Abstract
Objective
To document longitudinal symptom, quality-of-life and imaging response in patients with recurrent gynecological tumors treated with magnetic resonance guided high intensity focused ultrasound (MRgHIFU), and compare changes in patients with intra- versus extra-pelvic lesions.
Methods
Eleven symptomatic patients with painful recurrent gynecological tumors were treated with MRgHIFU (Profound Sonalleve) in a prospective single center study (NCT02714621). Pain scores, analgesic intake and quality-of-life metrics, whole tumor volume, and perfused tumor volume from Gadolinium-enhanced T1W imaging documented before and up to 90 days after treatment were compared between patients with intra- and extra-pelvic tumors.
Results
Two of five patients with intra-pelvic and three of six patients with extra-pelvic tumors were classified as responders (>2 point reduction in NRS pain score without analgesia increase or a > 25% reduction in analgesic use). Cohort reductions in worst pain scores were not significant for either group. Emotional functioning for the whole cohort improved, although physical functioning did not. Ablative thermal temperatures were achieved in three patients with extra-pelvic tumors, but in none whose tumors were intra-pelvic. Pain response did not correlate with thermal dose. Tumor volume increased by 18% immediately post-treatment in the extra-pelvic but not in the intra-pelvic group. Ratio of perfused to whole lesion volume decreased by >20% by day 30 in extra-pelvic, but not intra-pelvic tumors although at day 30 both extra-pelvic and intra-pelvic tumors increased in volume.
Conclusion
MRgHIFU treatments can be delivered safely to patients with recurrent gynecological tumors. Extra-pelvic tumors responded better than intra-pelvic tumors and showed immediate swelling and reduction in perfused volume by day 30.
Introduction
Recurrent gynecological cancer is associated with significant morbidity and, if uncontrolled, causes progressive pain and bleeding [Citation1]. Previously irradiated patients are often unsuitable for re-irradiation and their disease extent and location may exclude them from exenterative surgery. Additionally, response to systemic therapy is usually poor within the irradiated pelvis. Progressive symptoms negatively impact quality-of-life (QoL) in these patients.
High-intensity focused ultrasound (HIFU) is a precise thermally ablative technique that is increasingly being trialed in a number of tumor types [Citation2–4]. Using the Sonalleve device (Profound Medical, Canada) localized areas of high temperature (50–80 °C) are generated during an exposure at a focus described as a treatment ‘cell’ (unit of tissue to which energy is applied). This causes protein denaturation and coagulative necrosis leading to cell damage and death [Citation5]. Outside the focus, energy deposition is lower, so that pre- and post-focal tissues are spared thermal or mechanical damage. After an exposure, heat is dissipated to the surrounding tissues allowing repeated exposures to be carried out over a relatively short time [Citation6–8]. The thermal dose delivered is measured as 240 equivalent minutes (EM) at 43 °C. When undertaken under magnetic resonance (MR) imaging guidance, it is possible not only to target the HIFU beam geometrically but also to provide real-time feedback on temperature changes within the treatment cell and the surrounding tissue, using MR thermometry [Citation9,Citation10]. MR-guided HIFU (MRgHIFU) is ideal for use in the previously irradiated pelvis where re-irradiation is not an option because of potential morbidity to surrounding normal tissue.
Within the pelvis, MRgHIFU is now an established ablative therapy for symptomatic benign uterine fibroid disease where it has been shown to be a cost effective and safe treatment modality compared to surgery [Citation11–14]. After treatment, Gadolinium enhanced T1-weighted (Gd-T1W) imaging can show non-perfused regions indicative of tissue ablation, and assess changes in tumor size after treatment [Citation15]. Nevertheless, the feasibility of treating malignant disease with MRgHIFU, particularly when disease is located at deep intra-pelvic sites with overlying rectum has not been established. The purpose of this study therefore was to establish the feasibility of treating recurrent gynecological malignancy with MRgHIFU, to document longitudinal symptom, QoL and imaging response, and to compare responses between patients with intra-pelvic (within anatomical pelvis) versus extra-pelvic lesions.
