?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Microwaves (MWs) deliver relatively high temperatures into biological tissue and cover a large ablation zone. This study aims to evaluate the efficacy and effectiveness of water-cooled double-needle MW ablation arrays in assisting the hepatic transection of an in vivo pig model.
Methods
Our research program comprised computer modeling, tissue-mimicking phantom experiments, and in vivo pig liver experiments. Computer modeling was based on the finite element method (FEM) to evaluate ablation temperature distributions. In tissue-mimicking phantom and in vivo pig liver ablation experiments, the performances of the water-cooled MW ablation array and conventional clamp crushing liver resection were compared.
Results
FEM showed that the maximum lateral ablation diameter at 100 W output and a duration of 60 s was 3 cm (assessed at 50 °C isotherm). In the phantom, the maximum transverse ablation diameter of the double-needle MW ablation increased rapidly to 3 cm in 60 s at 50 W. The blood loss and blood loss per transection area in Group A were significantly lower than those in Group B (18 (7–26) ml vs. 34 (19–57) ml, and 2.4 (2–3.1) ml/cm2 vs. 6.9 (3.2–8.3) ml/cm2, respectively) (p < 0.05). The transection speed in Group A (2.6(1.9–3.8) cm2/min) was significantly faster than that in Group B (1.7(1.1–2.2) cm2/min) (p < 0.05).
Conclusion
In this experimental model, the new water-cooled MW array-assisted liver resection (LR) has the potential advantage of less blood loss and rapid removal than the conventional LR.
Introduction
Hepatocellular carcinoma (HCC), a frequently occurring cancer, is the third most common cause of death in cancer patients worldwide. Most HCC cases are associated with chronic hepatitis and cirrhosis caused by hepatitis B or C viral infection. Surgical excision is still the preferred treatment for patients with HCC cirrhosis [Citation1]. Unfortunately, most HCC cases involve underlying cirrhosis. Moreover, liver storage is usually inadequate in patients with cirrhosis, and mortality and morbidity do not decline after liver resection (LR) [Citation2].
A traditional LR procedure, known as clamp crushing LR, is usually performed using the Pringle procedure, which refers to an operation during liver surgery that involves the intermittent clamping of the inflow vascular pedicle [Citation3]. However, inflow occlusion can cause ischemic-reperfusion injury to the liver, especially in patients with cirrhosis. Recent innovations, such as the Cavitron ultrasonic surgical aspirator, bipolar diathermy [Citation4], staplers, and LigaSure technology [Citation5], are used to prevent bleeding during LR. However, all these devices are not suitable for cases of cirrhosis. Many studies have shown that LR used in HCC therapy results in an average blood loss of 600–1000 ml [Citation6], and the use of the Pringle maneuver is necessary to operate. Blood loss and blood transfusion are risk factors of postoperative morbidity and mortality and are related to tumor recurrence after HCC treatment [Citation7].
LR that uses heat coagulative necrosis through microwave (MW) or radiofrequency (RF) energy, such as TissueLink and Habib ablation needles [Citation8], has been widely used worldwide. In comparison with traditional LR, this precoagulated-assisted LR has the advantages of low blood loss and low blood transfusion rate [Citation9]. Precoagulated-assisted LR is recommended for patients with cirrhosis. However, its use is still controversial. Some studies have shown that precoagulated-assisted LR can lead to severe postoperative complications, such as abdominal abscesses, bile fistula, and bile duct damage [Citation10]. Some studies have also shown that MW-assisted LR (MW-LR) or RF-assisted LR (RF-LR) enables minimum blood loss and improves resection margins [Citation11,Citation12]. Therefore, in theory, this procedure may be beneficial for the survival of patients with HCC. Over the years, MW-LR has been performed in some patients with HCC at our center. As observed, the total survival rate and tumor-free survival rate of MW-LR in patients with HCC were better than those patients who underwent traditional LR [Citation13]. Moreover, we found that MW-LR followed the no-touch principle of tumor surgery closely. Therefore, we proposed and produced a new water-cooled MW ablation array to improve the efficiency of tumor ablation and liver transection. Here, we will evaluate the efficiency and efficacy of this device in liver parenchymal transection in a pig model.
