Abstract
Objective
The purpose of this study was to compare the efficacy of focused ultrasound (FU) and interferon drug therapy for cervical intraepithelial neoplasia 1 (CIN1) and chronic cervicitis associated with high risk human papillomavirus (HR-HPV) infection, as well as analyze the influencing factors.
Methods
A retrospective cohort study was performed from January 2017 to December 2019. A total of 592 patients were enrolled, of which 300 patients were treated with FU and 292 patients were treated with interferon drugs. Kaplan-Meier curves and a COX regression model were used to compare the curative effects of the two therapeutic methods using HR-HPV clearance as the main outcome. The relationship between age, HR-HPV infection type, pathological type, preoperative HR-HPV status and HR-HPV clearance were also analyzed.
Results
The median time for HR-HPV clearance was 6.00 months (95% CI: 5.24–6.76) in the FU group and 26.00 months (95% CI: 22.32–29.68) in the medication group. A significant difference was observed between the two groups (χ2 =198.902, p = 0.000). The HR-HPV clearance rate was 4.927 (95% CI 3.840–6.321; p = 0.000) times higher in the patients treated with FU than those treated with interferon drugs. In the FU group, no significant difference was observed in HR-HPV clearance rate between CIN1 and chronic cervicitis (χ2=0.660, p = 0.416), which was also insignificant between HR-HPV persistent and non-persistent infections (χ2=0.751, p = 0.386).
Conclusion
FU therapy can eliminate HR-HPV infections in a short period of time. Moreover, the treatment efficacy of FU was significantly superior to that of interferon drugs.
Introduction
Globally, cervical cancer remains the fourth most common cancer among women; however, it is the second most common cancer among women and has the third highest mortality rate in low- and middle-income countries (LMICs) [Citation1]. It is estimated that there were approximately 100,000 new cases of cervical cancer in China in 2015, accounting for 18.6% of the total number of cases in the world, and is responsible for 30,000 deaths [Citation2].
It has been well-established that a persistent HR-HPV infection is a ‘necessary’ cause of cervical cancer, and cervical intraepithelial neoplasia (CIN) is a group of cervical lesions closely related to cervical cancer, reflecting its continuous occurrence and development. Although the spontaneous regression rate of CIN1 can be as high as 60%[Citation3], women who are also infected with HR-HPV have a longer duration of lesions, a lower regression rate, and faster disease progression [Citation4]. Therefore, the active treatment of patients with an HR-HPV infection combined with CIN1 can block disease progression and reduce the risk of cervical cancer. Moreover, for women diagnosed with HR-HPV infection combined with CIN1 or chronic cervicitis at the first visit, positive HPV DNA tests, abnormal cytology results, and colposcopy during the observation follow-up are associated with different levels of psychological distress and financial burden [Citation5–8]. In particular, in developing countries like China, some patients urgently require treatment rather than observation and follow-up.
Ablation therapy is often used due to the high cost of excisional methods and their potential impact on future fertility combined with the high natural regression rate of low-grade cervical lesions related to HR-HPV infection [Citation9]. At the same time, drugs, especially interferon drugs, are also used as a treatment[Citation10]. Interferon (IFN) exerts antiviral, immunoregulatory, and antitumor effects, and also plays a critical role in the innate immune response against viral infections by inhibiting HPV replication [Citation11]. Previous studies have reported that while interferon has achieved favorable results for the treatment of anogenital warts [Citation12,Citation13], there are mixed reviews regarding the treatment of HPV infection-related cervical lesions [Citation14]; however, interferon drugs remain widely used for the treatment of HPV infection-related cervical lesions in China [Citation15].
Focused ultrasound (FU) is a newly developed noninvasive treatment technology. For the treatment of cervical diseases, FU represents a type of ablation treatment, but differs from the traditional treatment mode due to its ‘inside-out’ treatment method and immune stimulation [Citation16]. After years of development, FU has achieved good clinical effects for the treatment of CIN, persistent HR-HPV infection, and symptomatic cervical columnar epithelial ectopy [Citation17–20]. However, these studies have generally had a small sample size and short follow-up time, and have mainly focused on changes in clinical symptoms and pathological results. HPV-based testing is the basis for risk estimation of CIN3 + [Citation21]. Compared with cytology, the higher sensitivity and greater negative predictive value of HPV testing makes it suitable for long-term risk prediction [Citation22]. In this retrospective cohort study, we primarily discussed the clearance of HR-HPV infections closely related to cervical lesions after treatment, compared the efficacy of FU and interferon drugs, and analyzed the factors related to efficacy.