Materials and methods
Study population
This was a prospective single-center, institutional review board approved, feasibility study (NCT02714621). Patients with intra-pelvic and extra-pelvic recurrent gynecological malignancy unsuitable for other therapies, or who declined standard treatment, were recruited (January 2018–May 2019). Participants were symptomatic from their target lesion, defined as an 11-point Numerical Rating Scale (NRS) pain score of ≥4/10 despite analgesia and/or bleeding. All patients had pain, and two also had bleeding. Patients received the patient information sheet at least 24 h before providing written informed consent. They underwent an initial planning MRI scan in the treatment position on the HIFU device before treatment to ensure that the lesion was at least partly accessible to treatment without over-exposing critical structures (bowel, bladder, bone, nerves, and major blood vessels).
Patient preparation
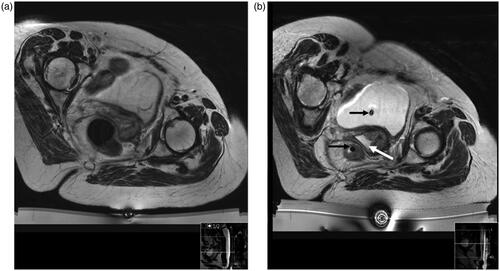
For centrally recurrent disease, patients commenced a low residue diet a week before treatment and self-administered a micolette® enema for 3 days. On the day of treatment, patients had the skin over the treatment area depilated to reduce air trapping. For those with intra-pelvic tumors, a urinary catheter was inserted and clamped following anesthesia, after filling the bladder with 200 ml of sterile 0.9% normal saline. This improved visualization of the tumor-bladder wall interface for centrally recurrent lesions and served to dissipate heat beyond the HIFU treatment focus. A second urinary catheter introduced concurrently into the rectum enabled release of rectal gas. Degassed gel (1:2 ultrasound gel to de-ionized water) was introduced via a catheter into the vagina to reduce air in the HIFU beam path (). Bladder and bowel preparation was not required in cases of previous pelvic exenteration (n = 2), popliteal (n = 1), or inguinal node treatments (n = 2).
Figure 1. Patient with central recurrence in position within the MRI scanner with the HIFU device in place. T2-W image at planning study (a) and on day of treatment (b) after bowel and bladder preparation. In b, urinary and rectal catheters (black arrows) are noted in situ. The vagina is filled with degassed gel (white arrow).
Patient positioning
The planning scan guided patient positioning on the day of treatment and placed the target lesion as close as possible to the center of the HIFU window. The supine oblique position was used for five patients with intra-pelvic lesions and three with extra-pelvic lesions; the prone oblique position was used for three patients with extra-pelvic lesions.
A dampened gel pad (Aquaflex, Parker Laboratories Inc., Fairfield, NJ) cut to size was placed between the patients’ skin and the HIFU window to ensure good acoustic contact, and degassed water was used to enhance acoustic coupling. In two patients, with skin irregularity overlying the treatment area, a 40 mm thick gel pad was custom sculpted to the patient’s anatomy to achieve acoustic contact with the HIFU window.
Treatments were performed with a Profound Sonalleve device within the bore of a 3 T Philips Achieva (Philips Medical Systems, Best, The Netherlands) under heavy conscious sedation (n = 4), spinal block with conscious sedation (n = 2), regional nerve block (n = 1), or general anesthesia (n = 4).
Imaging and image-guided treatment
T1W, T2W, and diffusion-weighted sequences with a field-of-view (FOV) that covered the entire region-of-interest were obtained pretreatment. A Dixon sequence was acquired before and after administration of 0.2 ml/kg gadolinium (Gd)-contrast agent. Sequence details are given in Supplementary Table S1. Proton Resonance Frequency Shift (PRFS)-based MR thermometry indicated the temperature change at and around the focus. Cells of 4 and 8 mm diameter were used with those located at the greatest depth delivered first, to avoid making exposures through already heated regions. The extent of treatment varied between patients and depended on the risk of exposure to surrounding structures. To reduce risk to the skin and subcutaneous tissue, the time allowed for cooling between each exposure always exceeded the minimum cooling periods mandated by the Sonalleve software.
The same MR imaging sequences were acquired at baseline and at follow-up visits. Gadolinium-enhanced scans were performed at the screening visit and post-treatment; they were omitted immediately pretreatment to avoid dissociation of the gadolinium chelate during heating. Immediately post-treatment, the T1W Dixon sequences (for registration with post-contrast images) also were re-acquired and then repeated following administration of 0.2 ml/kg Gd-contrast agent.
Data collection
Baseline demographic data and symptom assessment was done at screening. Symptoms were re-assessed for a week before treatment, for 30 days after treatment, and at days 60 and 90 post-treatment.