Materials and methods
Description of the water-cooled double-needle MW ablation device
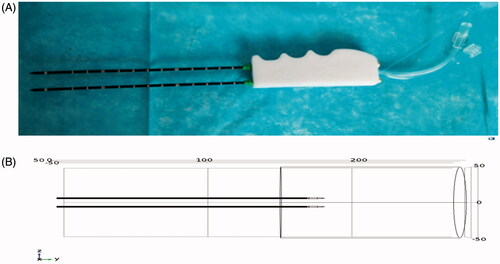
The MW-assisted device was manufactured by the ECO Microwave System Co., Ltd., Nanjing, China, as a prototype. The device is a hand-held instrument that contains the following parts:
Two 18 cm-long and 2 mm-wide needles for MW ablation. The power of each electrode can be set from 50 to 60 W, and a maximum of 150 W can be allocated.
Two coaxial cannulas, through which chilled water was circulated during ablation.
The probe can reduce electrode charring through uniform heating and apply sequential heating to achieve complete ablation in a large area. A photograph of the device is shown in , which shows the constructed probe prototype. During ablation, the MW electrodes are inserted into the liver, and the insertion depth is adjusted according to the specific distance, which ranges from 3 to 8 cm from clinical experience.
Computer modeling and temperature simulation
The simulation of liver ablation temperature distribution at 2450 MHz was reported in detail in our previous study [Citation14]. In brief, the simulation was performed with the finite element method (FEM) using COMSOL Multiphysics 5.2 as follows: The established 3D geometric model is shown in . The binary array for MW ablation surgery was placed in a liver model and had a radius of 35 mm, and a height of 110 mm. The internal and external conducting materials of the probe were made of copper, and the dielectric between the internal and external conductors was made of polytetrafluoroethylene.
The electromagnetic field is obtained by using the transverse magnetic equation in the form of [Citation15]:
(0-1)
(0-1)
Where εr is the relative dielectric constant of the liver tissue, σ is the conductivity, μr is the relative magnetic permeability (μr=1), and 8.852 × 10−12F/m is the vacuum permittivity. The specific absorption rate of the liver tissue for MWs can be obtained by solving the electromagnetic field distribution:
(0 Tables S1 and S22)
(0 Tables S1 and S22)
The ablation temperature field is solved by using Pennes’ bioheat equation, which distinguishes the heat transfer problem of biological tissue from that of general engineering material problems fundamentally in
(0 Tables S1 and S23)
(0 Tables S1 and S23)
Where Qb = WbCb(Tb−T) reflects the heat transferred by the blood flow into and out of the control volume (blood perfusion heat), Qm indicates the heat generated by metabolism (ignored in this study), and other parameters are denoted as follows:
ρ—density (kg/m3);
c—specific heat of the tissue (J/kg·°C);
K—thermal conductivity (W/(m·K));
T—temperature of the tissue (°C);
Wb—blood perfusion rate (kg/m3·s);
Cb—specific heat of the blood (J/kg·°C);
Tb— blood temperature (°C).
MW ablation of tissue-mimicking phantom
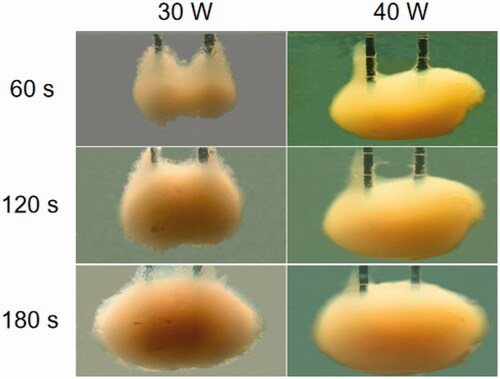
The tissue-mimicking phantom is a mixture of polyacrylamide gel phantom and bovine serum albumin [Citation15]. The temperatures of these phantoms were controlled at 24 °C [Citation16]. The procedure was performed by inserting the MW electrode into the phantom at a depth of 5 cm. For material reasons, the phantom could not withstand an output power of more than 50 W. We set the power to 30 and 40 W, and the procedure was continued for 60 to 180 s. The effect of the MW electrode was estimated based on the maximum diameter of the coagulation zone.
In vivo study on pig liver
After the in vivo study was approved by the local animal experimentation ethics committee, eight female Bama minipigs with a mean weight of 20–25 kg were used. The experiment was carried out in the operating room of the medical laboratory animal center of the People’s Hospital of Wuhan University. The animals were acclimatized under standard laboratory conditions at least a week before the surgery. The animals fasted overnight with free access to water before the operation. The operating room temperature was controlled at 24–26 °C.
