Abstract
Background
Radiofrequency ablation (RFA) is being considered as the favorable treatment option for unresectable colorectal cancer liver metastases (CRLM) receiving chemotherapy, yet there still exist challenges for recurrence after RFA. The present study aims to establish an effective nomogram to predict intrahepatic progression-free survival (PFS) and select RFA candidates.
Methods
Patients with unresectable CRLM treated with chemotherapy followed by RFA between 2010 and 2016 were enrolled in this study. The nomogram to predict intrahepatic PFS was established based on multivariable Cox regression analysis. The predictive performance of the nomogram was assessed according to the C-index, calibration plots and Kaplan–Meier curve.
Results
Of a total of 158 patients, the earlier new intrahepatic metastases over local tumor progression were observed in 157 patients during the follow-up, and the mean intrahepatic PFS was 16.9 ± 1.4 months in the present cohort. The optimal cutoff value of tumor size after chemotherapy was identified as 16 mm by X-tile analysis. Based on multivariate analysis, independent prognostic factors for intrahepatic PFS included primary positive lymph nodes, multiple metastases, tumor size >16 mm, no primary lesion resection, mutant KRAS and PD response after chemotherapy. The nomogram was established to predict intrahepatic PFS based on all independent factors, which achieved favorable discrimination and calibration.
Conclusion
This study firstly established the nomogram to predict intrahepatic PFS for unresectable CRLM patients receiving chemotherapy followed by RFA. It can facilitate the selection of RFA candidates, and help both surgeons and patients choose individualized regimens in the treatment decision.
Introduction
Colorectal cancer ranks as the third most common malignant tumor and has become the second leading cause of cancer-related death worldwide [Citation1]. With improved awareness and modern scanning techniques, nearly 80% of patients with colorectal cancer have developed colorectal liver metastases (CRLM) at diagnosis [Citation2]. And, 60–80% of CRLM patients will have local recurrence or new intrahepatic metastases after R0 resection [Citation3–5]. Although surgical resection remains the preferred treatment method for CRLM, most of these patients (about 70–90%) were not surgical candidates [Citation6,Citation7], and increasing evidence has demonstrated that liver metastases are the most common cause of death in patients with colorectal cancer [Citation2]. For those with a liver-limited unresectable disease that, because of involvement of critical structures, cannot be resected unless regression is accomplished, systemic chemotherapy is being increasingly considered in an attempt to improve survival benefit or potentially convert patients with unresectable metastatic disease to resectability [Citation8,Citation9].
Although systemic chemotherapy can partly achieve conversion therapy, systemic chemotherapy is difficult to fully achieve the high response rates for certain patients, thus resulting in the fact that some patients are not still suitable for surgical resection after chemotherapy. Fortunately, evidence on the use of radiofrequency ablation (RFA) as a reasonable treatment option for nonsurgical candidates and those with recurrent disease after hepatectomy with small liver metastases that can be treated with clear margins is growing [Citation10–13]. And the relevant reports analyzed the factors affecting oncological outcomes after RFA of CRLM, such as RAS gene, ablation margin and modified clinical risk score (CRS), embryonic origin of the primary lesion, etc. [Citation14–25]. Moreover, the previous studies also documented the favorable local control and long-term survival benefit for CRLM patients receiving RFA in addition to systemic chemotherapy [Citation26–28]. However, there still exist challenges for recurrence after RFA, and the definition of early recurrence and risk prognostic factors is ambiguous for unresectable CRLM patients receiving chemotherapy followed by RFA. Therefore, it is essential for the appropriate selection of adequate RFA candidates to make patients have the favorable benefit from RFA.
Along this line, the present study aims to establish a nomogram based on independent prognostic factors in an attempt to determine the criteria of RFA candidates and predict intrahepatic progression-free survival (PFS) for unresectable CRLM patients receiving chemotherapy followed by RFA. The proposed nomogram could provide individualized predictions for clinicians and patients with unresectable CRLM in the treatment decision process.