Patients completed diaries (day −7 to day 30) that detailed pain scores (0–10 scale), analgesic use and blood loss, (if any, by number of pads required per day). In addition, patients completed three validated questionnaires at screening, and on days 1, 7, 30, 60, and 90 after treatment. These were the Brief Pain Inventory short form (BPI), QLQ-C15-PAL and EQ-5D-5L. The EQ-5D-5L captured overall health status and the QLQ-C15-PAL assessed QoL more specifically [Citation16–18].
Adverse events (AEs) were classified using Common Terminology Criteria for Adverse Events (CTCAE) v4.1. They were further categorized as definitely/probably/possibly/unlikely device-related (from MRgHIFU), study-related (from study procedures), or unrelated to treatment.
Data analysis
The number, diameter, total volume, and location of treatment cells planned for each patient were recorded. The duration and the power of each sonication were noted; their product provided the applied acoustic energy of each sonication. Thermal changes were measured on PRFS by estimating ablative thermal dose volume, calculated as the product of 3 orthogonal maximum dimensions of the 240EM at 43 °C dose contour [Citation19,Citation20]. The sum of ablative thermal dose volumes for all sonications for each patient was V240EM. In addition, the maximum temperature recorded in the tumor during each sonication was used to calculate the mean maximum temperature (TM) from all sonications for each patient.
As all patients had pain, they were categorized as responders or non-responders by comparing their baseline NRS score with their average score for days 28–30. They were considered to be a responder if they had: (i) an improvement of ≥2 points in their reported pain at or after day 30, provided they had <20% increase in their analgesic use, (ii) ≥25% reduction in their analgesic use without change in their reported pain
T1W images were used to estimate any changes in maximum tumor diameter from baseline. Gd-T1W images were used to measure non-perfused volume (NPV), by drawing regions-of-interest (ROIs) on the baseline, immediate post-treatment and day 30/60/90 images. The total NPV was calculated from the product of summed ROI areas and slice thickness.
Statistical analyses
Analyses were descriptive due to the low number of patients in this feasibility study. Continuous variables were summarized using mean, standard deviation, median, quartiles, minima and maxima, and categorical variables using counts and percentages and Spearman’s non-parametric tests to assess differences between groups. Safety data were reported using the number of patients with adverse events using CTCAE v4.1 grade as assessed by patient reported outcomes, and clinical review and examination.
Results
Patients and treatments
Thirteen patients were recruited and 11 treatments performed in 10 patients (one patient had the same target pelvic lesions treated twice). shows patient demographics and tumor location. Two patients failed screening: one had no visible macroscopic disease and the other had a lesion lying deeper than the focal reach of the device.
Table 1. Patient characteristics of those treated.
Adverse events
There were no anesthesia-related complications. There were no serious adverse events (SAEs) observed during or immediately after any of the treatments. The most common treatment-related AEs were localized to the skin (n = 5) and pain flare (n = 2). Three patients experienced mild (Grade 1) skin erythema managed with ice packing immediately post-procedure; this resolved within 24 h. Additionally, patient 4 sustained a burn (which developed into a Grade 2 burn) to skin within the treatment path, due to a combination of the skin-to-skin interface of the groin fold and adjacent scar tissue. This was managed conservatively in the community by the tissue viability team. Persistent pain from this at day 60 contributed to this patients increasing pain scores. In patient 9, erythema observed over the sacral area developed into 2 small blisters that resolved within 30 days. In both these cases, energy/temperature changes at the skin were indicated by the thermometry feedback during treatment and had been noted as temperature increases consistent with skin erythema. As a skin burn was not anticipated, we continued treatment after a cooling period that extended 5 min beyond that recommended by the software.