Pentobarbital sodium (30 mg/kg) was slowly pushed through the ear vein before the operation. After endotracheal intubation, the mode of volume-controlled ventilation was selected, the oxygen flow rate was 2–4 L/min, respiratory rate was 16–20 breaths/min, tidal volume was 8–12 ml/kg, and the ratio of respiration and respiration was 1:1.5. The respiratory parameters were adjusted to maintain the SpO2 and PCO2 levels above 95% and between 35–45 mmHg, respectively. During the operation, inhalation anesthesia was used, and the isoflurane concentration was adjusted to 1–2 vol%. In general, the total volume of the intraoperative fluid was controlled at 500–1000 ml, in which the ratio of crystal to colloid was 2:1. After the operation, 250–500 ml of 5% glucose was infused slowly.
Midline laparotomy incision was performed on every animal from the xiphisternum to the umbilicus to expose their liver. The inferior surface of the liver was entirely isolated from the stomach and the intestine with gauze. The liver lobes of pigs are wide and deep, thereby leaving the liver independent and active. Therefore, for practical and anatomical reasons, the transection location was roughly found at the junction of the free part and the connecting part of each lobe.
The experiment involved two groups: Group B was treated with conventional clamp crushing hepatectomy and intermittent hepatic portal occlusion [Citation17]. The Pringle maneuver was carried out for 15 min each time at 5-min intervals. In Group A, a water-cooled, double-needle MW ablation device was used similar to Habib’s methods [Citation18]. The power of each electrode was set to 100 W, and each probe was applied for 60 s. The depth and probe distances were 2–3 cm. After completing ablation, the coagulated tissues of the liver overlapped and formed a coagulated zone along the transection plane. Then, a blunt dissector was used to separate the vessels along the predetermined plane, and the vessels with diameters larger than 2 mm were ligated and severed on the transverse plane. All liver resections were performed by Dr. Chen Zubin.
Outcome measures
Transection time: the total resection time comprised the time from the beginning of LR to complete hemostasis (including the time of microwave ablation);
Blood loss: the total amount of blood loss during transection comprised the blood volume in the aspirator and the gauze (weight difference between wet and dry gauze);
Transection area: obtained by the delineation of the transection plane;
Transection speed: the ratio of the transection area to the resection time;
Mean blood loss: the ratio of blood loss to the transection area;
Pathological examination
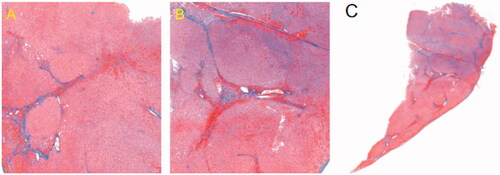
Biopsies of approximately 1 cm3 of the transection surface were taken upon completion of the resection. The specimens were fixed with 10% formalin, paraffin was embedded for light microscopy, and histopathological characteristics were assessed based on Masson’s trichrome stained sections.
Statistical analysis
The outcomes from both groups were compared and correlated. Collected data were statistically analyzed using paired t-test and Wilcoxon's signed rank test. p < 0.05 was considered statistically significant. Differences in variables were considered significant at a threshold of p < 0.05. Statistical analyses were performed using statistical software (SPSS version 22.0; IBM Corporation, Chicago, IL, USA).
Results
Computer modeling
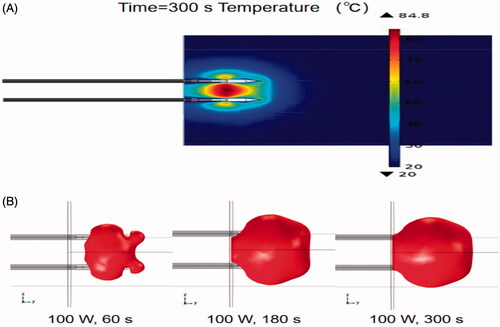
shows the temperature distribution of the double-needle MW electrode ablation in liver tissues simulated by using FEM after conducting sensitivity analyses. The average maximum temperature is 84.8 °C. is a simulated time sequence (60 s) of a 50 °C isothermal surface for the double-needle MW ablation when the total output power is 100 W. The diameter of the ablation zone quickly increased to 4 cm at 180 s.