Methods
Study population
This study was approved by the institutional review board of Beijing cancer hospital, and the requirement to obtain informed consent was waived. Between January 2010 and December 2016, patients with unresectable CRLM and undergoing systemic chemotherapy were analyzed. Among them, 360 patients received the combination of systemic chemotherapy with RFA. According to the established criteria, 158 patients treated with systemic chemotherapy followed by RFA were incorporated into this study. The inclusion criteria were showed as follows: (1) The diagnosis of CRLM was confirmed through radiological examinations when the primary lesion was diagnosed by histopathology; (2) the patients have received systemic chemotherapy before RFA; (3) the response to pre-RFA chemotherapy was evaluated. The exclusion criteria included, (1) other treatment methods were received after ablation and before intrahepatic progression, (2) patients who have no sufficient clinic-pathological information and the evaluation of chemotherapy response, (3) any other malignancy in the past 10 years, (4) other factors affecting outcomes, such as clinical significant cardiovascular, uncontrolled hypertension, bleeding disorders or coagulopathy and active infection. The criteria of unresectable CRLM was defined as follows, (1) no radical resection for primary lesion, (2) unresectable disease due to certain factors, such as involvement of critical structures, (3) having the risk of insufficient remnant liver volume after liver resection, (4) the patient’s status to fail to tolerate surgical resection.
The basic characteristics of patients in this study were collected, including age, gender (male or female), primary lesion site (right hemi-colon or left hemi-colon), primary T staging (T1–2 or T3–4), primary N staging (negative lymph nodes, N0; positive lymph nodes, N1+), primary M staging (no metastases at the diagnosis of the primary lesion, M0; metastases at the diagnosis of the primary lesion, M1), primary grading (I–III or IV), differentiation degree (high differentiation or non-high differentiation), type of metastases (synchronous or metachronous), number of metastases (solitary or multiple), tumor size, primary lesion resection (yes or no), extrahepatic metastases (yes or no), chemotherapy regimen (oxaliplatin-based and irinotecan-based), KRAS gene (wild and mutant), margin size (6–10 mm and 11–15 mm), CEA level, as well as modified clinical risk score (CRS; low risk, 0–1; intermediate risk, 2–3; high risk, 4–5) from a previous report [Citation16].
CRLM treatment and follow-up
The systemic chemotherapy was performed according to the basic status of patients. The systemic chemotherapy regimens mainly include oxaliplatin-based chemotherapy and irinotecan-based chemotherapy, such as XELOX, FOLFOX and FOLFIRI. The response to chemotherapy was evaluated after every two cycles, which was classified as partial response (PR), stable disease (SD), or progressive disease (PD) using the Cancer Response Evaluation Criteria In Solid Tumors (RECIST) [Citation29]. And, the response to chemotherapy was regarded as a non-progressive disease (nonPD) when the evaluation was PR or SD. The final decision to perform RFA after systemic chemotherapy was made during a multidisciplinary meeting including radiologists, oncologists and surgeons, as well as patients’ choice. The detail of RFA is shown in the Supplementary Materials.
Definitions of terminology
Definitions are based on the standardization by the International Working Group on Image-Guided Tumor Ablation [Citation30]. The tumor was treated according to protocol and was completely covered by ablation zones. The goal of each treatment was to create a zone of ablation at least 1 cm larger than the tumor’s largest diameter to achieve a minimum ablation margin of at least 5 mm uniformly all around the tumor [Citation17]. Tumor coverage was assessed by the immediate contrast-enhanced ultrasound (CEUS) following ablation, and supplementary ablation would be performed if the residual tumor was present. Technical success was defined as the treatment of the tumor according to protocol and complete coverage by no enhancement on immediate CEUS at the end of the procedure. Complete ablation was achieved when the entire ablated zone presented no enhancement on follow-up CECT/MRI at 1 month after ablation. Local tumor progression (LTP) was defined as the appearance of tumor foci at the edge of the ablation zone after the tumor had been documented as having achieved complete ablation. New metastases were defined as a lesion with similar characteristics but not contacting the original ablation zone in the liver. Progression-free survival (PFS) refers to the time interval between the date of performing RFA and the date of progression (including recurrence or new metastases) of the disease or death, whichever occurred first.
Statistical analysis
Continuous variables are represented as mean ± standard deviation (SD), and categorical variables are described as frequency or percentages. The X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to determine the optimal cutoff value of tumor size after systemic chemotherapy, which was identified according to the PFS. Univariate and multivariate analyses of various clinic-pathological factors were performed by Cox’s proportional hazard model to identify independent prognostic factors for PFS. A nomogram was established based on the results of the multivariable analysis. The predictive accuracy of the nomogram was measured with the concordance index (C-index) and assessed by calibration. Statistical analysis was performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) and R version 4.0.1 (http://www.r-project.org).
Results
Patient characteristics
In the present study, 158 patients with CRLM met the inclusion criteria and entered into the training cohort. The basic characteristics of patients were summarized in . Among all patients, the technical success rate was 100%, and the complete ablation was achieved in 99.4% of patients (157/158). The LTP rate was 0.6% before the occurrence of new intrahepatic metastases, and new intrahepatic metastases in the liver were observed in 158 patients (100%). The mean PFS was 16.9 ± 1.4 months after the follow-up. The postoperative 1-, 2-, 3-, 4- and 5-year PFS rates were 45.6, 24.0, 14.6, 8.2 and 5.1%, respectively.