Symptomatic response
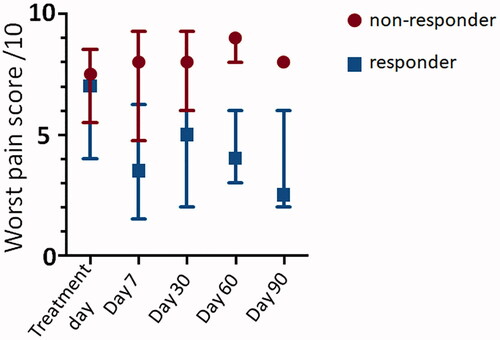
All patients had pain and completed their diaries from 7 days prior to treatment to day 30 post-treatment. The baseline diary pain score was the statistical mode of all the pretreatment patient reported scores. All patients completed day 30 follow-up with four patients well enough to complete all follow-up (Supplementary Table S2). Five patients were classified as responders (patients 1, 6, 7, 8, and 10), the other five (one treated twice) were non-responders. Differences in BPI pain scores between responders and non-responders were greater at day 7 than day 30 (, patient numbers too small for statistical analysis). Composite BPI severity score (range and median) at baseline, days 7 and 30 for responders was 1.5–6.0 and 5.0; 0.5–4.0 and 2.9; 0–4.75, and 4.3, respectively, and for non-responders, it was 2.75–8.0 and 5.0; 1.75–9.0 and 3.8, 1.5–8.25 and 4.3, respectively. This was supported by the average diary pain scores (range and median) at baseline and day 30 for responders (5.4–9.3 and 6.1; 3.8–7.0 and 5.0, respectively) and non-responders, (3.1–9.8 and 7.7; 0.8–10.0 and 7.6, respectively). ,b) illustrates changes in pain scores by response category and lesion location. Worst pain, but not least or current pain, improved in responders but not non-responders ().
Figure 2. Box and whisker plot summarizing percentage change in NRS pain scores for (a) responders (green, n = 5) and non-responders (red, n = 6) and (b) intra-pelvic (green, n = 5) versus extra-pelvic (blue, n = 6) tumors. Median (central line) and upper and lower quartiles are indicated by the upper and lower boundaries of the box. The whiskers denote maximum and minimum values.
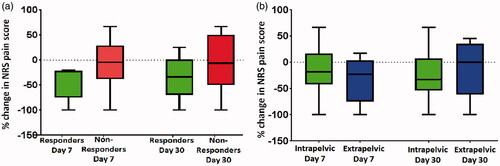
In patients with intra-pelvic lesions, two of five responded, two experienced pain progression at day 30 and one patient’s pain and analgesic use remained unchanged. Of patients with extra-pelvic lesions, three were responders, in all of whom imaging changes seen within their target tumor. However, patient 8 only achieved 25% reduction in pain score by day 60 ().
Table 2. Relationship of thermal energy delivered and dose to change in NRS pain score at each time-point.
Per vaginal (PV) bleeding was present at baseline in two patients. One had subjective improvement but this was not reflected in her diary record because of a continuing colorless discharge, which still required pads. The other patient with involved vaginal margin inaccessible had no reported change in bleeding (mean severity score 3, requiring three pads per day).
Relating pain scores to thermal dose delivered
Three patients (all extra-pelvic) had 240 EM dose contours within the tumor (). Lack of 240 EM in all but one of the patients with intra-pelvic tumors meant that mean focal temperatures were <55 °C. In this small patient cohort, there was no observable relationship between thermal dose delivered and percentage change in pain score or between V240EM (where recordable) and change in pain score.
Quality-of-life measures
Using the EORTC-C-15PAL for the whole cohort, the data showed that although physical functioning did not improve with time, emotional functioning did; other symptoms remained stable (). Three responders improved in their physical functioning, emotional functioning, and overall QoL with increasing time after treatment. Three non-responders declined in their physical functioning, emotional functioning, and overall QoL with increasing time after treatment. Two patients (one treated twice) experienced no change in their QoL. The EQ-5D-5L showed clear improvement in the index value and in the visual analogue pain score for patient 1 with time from treatment, and clear decline in patient 11, but data from all other patients was variable, making it less useful than the EORTC-C-15PAL and Supplementary Figure S1).
Table 3. Percentage change in mean scores for each QoL feature.
Imaging changes after treatment
One extra-pelvic tumor was ablated entirely (no longer visible following contrast administration, ); the other four all showed an immediate increase in total tumor volume (18.3 ± 5.4%). Intra-pelvic tumors showed no significant increase in volume immediately post-treatment (0 ± 10.8%). At days 7 and 30 both extra-pelvic and intra-pelvic tumors demonstrated growth ( and Supplementary Table S3).
Figure 4. Axial T1W image with fat suppression pulse and after contrast enhancement with gadolinium chelate before and after treatment of an extra-pelvic tumor. The pretreatment image shows the enhancing nodule of recurrent tumor in the left ischio-rectal fat (yellow arrow). Immediately post-treatment there is complete ablation of this enhancing lesion (yellow arrow).