Materials and methods
This study design was approved by the ethics committees of both Chongqing Medical University and Affiliated Hospital of North Sichuan Medical College (Reference: 2020ER124-1, 28 October 2020). Patient data were suitably anonymized and protected according to national standards.
Study population
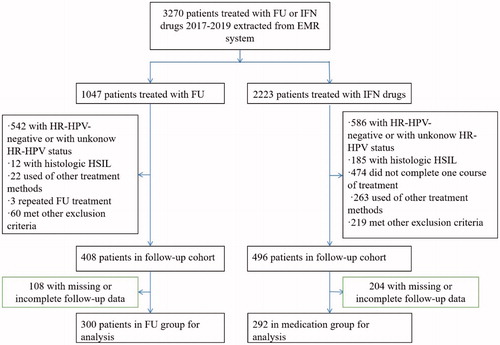
The clinical data of 1047 women who underwent FU therapy between January 2017 and December 2019 at the Department of Obstetrics and Gynecology of Affiliated Hospital of North Sichuan Medical College, located in the city of Nanchong in southwest mainland China, were retrospectively analyzed. There were 2223 women who were treated with interferon drugs during the same period, who were extracted from the outpatient electronic medical record (EMR) system as a control group. All patients were traced back to the time at which their first test was found to be HR-HPV positive in the EMR system. The inclusion criteria consisted of: women ≥ 18 years; no prior history of total hysterectomy or cervical resection; the HPV DNA test showed HR-HPV infection, the cytology results were negative for intraepithelial lesion or malignancy (NILM), atypical squamous cells of undetermined significance (ASC-US), or low-grade squamous intraepithelial lesion (LSIL), and colposcopy and cervical biopsy revealed CIN1 or chronic cervicitis; no vaginal medications were being used at the time of testing; and available data from at least one follow-up (HR-HPV testing and liquid-based cytology or cervical biopsy). The exclusion criteria consisted of: women with cytology results revealing atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H) or more severe cytology results than ASC-H; women with histological HSIL and cervical cancer; use of other treatment methods (e.g., LEEP or microwave); comorbidities consisting of serious systemic diseases (e.g., severe heart, liver, kidney, blood system, and autoimmune diseases); had not completed one course of medication treatment; pregnant and lactating women; and patients with acute inflammation of the reproductive tract. From the original subject pool, a total of 592 women who satisfied the inclusion criteria were selected and enrolled into the study. presents a flow diagram for patient selection.
Methods
The focused ultrasound equipment (Model-CZF Focused Ultrasound Therapeutic Device for Gynecology) used for treatment was manufactured by Chongqing Haifu Medical Technology Co, Ltd. (Chongqing, China), with a therapeutic power of 3.5 W–4.5 W, working frequency of 10.0 MHz, and impulse of 1000 Hz. Treatment was performed by one trained and qualified physician. Patients were placed in the lithotomy position and a sterile speculum was used to expose the cervix. The cervix was disinfected and an ultrasound coupling gel was applied prior to treatment. The treatment probe was placed in close contact with the cervix, and a circular scanning was performed from the lesion area to the normal area. The treatment range was needed to exceed the edge of the lesion by approximately 2 mm. The treatment typically lasted for 3–5 min until the lesion presented as a depressed region with modest introversion of the external cervical aperture. All patients included in the study received one FU treatment.
Medications included: recombinant human interferon α2 b vaginal effervescent capsule (800,000 IU/piece, Shanghai Huaxin Biotechnology Co., Ltd.); recombinant human interferon α2 b cream (2,000,000 IU/branch, Anhui Anke Biotechnology Co., Ltd.); recombinant human interferon α2 b vaginal suppository (500,000 IU/piece, Changchun Institution of Biological Products Co., Ltd.); recombinant human interferon α2 b gel (1,000,000 IU/branch, Zhaoke Pharmaceutical Co., Ltd.); and recombinant human interferon α2 b vaginal effervescent tablets (500,000 IU/piece, Beijing Kawin Technology Share-Holding Co., Ltd.). The above drugs were used in accordance with the manufacturer’s instructions or the doctor's advice, and used for at least one course of treatment.