MW ablation of tissue-mimicking phantom
shows the image of the double-needle MW ablation in the tissue-mimicking phantom at different times (e.g., 60, 120, and 180 s) with 30 and 40 W power. At 60 s, the diameter of the ablation area increased rapidly to 2.5 cm, and the diameter gradually reached 4 cm at 40 W and 180 s.
Performance of the MW ablation on in vivo pig liver
All the animal subjects tolerated the procedures well during the operation. No relevant complications were observed with any method during the operative procedure. The animals were killed with an overdose of sodium pentobarbital on the third postoperative day. The abdomen was then inspected for short-term complications. There was no obvious bleeding and bile leakage in all animals.
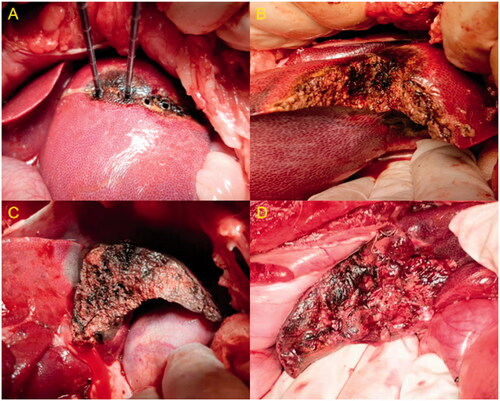
During the transection, one or two veins had a diameter larger than 3 mm in each transverse section. In almost all cases of Group B, these large vessels required at least one or two sutures to achieve complete hemostasis (). On the contrary, complete hemostasis in Group A was always achieved using the MW device alone without the need for hemostatic sutures in any of the cases (). Therefore, the mean blood loss during each hepatic transection was less than that of the conventional hepatectomy.
Figure 4. (A) Rear view and chief inner vessels of a pig liver. (B) Schematic anterior view of the lobar anatomy of a pig liver and the level of transections is depicted by dotted lines.
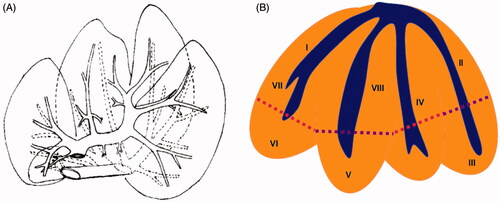
Figure 5. (A–C) Steps in the hepatic transection with a water-cooled double-needle MW ablation device. (D) Transverse section of routine hepatectomy with intermittent hepatic portal occlusion.
The outcome measures are shown in . The average dissection area in the two groups was similar, that is, 9.3 (8.5–11.2) cm2 in Group A, 9.8 (8.7–10.9) cm2 in Group B. demonstrates the total amount of blood loss in Group A as 18 (7–26) ml, which was lower than that of the other group with a p-value lesser than 0.05. shows the bleeding rates, which consist of blood loss and dissection area. The average bleeding rate in Group A was 2.4 (2–3.1) ml/cm2, which was less than that of Group B (6.9 (3.2–8.3) ml/cm2) with statistical significance (p < 0.05). The resection speed in Group A was significantly faster than that of Group B (p < 0.05).
Table 1. Results of the in vivo studies that compare MW ablation with hepatectomy.
Pathological examination
Histological findings showed coagulated hepatocytes after MW ablation on the MW ablated surfaces (). MW ablation caused extensive coagulation and fixation of hepatocytes in a short time.
Discussion
In this study, we designed and manufactured an MW ablation array prototype that included a power divider, two ablation electrodes, and a water-cooled circulatory system. The ablation performed by using the water-cooled double-needle MW ablation device in computer simulation showed that the maximum lateral ablation diameter was 3 cm at 100 W output for a duration of 60 s (assessed at 50 °C isotherm). In the tissue-mimic phantom, the maximum transverse ablation diameter of the double-needle MW ablation increased rapidly to 3 cm in 60 s at 40 W. In the in vivo pig model, each probe was applied for 60 s at 150 W input power, thereby producing a coagulated area with a width of 3 cm along the intended transection line in the liver efficiently. After the completion of individual ablation, the coagulated tissues of the liver parenchyma overlapped and formed a completely coagulated zone along the intended transection plane. Subsequently, liver transection could be performed bloodlessly and easily along this coagulated plane. Our results show that this water-cooled double-needle MW ablation device is a suitable device for precoagulated-assisted LR procedures.