Table 1. The baseline characteristics of CRLM patients.
Optimal cutoff value of tumor size after chemotherapy
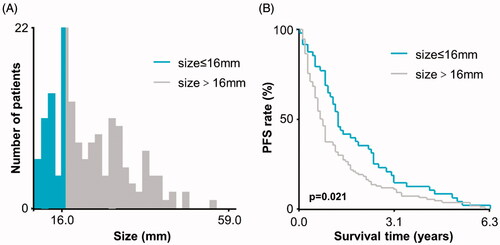
The tumor size of 16 mm was identified as the optimal cutoff value based on the interaction between tumor size and PFS through X-tile analysis (). In the subsequent analysis, the patients with CRLM were divided into small-size (≤16 mm) and large-size (>16 mm) groups according to tumor size after chemotherapy.
Prognostic factors for PFS in training cohort
In the univariate analysis, including primary lymph nodes, a number of metastases, size of metastases, KRAS gene, primary lesion resection, chemotherapy response, CEA level and modified CRS were related to a decreased PFS. Six independent prognostic factors for PFS were identified in multivariate analysis, including primary lymph nodes (HR = 1.539, 95%CI: 1.010–2.340, p = 0.040), the number of metastases (HR = 0.594, 95%CI: 0.415–0.850, p = 0.004), size of metastases (HR = 0.569, 95%CI: 0.415–0.814, p = 0.002), primary lesion resection (HR = 0.424, 95%CI: 0.191–0.942, p = 0.040), KRAS gene (HR = 0.572, 95%CI: 0.406–0.808, p = 0.001) and chemotherapy response (HR = 1.692, 95%CI: 1.160–2.468, p = 0.006) ().
Table 2. Univariate and multivariate analysis of influencing factors associated with PFS.
Construction of a prognostic nomogram for intrahepatic PFS
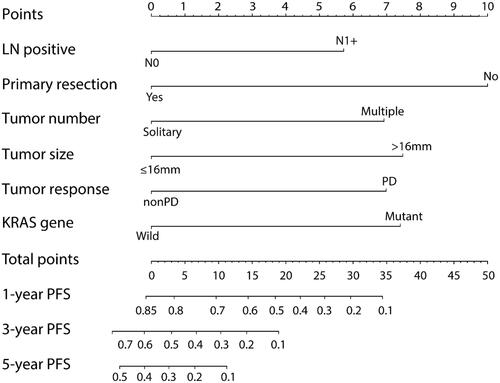
Subsequently, a prognostic nomogram with point scales was established according to independent prognostic factors (). Based on the multivariable Cox model, these independent prognostic factors were assigned a specific point scales as follows: primary N1+, 5.7 points; multiple metastases, 6.9 points; the size of metastases >16 mm, 7.5 points; no primary lesion resection, 10 points; mutant KRAS, 7.4 points; PD response after chemotherapy, 7.0 points. The sum of the points for each variable was plotted on the total points axis, and the total points for the scores ranged from 0 to 50. The estimated probabilities of PFS after RFA at 1, 3, and 5 years were obtained by drawing a line horizontally from the plotted total points axis straight to the survival axis, and the C-index for PFS prediction was 0.705 (95%CI: 0.664–0.746).
Figure 2. Nomograms express the results of prognostic models using clinic-pathological characteristics to predict PFS of CRLM patients. Nomograms can be explained by adding up the points assigned to each variable, which is indicated at the top of scale. The total points can be converted to predicted 1-, 3- and 5-year probability of recurrence for a patient in the lowest scale.
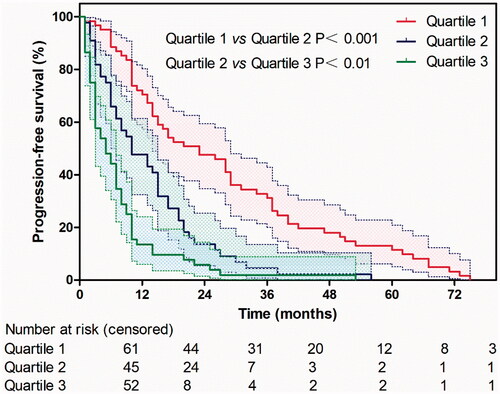
A calibration plot for the probability of 1-, 2-, 3-, 4- and 5-year PFS ( and Figure S1) demonstrated favorable calibration between the prediction by the nomogram and the actual observation. The survival curves stratified by quartiles of the nomogram-predicted score are shown in . Patients with the lowest predicted PFS (Quartile 3, total points above 25, mean PFS: 7.6 ± 1.2 months) exhibited substantially worse survival than those in Quartile 1 (total points 0–15, mean PFS: 27.5 ± 2.6 months) and Quartile 2 (score 16–25, mean PFS: 13.1 ± 1.7 months), whose difference between them was statistically significant (Quartile 1 vs Quartile 2, p < 0.001; Quartile 2 vs Quartile 3, p < 0.01).