Follow up
Patients were advised to avoid sexual intercourse, bath and swimming for two months after FU treatment or while receiving vaginal medication. Patients in the FU group were followed up for one week after treatment to observe whether abnormal vaginal discharge was present. The patients were then advised to undergo HPV testing, cytological testing, and if necessary, a cervical biopsy under colposcopy every 6 months as a follow-up.
HPV testing
A High-Risk Human Papillomavirus Genotyping Real Time PCR Kit (Shanghai ZJ Bio-Tech Co., Ltd. China) was used for HPV testing. This kit tests for 15 HR-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 82).
Cervical cytology assessment, colposcopy, and biopsy
Cervical cytology reports followed The Bethesda System 2001 [Citation23]. Terminology for reporting the results of cervical cytology and biopsy samples acquired from the colposcopy were analyzed by expert pathologists. All CIN diagnoses were obtained from the cervical tissue samples acquired from the colposcopy-directed punch biopsy.
Definitions
A persistent HR-HPV infection was defined as the presence of the same type-specific HPV DNA in repeated sampling after 6 months. HR-HPV clearance was defined as all HR-HPV subtypes becoming negative.
To investigate whether different HR-HPV genotypes and infection with multiple HR-HPV types could affect HR-HPV clearance, the patients were classified to four types: single type infection with HPV16 or 18 (Type I); co-infection with HPV16/18 (Type II); single type infection without HPV16 or 18 (Type III); co-infection without HPV16/18 (Type IV) [Citation24]. The four types were combined and analyzed according to the presence or absence of HPV16/18 infection, Types I and II were combined into HPV16/18 infection, and Types III and IV were combined into non-HPV16/18 infection; according to whether they were single type infection or multiple type infection, Types I and III were combined into single HR-HPV infection, and Types II and IV were combined into multiple HR-HPV infection. We used HR-HPV clearance as the outcome variable, and the time between the date of HR-HPV detection and clearance, or the date of the last follow-up as the outcome time.
Statistical analysis
Statistical analysis was performed using SPSS Version 22.0. Chi-squared tests and t-tests were employed to compare the differences in age, HR-HPV infection type, and accumulated clearance rate of HR-HPV infection between the FU group and medication group. The Kaplan-Meier method was used to calculate the HR-HPV clearance rate. A log-rank test was employed to compare the difference in the HR-HPV clearance rate between the two groups. Further univariate and multivariate analyses were conducted using a Cox proportional hazards regression analysis to examine whether the HR-HPV clearance rate was associated with age, HR-HPV infection type, and treatment method. Statistical tests included a two-sided test and statistical significance was considered at p < 0.05.
Results
A total of 592 women fulfilled our inclusion criteria. Of these, 300 (50.7%) were treated with FU, whereas the remaining 292 (49.3%) were treated with interferon drugs. The mean age of these patients was 40.72 ± 9.15 years old, ranging from 18 to 69, of which 82 (13.9%) were younger than 30 years old. There was no difference in age between the two groups (40.25 ± 8.94 years old in the FU group vs. 41.19 ± 9.35 years old in the medication group, t=-1.231, p = 0.219). The median course of medication treatment was 5 courses, the 25th percentile of treatment course was 3 courses, and the 75th percentile of treatment course was 8 courses.
The distribution of the HPV subtypes in the study population was listed in . The most common subtype was HPV52 (29.73%), followed by HPV58 (18.07%), HPV16 (15.71%), HPV39 (11.66%), HPV51 (9.46%), and HPV18 (8.11%). shows the distribution of different HR-HPV infection types in the two groups. The most prevalent HR-HPV infection type in the two groups was Type III, which was 57.3% (172/300) in the FU group and 65.1% (190/292) in the medication group. In the FU group, Type I, Type IV, and Type II were second only to Type III, accounting for 19.3% (58/300), 12.3% (37/300), and 11.0% (33/300), respectively. In the medication group, Type IV, Type I, and Type II were second only to Type III, accounting for 19.2% (56/292), 9.2% (27/292), and 6.5% (19/292), respectively. There was a significant difference in the HR-HPV infection types between the two groups (χ2=19.747, p = 0.000).
Table 1. Distribution of HR-HPV subtypes in the study population.
Table 2. HR-HPV infection types in each group.