Some studies have reported precoagulated-assisted LR, including RF-LR or MW-LR, which have the advantages of having the least blood loss and few inflow occlusions [Citation19,Citation20]. Habib’s bipolar RF device is currently the most widely available tool for precoagulated LR [Citation21]. MW-LR is another precoagulated method used by some surgeons [Citation22,Citation23]. In our previous study, we reported that the use of MW-LR was more suitable for HCC patients with cirrhosis than clamp-crush LR because precoagulated-assisted LR enabled minimum blood loss and improved resection margins. Traditional LR was performed accompanied by the mobilization (e.g., rotating, lifting, and stretching) of the liver, which could compress tumor cells, thereby causing distant metastasis through the vessels around the tumor. By contrast, precoagulated-assisted LR could seal the vessels around the tumor and achieved a negative resection margin, which decreased tumor recurrence [Citation13]. However, MW-LR with single-needle devices is primitive and slow [1Citation4]. Thus, we designed and produced a water-cooled double-needle MW ablation device to achieve efficient liver transections.
We used a water-cooled double-needle MW ablation device to evaluate the efficiency of division and hemostasis in the tissue of an in vivo pig liver model. We observed a six-fold reduction in the mean blood loss relative to the transection surface when using the test device compared with the conventional hepatectomy method. In addition, a 40% average increase in the mean transection speed was observed when the test device was used compared with the conventional hepatectomy group. The time needed for hemostasis was reduced significantly because of venous hemorrhage with a diameter that was larger than 3 mm. These figures are especially meaningful because the transected area in both groups was similar. The results showed that the device could improve the efficiency of the operation.
Only needlelike MW electrodes could be manufactured to be used during liver surgery because of physical reasons. By contrast, a device that used RF energy was more easily manufactured into different shapes and were widely available. Few bipolar RF electrodes were not used in liver surgery. The use of Tissuelink, an RF device, was effective in preventing bleeding during liver transection [Citation24]. Recently, the use of a pencil-type RF electrode was useful in both open and laparoscopic liver surgery. However, RF devices easily caused tissue carbonization and often blocked the flow of current. MW ablation was more potent than RF energy without the limitation of tissue carbonization [Citation25].
There are several limitations to this study. Firstly, only needlelike MW electrodes are available because of physical reasons, and the shape of the device may damage the main blood vessels or the main hepatic ducts during the operation. Therefore, the device should be used with caution near these structures, such as the hepatic hilum. Secondly, the use of MW arrays in laparoscopic surgery becomes difficult because of the water-cooled and hand-held parts. Thus, the device requires further miniaturization for minimally invasive surgery. Finally, the tested method has been compared with conventional hepatectomy, and the effect was improved evidently. Further research may help patients select the best treatment using this method.
Conclusion
The use of the proposed water-cooled and hand-held parts enables MW arrays to address parenchymal division and hemostasis simultaneously, thereby reducing blood loss and transection time. Further experience should confirm these results.
Author contributions
Zu-Bing Chen and Liang Lang designed and coordinated the research plan and conducted computer modeling studies; Qiang Yang and Wei Li carried out the MW ablation of tissue-mimicking phantom and pig liver model; Yuxin Du participated in the design of technological improvements. All authors have read and approved the final manuscript.
Disclosure statement
Dr. Zu-Bing Chen has applied for a patent relating to the content of the manuscript. All other authors declare that they have no competing interests.
Additional information
Funding
References
- Yao P, Chu F, Daniel S, et al. A multicentre controlled study of the inline radiofrequency ablation device for liver transection. HPB. 2007;9(4):267–271.
- Wang Z, Luo H, Coleman S, et al. Bicomponent conformal electrode for radiofrequency sequential ablation and circumferential separation of large tumors in solid organs: development and in vitro evaluation. IEEE Trans Biomed Eng. 2017;64(3):699–705.
- Michielsen P, Ho E. Viral hepatitis B and hepatocellular carcinoma. Acta Gastroenterol Belg. 2011;74(1):4–8.
- Ricks R, Hopcroft S, Powari M, et al. Tissue penetration of bipolar electrosurgery at different power settings. BJMMR. 2017;22(1):1–6.