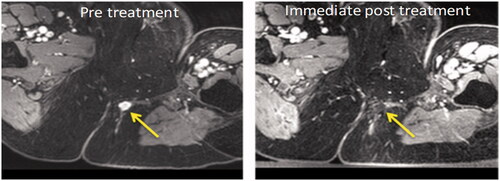
Table 4. Whole tumor and enhancing tumor volumes at baseline and longitudinal changes over 30 days.
Ratios of enhancing to whole tumor volumes at baseline ranged from 0.47 to 1.0. Immediately post-treatment there was a decrease in this ratio for extra-pelvic tumors (three patients showed >10% reduction, 1 showed a 46% increase), but no change in the intra-pelvic ones ( and ). However, variability in the extra-pelvic tumors was high. Necrosis of gluteal fat was noted in the pre-focal region in two subjects by day 30 (increased signal-intensity on fat-suppressed images with associated loss in tissue volume).
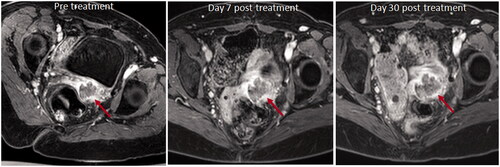
Figure 5. Axial T1W images with fat suppression pulse and after contrast enhancement with gadolinium chelate before and after treatment of an intra-pelvic tumor. The pretreatment image shows the non-enhancing tumor mass at the vaginal vault on the left (red arrow). There is no substantial change in tumor volume post treatment, either at Day 7 or Day 30, and no change in the relative enhancing and non-enhancing components (red arrows).
Discussion
This study demonstrates the feasibility of performing MRgHIFU in patients with recurrent gynecological tumors and indicates that better pain control is achieved in extra-pelvic than intra-pelvic tumors. This was primarily related to the greater depth of the central pelvic and side-wall recurrences compared to the extra-pelvic recurrences, which resulted in more dissipation of ultrasound energy in pre-focal tissues in the former. A study in pancreatic cancer indicated that a 1-cm increase in depth decreased ablation by 30.7%. At <7 cm posterior tumor depth (from computerized tomography scan), ablation as assessed by the non-perfused post treatment volume was nearly 10 times greater than at depths >7 cm [Citation21]. In our series, the depth of the closest tumor border was >8 cm for intra-pelvic lesions and between 3 and 8 cm for extra-pelvic lesions. Experimental data also indicate that layering of fat and muscle interfaces, as encountered in our study, repositions and broadens the focus resulting in suboptimal focal temperature rises [Citation22]. This was particularly problematic when delivering HIFU through the gluteal region which has a predominance of fat. Also, fat and muscle lay in uneven layers in a supine oblique position. In future, the use of higher powers and longer focal length transducers will be essential for effective delivery of HIFU particularly to intra-pelvic tumors.
Previous data on the use of HIFU for treating intra-pelvic tumors in the context of radiotherapy are confined to prostatic ablations. Even in this application where HIFU has been used to successfully treat radiorecurrent prostate cancer [Citation23] and conversely salvage radiotherapy for recurrent prostate cancer has shown encouraging outcomes after initial treatment with HIFU [Citation24], true combinations of HIFU and radiotherapy are in their infancy. A very early study of HIFU prior to radiotherapy for organ preservation in testis cancer showed that disease recurred in 1 of 4 patients who did not receive radiotherapy after HIFU [Citation25]. More recently, a safety trial delivering HIFU to 30 patients with pancreatic cancer receiving chemotherapy or chemoradiotherapy reported adverse events in 10% and no serious adverse events [Citation26]. The utility of HIFU-radiotherapy combinations therefore remains to be evaluated.
Although no significant V240EM was seen in any of the intra-pelvic tumors treated, some pain responses were evident. This indicates either that a significant V240EM is not necessary for a symptomatic response, or that the HIFU procedure had a powerful placebo effect. In a multicenter randomized controlled trial treating bone metastases, pain responses were seen in 20% of patients in the placebo arm, with 5.7% showing a complete response [Citation27]. The response rate in our series was greater than this even when ablative temperatures were not reached. This suggests that sub-ablative heating changes may well cause physiological changes at a cellular level, possibly due to activation of vibration sensitive ion channels modulating the function of sensory nerve fibers and resulting in symptomatic improvement. Alternatively, the PRFS might have underestimated temperature rises because of difficulty in placing the pre-focal monitoring slice away from fat (e.g., in patient 11, where immediate post-treatment changes on contrast-enhanced images in the treated region were seen). As by their nature several of these tumors had a non-perfused component pretreatment, any tumor growth would result in an increase this non-perfused component making comparisons between non-perfused volumes and pain scores or thermal dose poorly indicative of response in this small pilot cohort.