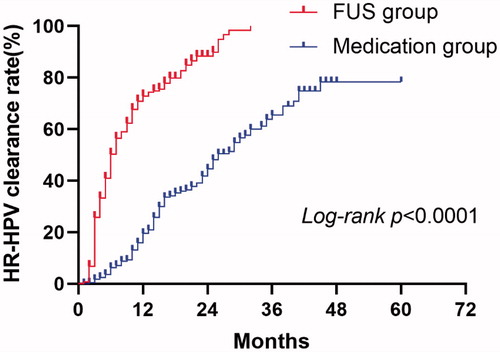
The time for the two patient groups, including the study outcome time and the median time for HR-HPV clearance in the two groups are shown in . We found that the shortest time for the study outcome in the FU group was 1 month, which was the same as that in the medication group. The longest time was 32 months in the FU group and 60 months in the medication group. The median time for HR-HPV clearance was 6.00 months (95% CI: 5.24–6.76) in the FU group and 26.00 months (95% CI: 22.32–29.68) in the medication group, which was significantly different between the groups (χ2=198.902, p = 0.000).
Table 3. Study outcome time and median time for HR-HPV clearance in each group.
The accumulated clearance rate of HR-HPV infection at the 6th and 24th months were 47.3% and 72.3%, respectively in the FU group, 5.8% and 31.5%, respectively in medication group, with significant differences observed between the groups (p = 0.000 at the 6th month and p = 0.000 at the 24th month) ().
Table 4. Accumulated clearance rate of HR-HPV infection in each group.
As shown in , the univariate Cox regression analysis showed that FU treatment and single HR-HPV infection were significantly associated with an increased HR-HPV clearance rate (HR 4.854; 95% CI 3.799–6.203, p = 0.000 and HR 1.613; 95% CI 1.227–2.121, p = 0.001, respectively). In the multivariate Cox regression analysis, FU treatment and single HR-HPV infection were significantly associated with an increased HR-HPV clearance rate (HR 4.927; 95% CI 3.840–6.321, p = 0.000 and HR 1.534; 95% CI 1.163–2.023, p = 0.002, respectively). Age (30 years or older vs younger than 30 years) and HPV16/18 status (non-HPV16/18 infection vs HPV16/18 infection) were included in this regression model and were not found to be the significant co-variate (HR 0.880; 95% CI 0.646–1.200, p = 0.419 and HR 0.915; 95% CI 0.711–1.178, p = 0.491, respectively).
Table 5. Univariate and multivariate Cox regression analyses for analyzing variables associated with the HR-HPV clearance rate.
represents the Kaplan-Meier curves for the HR-HPV clearance rate according to outcome time (months) according to the treatment methods. The HR-HPV clearance rate in the FU group was always higher than that of the medication group (χ2=198.902, p = 0.000).
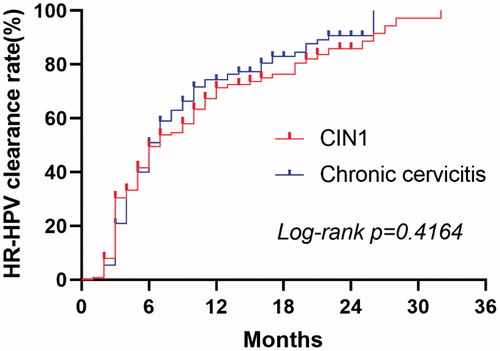
The subgroup analysis involving only FU therapy showed that the median time for HR-HPV clearance was 7.00 months (95% CI: 4.94–9.06) in patients with CIN1 and 6.00 months (95% CI: 5.11–6.89) in patients with chronic cervicitis. There was no significant difference in the HR-HPV clearance rate between the two pathological types (χ2=0.660, p = 0.416). shows the Kaplan Meier curves of the HR-HPV clearance rate for patients in the FU group stratified into pathological types.