Figure 3. Calibration curves for 3-year PFS using nomograms with clinic-pathological characteristics are shown. The X-axis is nomogram-predicted probability of PFS and Y-axis is actual PFS. The reference line was marked as gray and indicates perfect calibration.
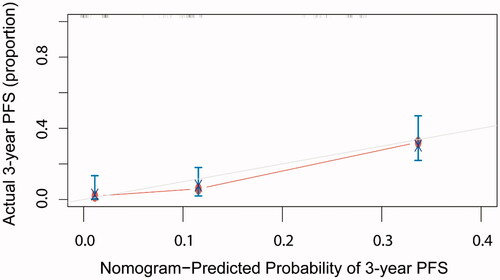
Figure 4. The survival curves stratified by quartiles of the nomogram-predicted score. Patients with the lowest predicted PFS (Quartile 3, total points above 25, mean PFS: 7.6 ± 1.2 months) exhibited substantially worse survival than those in Quartile 1 (total points 0–15, mean PFS: 27.5 ± 2.6 months) and Quartile 2 (score 16–25, mean PFS: 13.1 ± 1.7 months), whose difference between them was statistically significant (Quartile 1 vs Quartile 2, p < 0.001; Quartile 2 vs Quartile 3, p < 0.01).
Discussion
In the present study, the nomogram was successfully developed to predict PFS for unresectable CRLM patients receiving chemotherapy followed by RFA. In this model, six variables were identified as independent prognostic factors by the multivariable Cox regression analysis and were incorporated into the nomogram. Each prognostic factor played a different weight in predicting intrahepatic PFS after chemotherapy followed by RFA, which would be quantified and specified in the treatment decision.
Actually, previous efforts mainly focused on the influence of clinic-pathological factors on outcomes for CRLM patients treated with RFA, such as tumor staging, tumor number [Citation27,Citation31], yet few studies have addressed the effect of chemotherapy response on survival benefit. While our results go well beyond previous findings as we found that the outcome was closely associated with the chemotherapy response for patients receiving chemotherapy followed by RFA. The present study indicated that patients with high chemotherapy response rates could have a longer intrahepatic PFS compared to patients with low chemotherapy response after RFA. For this reason, studies previously demonstrated that by comparing with CRLM from hindgut origin, CRLM from midgut origin was associated with worse pathologic response to chemotherapy and worse survival after resection and ablation [Citation24,Citation32]. Along with this, we spectated that the embryonic origin of the primary lesion may predict pathologic response to chemotherapy and survival in patients undergoing RFA for CRLM, to some extent. In addition, chemotherapy resistance may result from cancer cells by arresting cell cycle at G0/G1 phase, which could escape chemotherapy-induced cell death by entering stemness; whereas cancer stemness could further induce the tumor growth and maintenance due to the capacity of self-renewal, self-differentiation, and high tumorigenicity [Citation33–36]. Moreover, previous studies also documented that the density of tumor-infiltrating lymphocytes (TILs) in the invasive margin of CRLM revealed a strong association with chemotherapy response, and the low chemotherapy response rates represented fewer TILs for CRLM [Citation37–39]. Collectively, the high chemotherapy response rates can be considered as the important indicator of favorable prognosis for patients treated with chemotherapy followed by RFA. Meanwhile, these results also provide an important reference for the doubt whether patients with high chemotherapy response continue receiving chemotherapy at the risk of chemotherapy resistance or perform RFA.
In addition to the influence of chemotherapy response on intrahepatic PFS after ablation, RAS mutation status is also increasingly being considered as the biological predictor of patterns of response and recurrence after chemotherapy and resection of CRLM [Citation40,Citation41], and the present study displayed that the outcomes were also worse following liver ablation in CRLM patients with KRAS mutation. In consistent with the present study, the previous report has documented that RAS mutation predicts positive resection margins and narrower resection margins in CRLM patients undergoing resection, suggesting different pathological and phenotypic features in patients with mutant and wild-type RAS [Citation41]. Notably, the minimal ablation margin and RAS mutation status may interact as independent predictors of local tumor progression (LTP) following CRLM ablation [Citation19–21].