The volume of tumor treated in this series was much smaller than the gross tumor volume, primarily because a significant proportion of the tumor was beyond the reach of the transducer focus. We always intended to assess only the feasibility of treatment for pain control rather than full-scale debulking. Imaging changes were, therefore, only expected at the site of the ablation. In fibroid ablation, where non-perfused volumes have been measured at ∼20% of the treated fibroid [Citation28], the ratio of the non-perfused fibroid post-treatment (indicating ablated volume) to thermal dose volume has been shown to be >1 [Citation29]. This means that the extent of ablation was greater than the volume in which an ablative thermal dose was achieved, and indicates spreading of thermal effects to surrounding tissue. In extra-pelvic tumors from our series where theV240EM was small compared to gross tumor volume, a sustained symptomatic response was evident. It is possible that, as with the fibroid data, ablative necrosis and physiological damage to surrounding tissue was attained even though a much smaller volume was treated.
Where thermal doses were achieved in the extra-pelvic tumors, there was an immediate increase in tumor volume, mainly in the non-perfused compartment, indicative of inflammation and edema. All tumors increased in volume with time indicating tumor progression, although alterations in the pace of progression cannot be estimated in this pilot study in the absence of a randomized control group. Nevertheless, some improvement in symptoms was achieved in 45% of treatments in this palliative care setting.
The same QoL measures as used for palliative radiotherapy indicated that emotional functioning improved although other measures did not. This indicates that on-going counseling and hospital visits with health care professionals provide valuable support for palliative care patients, despite the additional effort in attendance. Previous data on alleviation of pain relates mostly to bone metastases for both palliative radiotherapy [Citation30] and for HIFU [Citation31] where a randomized controlled trial showed that QoL improvements do not solely represent a placebo effect [Citation27]. An international multicenter trial of 20 patients that used the QLQ-C15-PAL and QLQ-BM22 questionnaires also showed clinically significant improvements in QoL in the 53% of patients who were classified as responders at day 30 but not in the 47% of patients classed as non-responders at this time point [Citation32]. In pelvic cancer, the impact of palliative treatments on QoL is poorly studied. A recent pilot data set from 25 patients treated with radiotherapy where the baseline symptoms were pain (48%), bleeding (40%), bleeding/pain (8%), and intestinal sub-occlusion (4%) showed that the improvement in well-being was 64% and in ability to perform daily activities 48% [Citation33], which mirrors the emotional and physical functioning metrics in our patient cohort.
As with other series, skin erythema is the commonest reported adverse event following MRgHIFU. Some degree of skin erythema was seen in half our patients. A very large series of more than 27,000 patients from 19 centers across China where HIFU was used to treat benign uterine disease indicated that the incidence of skin erythema was 0.32%, skin blistering 0.07% and skin burn 0.14% [Citation34]. This was much lower than in our study, where patients from an older, post-menopausal group, had often received previous radiation, or had adjacent surgical scars distorting the treatment site compromising optimal skin contact. Scar tissue was particularly problematic in 1 of our patients where a large inguinal scar and an overlying skinfold compromised acoustic contact. Factors significantly associated with thermal injury to skin, in a univariate logistic regression analysis of 892 cases, were related to sonication time, sonication time per hour, total energy deposited, distance from uterine fibroid ventral side to skin, volume of uterine fibroids, abdominal wall scar, abdominal wall thickness, and body mass index; in a multivariate analysis, however, total energy, abdominal wall scar and abdominal wall thickness only were significant [Citation35]. To overcome the problem of abdominal scars, acoustic patches on the skin, which reflect the ultrasound energy from scars, have been introduced [Citation36] and increase eligibility for MRgHIFU [Citation37] without compromising the efficacy of the treatment [Citation38]. We did not use acoustic patches because of anatomical distortion associated with major previous oncological surgery. In the future, acoustic patches may well avoid skin erythema in this patient group.