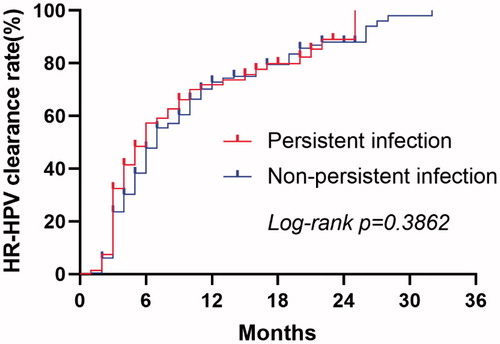
The subgroup analysis of the preoperative HR-HPV infection status (persistent infection or non-persistent infection) in the FU group showed that the median time for HR-HPV clearance was 6.00 months (95% CI: 4.436–7.564) in patients with persistent infection and 7.00 months (95% CI: 6.208–7.792) in patients with non-persistent infection. There were no significant differences in the HR-HPV clearance rate between the subgroups (χ2=0.751, p = 0.386). shows the Kaplan Meier curves of the HR-HPV clearance rate for patients in the FU group stratified into the preoperative HR-HPV infection status.
In addition, it is important to note that a 31-year-old patient in the medication group failed to clear the HPV16 infection and progressed to histological HSIL after 42 months. No disease progression was observed in the FU group.
Discussion
As a novel and noninvasive ablation therapy, FU represents a promising treatment for cervical lesions. Our previous studies have shown that it was feasible and effective for the treatment of patients with HPV-infected cervicitis and CIN1 [Citation18,Citation19]. Compared with laser ablation, FU therapy had equivalent therapeutic efficacy, but the post-operative bleeding and reactive discharge were substantially lower in the patients who underwent FU therapy [Citation17]. FU therapy is simple to perform and can be repeated. Moreover, patients do not need to receive anesthesia. FU is also a pollution-free operation that does not generate odors or smoke, burning tissue, or radiation.
On the other hand, some patients with HR-HPV infection-related low-grade cervical lesions are willing to select more conservative treatment as opposed to not undergoing interventions. At this time, interferon drugs have become a hopeful choice; Interferon drugs are costly. Thus, if an HR-HPV infection cannot be eliminated within a short period after interferon use, it will inevitably increase the psychological burden on the patients, and to a certain extent, the economic burden as well. Therefore, we compared FU and interferon drug treatments based on clinical reality, in order to determine the better choice for patients.
Based on a large sample and long-term retrospective cohort study, our results indicated that the rate of HR-HPV clearance after FU treatment was 4.927 (95% CI: 3.840–6.321) times higher than that of the interferon drugs, and the median time for HR-HPV clearance was significantly shorter than medication treatment (6.00 months [95% CI: 5.24–6.76] vs 26.00 months [95% CI: 22.32–29.68]). Moreover, the subgroup analysis showed that FU treatment was equally effective for CIN1 and chronic cervicitis, as well as for HR-HPV persistent and non-persistent infections. In terms of FU efficacy as an ablative treatment, our findings were consistent with the existing literature. Pinder et al. reported that 6 months after cryotherapy, thermal ablation, and LEEP treatment, the HR-HPV-negative rates were 40%, 42%, and 47%, respectively, whereas we found a rate of 47.3% (142/300) [Citation25]. Moreover, the study by Aerssens et al. found that during the two-year follow-up period after cryotherapy or LEEP, the clearance rate of HPV (including low risk and high risk) was 72.9%, whereas our finding was 72.3% (217/300) [Citation26]. These findings show that FU treatment is not less effective than any other ablative treatment. However, in our study, the HR-HPV clearance rate was lower than that reported by Li et al. at 75% (15/20) six months after treatment, which may be related to the difference in sample size and HR-HPV detection methods (The Hybrid Capture II [HC-2] test in Li’s study) [Citation18]. In the study conducted by Fu et al. [Citation19], the HPV (including high-risk and low-risk) negative rate was 85.71% (24/28) three months after FU treatment, whereas our study only focused on HR-HPV-positive patients. We did not strictly select patients for FU therapy based on age, type of transformation zone, HR-HPV status, etc. and some patients were not followed up strictly according to our scheduled time. Thus, these may be reasons that the HR-HPV negative rate was lower than other similar reports, and will need to be considered in future prospective, multicenter, and large sample studies.
