–104.
- Oosterhof GON, Cornel EB, Smits GAHJ, Debruyne FMJ, Schalken JA. Influence of high-intensity focused ultrasound on the development of metastases. Eur Urol. 1997;32:91–95.
- Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, et al. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217–1222.
- Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses – inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17:49–64.
- Den Brok MHMGM, Sutmuller RPM, Van Der Voort R, Bennink EJ, Figdor CG, Ruers TJM, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004; 64:4024–4029.
- Gillies MJ, Lyon PC, Wu F, Leslie T, Chung DY, Gleeson F, et al. High-intensity focused ultrasonic ablation of sacral chordoma is feasible: a series of four cases and details of a national clinical trial. Br J Neurosurg. 2017;31:446–451.
- Cranston D. High intensity focused ultrasound. Sci Omega Rev UK. 2014.
- Ter Haari G, Sinnetts D, Rivensi I. High intensity focused ultrasound-a surgical technique for the treatment of discrete liver tumours. Phys Med Biol. 1989;34:1743–1750.
- Cranston D. A review of high intensity focused ultrasound in relation to the treatment of renal tumours and other malignancies. Ultrason Sonochem. 2015;27:654–658.
- Leslie T, Ritchie R, Illing R, Ter Haar G, Phillips R, Middleton M, et al. High-intensity focused ultrasound treatment of liver tumours: post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br J Radiol. 2012;85:1363–1370.
- Illing RO, Kennedy JE, Wu F, Ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–895.
- Leslie TA, Kennedy JE, Illing RO, Haar GRT, Wu F, Phillips RR, et al. High-intensity focused ultrasound ablation of liver tumours: can radiological assessment predict the histological response? Br J Radiol. 2008;81:564–571.
- Ritchie RW, Leslie T, Phillips R, Wu F, Illing R, Ter Haar G, et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010; 106:1004–9.
- Marberger M, Schatzl G, Cranston D, Kennedy JE. Extracorporeal ablation of renal tumours with high-intensity focused ultrasound. BJU Int Suppl. 2005;52–55.
- Chakera A, Leslie T, Roberts I, O’Callaghan CA, Cranston D. A lucky fall? Case report. Transplant Proc. 2010; 42:3883–3886.
- Ritchie R, Collin J, Coussios C, Leslie T. Attenuation and de-focusing during high-intensity focused ultrasound therapy through peri-nephric fat. Ultrasound Med Biol. 2013;39:1785–1793.
- Dickinson L, Ahmed HU, Kirkham AP, Allen C, Freeman A, Barber J, et al. A multi-centre prospective development study evaluating focal therapy using high intensity focused ultrasound for localised prostate cancer: The INDEX study. Contemp Clin Trials. 2013; 36:68–80.
- Hamdy FC, Elliott D, Le Conte S, Davies LC, Burns RM, Thomson C, et al. Partial ablation versus radical prostatectomy in intermediate-risk prostate cancer: The PART feasibility RCT. Health Technol Assess (Rockv). 2018; 22:1–96.
- Lyon PC, Rai V, Price N, Shah A, Wu F, Cranston D. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschall der Medizin. 2020;41:550–556.
- Chen J, Li Y, Wang Z, McCulloch P, Hu L, Chen W, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG An Int J Obstet Gynaecol. 2018; 125:354–364.
- Chetan MR, Lyon PC, Wu F, Phillips R, Cranston D, Gillies MJ, et al. Role of diffusion-weighted imaging in monitoring treatment response following high-intensity focused ultrasound ablation of recurrent sacral chordoma. Radiol Case Reports. 2019;14:1197–1201.
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015; 31:302–309.
- Fry WJ, Barnard JW, Fry FJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science. 1955; 122: 1091.
- Lyon PC, Gray MD, Mannaris C, Folkes LK, Stratford M, Campo L, et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. Lancet Oncol. 2018; 19:1027–1039.
- Paverd C, Lyka E, Elbes D, Coussios C. Passive acoustic mapping of extravasation following ultrasound-enhanced drug delivery. Phys Med Biol. 2019;64: 045006.
- Kwan JJ, Myers R, Coviello CM, Graham SM, Shah AR, Stride E, et al. Ultrasound-propelled nanocups for drug delivery. Small. 2015;11:5305–5314.
- Qiao S, Elbes D, Boubriak O, Urban JPG, Coussios CC, Cleveland RO. Delivering focused ultrasound to intervertebral discs using time-reversal. Ultrasound Med Biol. 2019;45:2405–2416.