We were limited in our patient positioning by having to work within the confines of our 60 cm bore Achieva scanner, since the lesions were predominantly sited at the widest point of the body at the hip joints. This meant that the prone or supine oblique positions had to be accommodated often across the narrowest dimension of the bore. Wide bore or open scanners will address this in future. Adjacent or overlying bowel can also limit the delivery to a pelvic target. In our series, adjacent bowel was problematic for lesions at the vaginal vault, where adjacent rectum lay in the beam path. As our main objective was pain control rather than lesion ablation, we were able to modify our treatment plan to accommodate this. Where more definitive treatment of intra-pelvic tumors is needed, strategies to deflate or displace the rectum away from the treatment site are needed. Fat necrosis has not formally been reported as a side-effect of HIFU, however, HIFU lipolysisis used for cosmetic body sculpting, so the induction of fat necrosis is well-established [Citation39,Citation40]. The cosmetic effects of fat necrosis were not a consideration in our participants for whom symptom palliation was an overriding objective.
This study, albeit of a small cohort size, shows that MRgHIFU is feasible for palliating recurrent gynecological cancer, is associated with little in terms of unexpected or severe side-effects and can be integrated into the clinical cancer pathway. The current technology is limited by its inability to target tumors deep within the pelvis (vaginal vault and pelvic side-wall disease) was limited by current hardware. Pain at these common sites of recurrence is often the most challenging to manage successfully with standard analgesic agents. Future improvements in device technology should enable effective targeting of deeper tissues.
Ethical approval
Ethical approval for the clinical study (NCT02714621) was granted from the NHS Health Research Authority (REC reference: 15/WM/0470).
Author contributions
GI: Data curation, Formal analysis; Investigation, Writing first draft, Writing review and editing; SLG: Methodology, Investigation, Writing review and editing; AT: Funding acquisition, Supervision, Writing review and editing; MRDB: Methodology, Investigation, Writing review and editing; IR: Methodology, Validation, Writing review and editing; RG-W: Formal analysis, Writing review and editing; GtH: Conceptualization; Funding acquisition, Supervision, Resources, Writing review and editing; NMdS: Conceptualization, Funding acquisition, Investigation, Supervision, Resources, Project administration, Writing first draft, Writing review and editing.
Acknowledgements
The authors acknowledge support from the NIHR Biomedical Research Centre and Clinical Research Facility in Imaging at the RMH, and the Cancer Research Network. Also CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC & Department of Health C1060/A10334, C1060/A16464, equipment support from Philips Healthcare and Profound Medical, and ultrasound physics support from the Focused Ultrasound Foundation. The authors thank Ari Partenenfor his dedicated help and advice on system set-up.
Disclosure statement
No conflicting competing interests declared by any author.
Data availability statement
Imaging data is available through the Cancer Research UK Imaging data repository (contact via study CI, N deSouza).
Additional information
Funding
References
- Gressel GM, Dioun SM, Richley M, et al. Utilizing the Patient Reported Outcomes Measurement Information System (PROMIS®) to increase referral to ancillary support services for severely symptomatic patients with gynecologic cancer. Gynecol Oncol. 2019;152:509–513.
- Cordeiro ER, Cathelineau X, Thuroff S, et al. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int. 2012;110:1228–1242.
- Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:16480–16488.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2019;40:625–637.
- Sibille A, Prat F, Chapelon JY, et al. Extracorporeal ablation of liver tissue by high-intensity focused ultrasound. Oncology. 1993;50:375–379.
- Jolesz FA, Hynynen K, McDannold N, et al. MR imaging-controlled focused ultrasound ablation: a noninvasive image-guided surgery. Magn Reson Imaging Clin N Am. 2005;13:545–560.
- Rabkin BA, Zderic V, Crum LA, et al. Biological and physical mechanisms of HIFU-induced hyperecho in ultrasound images. Ultrasound Med Biol. 2006;32:1721–1729.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31:302–309.
- Huisman M, Lam MK, Bartels LW, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Ther Ultrasound. 2014;2:16.
- Pron G. Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15:1–86.
- Zhang L, Chen WZ, Liu YJ, et al. Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol. 2010;73:396–403.
- O'Sullivan AK, Thompson D, Chu P, et al. Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care. 2009;25:14–25.
- Chen J, Li Y, Wang Z, et al., Committee of the Clinical Trial of HIFU versus Surgical Treatment for Fibroids. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125:354–364.
- Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–430.