For interferon drugs, the findings of our study confirmed the previous view that the treatment is expensive and there is limited efficacy. There is no evidence that interferon was of value for the treatment of either low or high grade HPV-associated disease, and it is not recommended for routine clinical practice for the treatment of these lesions [Citation27,Citation28]. Six months after treatment with interferon drugs, the cumulative HR-HPV negative rate was only 5.8% and the cumulative HR-HPV negative rate was only 16.1% at 12 months. Zhang et al. found that for patients with an HR-HPV infection without cervical lesions, no interventions were taken, and the cumulative virus clearance rates at 6 and 12 months were 7.8 and 43.1%, respectively [Citation29]. In the present study, the efficacy of the interferon drugs was even lower than that of the nonintervention group reported by Zhang et al; however, these results should be interpreted with caution, as only one of the 292 patients in the medication group who did not clear the HPV16 infection progressed to histological HSIL after 42 months. This indicates that some of the patients whose HR-HPV test did not become negative were actually reinfected rather than cases of a persistent infection.
We also found that in our research cohort, the top three most common subtypes were HPV52 (29.73%), HPV58 (18.07%), and HPV16 (15.71%), which is highly consistent with the results of other studies. A cross-sectional population-based study from Shanxi province, China, showed that the most frequent HR-HPV genotypes were 16, 52, and 58, and HPV52 was the most frequently detected genotype among the HR-HPV-positive CIN1 patients [Citation30]. Mai et al. reported the most prevalent HPV subtypes to be HPV52, HPV58, and HPV16 in Shenzhen city, China[Citation31]. Moreover, the study by Ma et al. found that there was a higher prevalence of HPV52, HPV16, and HPV58 in Beijing China[Citation32]. Another study from western China also showed that the most common HR-HPV genotype in CIN1 was HPV52 [Citation33]. These studies demonstrate that both HPV52 and 58 were more common among the general population in China compared to that in developed countries, and more attention should be paid to this phenomenon.
In line with other studies from Oyervides [Citation34] and De Brot [Citation35], we found that multiple HR-HPV infection was associated with HR-HPV persistence. The HR-HPV clearance rate with single HR-HPV infection was 1.534 (95% CI: 1.163–2.023, p = 0.002) times higher than that with multiple HR-HPV infection. However, we did not find an age (30 years or older vs younger than 30 years old) and HPV16/18 infection to be associated with HR-HPV clearance.
The main limitations of this study included the retrospective nature and absence of some basic characteristics data of patients; however, these weaknesses reflected the realities of clinical practice. Another limitation was that although we have confirmed that the efficacy of FU for the treatment of low grade cervical lesions associated with HR-HPV infection was significantly better compared to that of interferon drugs, the advantages and disadvantages compared with other ablation therapies remains unknown. We are planning to address this question in a prospective, multicenter, randomized controlled study.
In conclusion, our study found that FU therapy had a significantly higher HR-HPV clearance rate and shorter clearance time compared to interferon drug therapy, and It was also an effective treatment for persistent HR-HPV infection. Thus, in economically underdeveloped areas, FU represented the better choice for patients who urgently require treatment, as it also helped to reduce their economic and psychological burden during follow-up. Interferon drugs were not recommended for the treatment of cervical lesions associated with HR-HPV infection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):22–36.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China. CA Cancer J Clin. 2016;66(2):115–132.,
- Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–192.
- Liu M, Yan X, Zhang M, et al. Influence of human papillomavirus infection on the natural history of cervical intraepithelial neoplasia 1: a meta-analysis. Biomed Res Int. 2017;2017:8971059.
- Waller J, McCaffery K, Kitchener H, et al. Women's experiences of repeated HPV testing in the context of cervical cancer screening: a qualitative study. Psychooncology. 2007;16(3):196–204.
- Henk HJ, Insinga RP, Singhal PK, et al. Incidence and costs of cervical intraepithelial neoplasia in a us commercially insured population. J Low Genit Tract Dis. 2010;14(1):29–36.,
- Kola-Palmer S, Walsh JC. Correlates of psychological distress immediately following colposcopy. Psychooncology. 2015;24(7):819–824.
- Mizukami A, Kaise T, Van Kriekinge G. Resource use and cost of treating human papillomavirus-related lesions in japanese women. Value Health Reg Issues. 2018;15:56–62.
- Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ. 2016;354:i3633.,
- Xiong Y, Cui L, Bian C, et al. Clearance of human papillomavirus infection in patients with cervical intraepithelial neoplasia: A systemic review and meta-analysis. Medicine (Baltimore). 2020;99(46):e23155.
- Terenzi F, Saikia P, Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. Embo J. 2008;27(24):3311–3321.
- Eron LJ, Judson F, Tucker S, et al. Interferon therapy for condylomata acuminata. N Engl J Med. 1986;315(17):1059–1064.