- Patrlj L, Tuorto S, Fong Y. Combined blunt-clamp dissection and LigaSure ligation for hepatic parenchyma dissection: postcoagulation technique. J Am Coll Surg. 2010;210(1):39–44.
- Vogl TJ, Nour-Eldin NA, Hammerstingl RM, et al. Microwave ablation (MWA): basics, technique and results in primary and metastatic liver neoplasms –review article. Rofo. 2017;189(11):1055–1066.
- Yamazaki S, Takayama T. Management strategies to minimize mortality in liver resection for hepatocellular carcinoma. Jpn J Clin Oncol. 2017;47(10):899–‐908.
- Jayant K, Sodergren MH, Reccia I, et al. A systematic review and meta-analysis comparing liver resection with the Rf-based device Habib™-4X with the clamp-crush technique. Cancers 2018;10(11):428.
- Clasen S, Rempp H, Schmidt D, et al. Multipolar radiofrequency ablation using internally cooled electrodes in ex vivo bovine liver: correlation between volume of coagulation and amount of applied energy. Eur J Radiol. 2012;81(1):111–113.
- Zhang E-, Yang LF, Wu Z-B, et al. Therapeutic efficacy of percutaneous microwave coagulation versus liver resection for single hepatocellular carcinoma ≤3 cm with Child-Pugh A cirrhosis. Eur J Surg Oncol. 2016;42(5):690–697.
- Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with hepatic resection for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. World J Surg Onc. 2019;17(1):98.
- Giorgio A, Gatti P, Montesarchio L, et al. Intrahepatic cholangiocarcinoma and thermal ablation: long-term results of an Italian retrospective multicenter study. J Clin Transl Hepatol. 2019;7(X):1–292.
- Chen ZB, Qin F, Ye Z, et al. Microwave-assisted liver resection vs. clamp crushing liver resection in cirrhosis patients with hepatocellular carcinoma. Int J Hyperthermia. 2018;34(8):1359–1366.
- Chen D-i, Du Y-X, Chen Z-B, et al. Computer modeling and in vitro experimental study of water-cooled microwave ablation array. Minim Invasive Ther Allied Technol. 2021; 30(1):12–19.
- Saito K, Taniguchi T, Yoshimura H, et al. Estimation of SAR distribution of a tip-split array applicator for microwave coagulation therapy using the finite element method. IEICE Trans Electron. 2001; E84C(7):948–954.
- Wang Z, Aarya I, Gueorguieva M, et al. Image-based 3D modeling and validation of radiofrequency interstitial tumor ablation using a tissue-mimicking breast phantom. Int J Comput Assist Radiol Surg. 2012;7(6):941–948.
- Zhou XD, Tang ZY, Yang BH, et al. Experience of 1000 patients who underwent hepatectomy for small hepatocellular carcinoma. Cancer. 2001;91(8):1479–1486.
- Weber JC, Navarra G, Jiao LR, et al. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236(5):560.
- Ni Y, Yang X, Cui J, et al. Combined microwave ablation and antiangiogenic therapy to increase local efficacy. Minim Invasive Ther Allied Technol. 2019;29(2):107–113.
- Jiang T, Kelekis A, Zhao Q, et al. Safety and efficacy of percutaneous microwave ablation for post-procedural haemostasis: a bi-central retrospective study focusing on safety and efficacy. Br J Radiol. 2020;93(1106):20190615.
- Cassinotto C, Denys A, Gay F, et al. Radiofrequency ablation of liver tumors: no difference in the ablation zone volume between cirrhotic and healthy liver. Cardiovasc Intervent Radiol. 2018;41(6):905–‐911.
- Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137.
- Dimitri M, Staderini F, Brancadoro M, et al. A new microwave applicator for laparoscopic and robotic liver resection. Int J Hyperthermia. 2019;36(1):75–86.
- Geller DA, Tsung A, Maheshwari V, et al. Hepatic resection in 170 patients using saline-cooled radiofrequency coagulation. HPB. 2005;7(3):208–213.
- Suwa K, Seki T, Tsuda R, et al. Short term treatment results of local ablation with water-cooled microwave antenna for liver cancer: comparison with radiofrequency ablation. Mol Clin Oncol. 2020;12(3):230–236.