- Groenvold M, Petersen MA, Aaronson NK, et al., EORTC Quality of Life Group. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42:55–64.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138.
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008;27:376–390.
- Giles SL, Winfield JM, Collins DJ, et al. Value of diffusion-weighted imaging for monitoring tissue change during magnetic resonance-guided high-intensity focused ultrasound therapy in bone applications: an ex-vivo study. Eur Radiol Exp. 2018;2:10.
- Ge HY, Miao LY, Xiong LL, et al. High-intensity focused ultrasound treatment of late-stage pancreatic body carcinoma: optimal tumor depth for safe ablation. Ultrasound Med Biol. 2014;40:947–955.
- Giles SL, Rivens I, Imseeh G, et al. Magnetic Resonance guided high intensity focused ultrasound for treating recurrent gynaecological tumors: effect of pre-focal tissue characteristics on target heating. J Imaging Interv Radiol. 2020;3:1–10.
- Jones TA, Chin J, McLeod D, et al. High intensity focused ultrasound for radiorecurrent prostate cancer: a North American Clinical Trial. J Urol. 2018;199:133–139.
- Pasticier G, Chapet O, Badet L, et al. Salvage radiotherapy after high-intensity focused ultrasound for localized prostate cancer: early clinical results. Urology. 2008;72:1305–1309.
- Madersbacher S, Kratzik C, Susani M, et al. Transcutaneous high-intensity focused ultrasound and irradiation: an organ-preserving treatment of cancer in a solitary testis. Eur Urol. 1998;33:195–201.
- Sofuni A, Moriyasu F, Sano T, et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol. 2014;20:9570–9577.
- Hurwitz MD, Ghanouni P, Kanaev SV, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106:dju082.
- Voogt MJ, Trillaud H, Kim YS, et al. Volumetric feedback ablation of uterine fibroids using magnetic resonance-guided high intensity focused ultrasound therapy. Eur Radiol. 2012;22:411–417.
- Yoon SW, Cha SH, Ji YG, et al. Magnetic resonance imaging-guided focused ultrasound surgery for symptomatic uterine fibroids: estimation of treatment efficacy using thermal dose calculations. Eur J Obstet Gynecol Reprod Biol. 2013;169:304–308.
- McDonald R, Chow E, Rowbottom L, et al. Quality of life after palliative radiotherapy in bone metastases: a literature review. J Bone Oncol. 2015;4:24–31.
- Dababou S, Marrocchio C, Scipione R, et al. High-intensity focused ultrasound for pain management in patients with cancer. Radiographics. 2018;38:603–623.
- Harding D, Giles SL, Brown MRD, et al. Evaluation of quality of life outcomes following palliative treatment of bone metastases with magnetic resonance-guided high intensity focused ultrasound: an international multicentre study. Clin Oncol (R Coll Radiol). 2018;30:233–242.
- Farina E, Macchia G, Siepe G, et al. Palliative short-course radiotherapy in advanced pelvic cancer: a phase II study (SHARON Project). Anticancer Res. 2019;39:4237–4242.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35:56–61.
- Yin N, Hu L, Xiao ZB, et al. Factors influencing thermal injury to skin and abdominal wall structures in HIFU ablation of uterine fibroids. Int J Hyperthermia. 2018;34:1298–1303.
- Gorny KR, Chen S, Hangiandreou NJ, et al. Initial evaluation of acoustic reflectors for the preservation of sensitive abdominal skin areas during MRgFUS treatment. Phys Med Biol. 2009;54:N125–N133.
- Yoon SW, Seong SJ, Jung SG, et al. Mitigation of abdominal scars during MR-guided focused ultrasound treatment of uterine leiomyomas with the use of an energy-blocking scar patch. J Vasc Interv Radiol. 2011;22:1747–1750.
- Keserci B, Duc NM. Volumetric magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids through abdominal scars: the impact of a scar patch on therapeutic efficacy and adverse effects. J Ther Ultrasound. 2017;5:22.
- Jewell ML, Weiss RA, Baxter RA, et al. Safety and tolerability of high-intensity focused ultrasonography for noninvasive body sculpting: 24-week data from a randomized, sham-controlled study. Aesthet Surg J. 2012;32:868–876.
- Lee HJ, Lee MH, Lee SG, et al. Evaluation of a novel device, high-intensity focused ultrasound with a contact cooling for subcutaneous fat reduction. Lasers Surg Med. 2016;48:878–886.