- Rockley PF, Tyring SK. Interferons alpha, beta and gamma therapy of anogenital human papillomavirus infections. Pharmacol Ther. 1995;65(2):265–287.
- Koromilas AE, Li S, Matlashewski G. Control of interferon signaling in human papillomavirus infection. Cytokine Growth Factor Rev. 2001;12(2-3):157–170.
- Shi HJ, Song HB, Zhao QY, et al. Efficacy and safety of combined high-dose interferon and red light therapy for the treatment of human papillomavirus and associated vaginitis and cervicitis: A prospective and randomized clinical study. Medicine. 2018;97(37):e12398
- van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–258.,
- Chen J, Zhou D, Liu Y, et al. A comparison between ultrasound therapy and laser therapy for symptomatic cervical ectopy. Ultrasound Med Biol. 2008;34(11):1770–1774.,
- Li CZ, Wang ZB, Yang X, et al. Feasibility of focused ultrasound therapy for recurrent cervicitis with high-risk human papillomavirus infection. Ultrasound Obstet Gynecol. 2009;34(5):590–594.,
- Fu Z, Fan Y, Wu C, et al. Clinical efficacy and mechanism for focused ultrasound (FUS) in the management of cervical intraepithelial neoplasia 1 (CIN1). Int J Hyperthermia. 2020;37(1):339–345.
- Li C, Xiong X, Li Y, et al. Therapeutic effects of focused ultrasound in 4014 patients with symptomatic cervical ectopy. Ultrasound Med Biol. 2013;39(4):604–610.,
- Perkins RB, Guido RS, Castle PE, et al.; A.R.-B.M.C.G. Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102–131.
- Mark S, Kinney WK, *, Cheung LC, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. JNCI J Natl Cancer Inst. 2018;110:1–8.
- Diane Solomon M, Diane Davey M, Robert Kurman M, et al.; Bethesda 2001 Workshop. The 2001 bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119.
- Wang Y, Xue J, Dai X, et al. Distribution and role of high-risk human papillomavirus genotypes in women with cervical intraepithelial neoplasia: a retrospective analysis from Wenzhou, southeast China. Cancer Med. 2018;7(7):3492–3499.
- Pinder LF, Parham GP, Basu P, et al. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: pilot phase of a randomised controlled trial. Lancet Oncol. 2020;21(1):175–184.
- Aerssens A, Claeys P, Garcia A, et al. Natural history and clearance of HPV after treatment of precancerous cervical lesions. Histopathology. 2008;52(3):381–386.
- Stanley M, Chapter 1. Genital human papillomavirus infections–current and prospective therapies. J Natl Cancer Inst Monogr. 2003;2003(31):117–124.
- Hampson L, Martin-Hirsch P, Hampson IN. An overview of early investigational drugs for the treatment of human papilloma virus infection and associated dysplasia. Expert Opin Investig Drugs. 2015;24(12):1529–1537.
- Zhang W, Gong X, Wu Q, et al. The clearance of high-risk human papillomavirus is sooner after thin loop electrosurgical excision procedure (t-LEEP). J Invest Surg. 2019;32(6):560–565.
- Zhao XL, Hu SY, Zhang Q, et al. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017;28(4):e30.
- Mai Q, Yang X, Cheng H, et al. Prevalence and genotype distribution of human papillomavirus among women with cervical lesions in Shenzhen city, China. Hum Vaccin Immunother. 2020;17(4):965–971.
- Ma L, Lei J, Ma L, et al. Characteristics of women infected with human papillomavirus in a tertiary hospital in Beijing China, 2014-2018. BMC Infect Dis. 2019;19(1):670.,
- Li K, Yin R, Li Q, et al. Analysis of HPV distribution in patients with cervical precancerous lesions in Western China. Medicine. 2017;96(29):e7304
- Oyervides-Muñoz MA, Pérez-Maya AA, Sánchez-Domínguez CN, et al. Multiple HPV infections and viral load association in persistent cervical lesions in mexican women. Viruses. 2020;12(4):380.
- De Brot L, Pellegrini B, Moretti ST, et al. Infections with multiple high-risk HPV types are associated with high-grade and persistent low-grade intraepithelial lesions of the cervix. Cancer Cytopathol. 2017;125(2):138–143.