We do, however, note that the interaction between ablation margin and intrahepatic PFS was not obvious according to subgroup analysis in the present study. For this reason, it may be ascribed to the influence of uncoordinated sample size between the group with a margin size of 6–10 mm and the group with margin size of 11–15 mm, yet more mainly to the different end-point event of PFS due to earlier new intrahepatic metastases over LTP for nearly all patients in our cohort. In other words, the end-point event of intrahepatic PFS was new intrahepatic metastases in current studies, not LTP as previously reported. Although studies previously displayed that LTP may be attributed to the growth of microscopic viable tumor at the site of ablation and pointed to the potential effect of ablation margin on LTP [Citation14,Citation42,Citation43], the influence of ablation margin on new metastases need to be continually explored. In addition, more previous studies showed that ablation margins greater than 5 mm are essential for satisfactory local tumor control [Citation14,Citation16–18,Citation22,Citation23]. While there was an ablation zone with a minimal margin uniformly larger than 5 mm in the current study cohort, so there may need a longer follow-up time to determine the outcome difference between subgroups. And, some new approaches need to be continually explored to offer a potential real-time biomarker and lower incomplete ablation rates, such as fluorescent tissue assessment and diffuse reflectance spectroscopy [Citation44,Citation45].
Meanwhile, the current study also clearly demonstrated that the size of metastases obviously affected PFS after ablation. However, the optimal cutoff value of 16 mm in the present study was contradictory to the previous cutoff value of 30 mm [Citation15,Citation16]. The previous report showed that the risk for LTP decreased by 46% for each 5-mm increase in minimal margin size, whereas each additional 5-mm increase in tumor size increased the risk of LTP by 22% [Citation17], so the results may be explained according to the interaction between tumor size and ablation margin, to some extent. Moreover, the difference of end-point events in different cohorts should be equally not ignored, the influence of tumor size on new metastases after ablation should be further explored, like the current study. In addition, the present study also assessed the use of modified CRS including tumor size for ablation of CRLM. The results indicated that although the modified CRS may be considered as a prognostic tool for ablation of CRLM according to univariate analysis, it was still not the independent prognostic factor for intrahepatic PFS after ablation, which was consistent with the results of the previous study [Citation16]. However, the outcome difference between low-risk CRS (0–2) and high-risk CRS (3–5) was significant according to univariate analysis and multivariate analysis (data not shown). This reason may be interpreted based on the cutoff value and weight for each influencing factor in modified CRS, and the cutoff value and weight of each influencing factor should be further defined to better use in ablation.
Of course, we acknowledged the limitations of this study. All clinical data were collected from the single institutions, which may induce the bias since the RFA efficacy partly depends on the expertise and experience of operators. In addition, there was some improvement space for the nomogram because the prediction calculation was based on the statistical significance within the collected factors, yet it is possible that there exist other unknown factors that will affect the outcome.
In conclusion, the present study firstly established the nomogram model to predict intrahepatic PFS for unresectable CRLM patients receiving chemotherapy followed by RFA. It can be used to select RFA candidates in an attempt to achieve better benefit from RFA and could help both surgeons and patients choose personalized regimens in the treatment decision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Ren L, Zhu D, Benson AB, 3rd, et al. Shanghai international consensus on diagnosis and comprehensive treatment of colorectal liver metastases (version 2019). Eur J Surg Oncol. 2020;46(6):955–966.
- D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096–1103.
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–448.
- Liu W, Wang K, Han Y, et al. Nomogram predicted disease free survival for colorectal liver metastasis patients with preoperative chemotherapy followed by hepatic resection. Eur J Surg Oncol. 2019;45(11):2070–2077.
- Hackl C, Neumann P, Gerken M, et al. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810.
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683.
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657.
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47.
- Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines. Colon Cancer, Version 2. 2019. J Natl Compr Canc Netw. 2019. Available from: www.nccn.org/patients
- Elias D, De Baere T, Smayra T, et al. Percutaneous radiofrequency thermoablation as an alternative to surgery for treatment of liver tumour recurrence after hepatectomy. Br J Surg. 2002;89(6):752–756.
- Nieuwenhuizen S, Puijk RS, van den Bemd B, et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: multidisciplinary consensus document from the COLLISION trial group. Cancers. 2020;12(7):1779.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280(3):949–959.
- Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22(6):755–761.
- Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology. 2016;278(2):601–611.
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275.
- Jiang BB, Yan K, Zhang ZY, et al. The value of KRAS gene status in predicting local tumor progression of colorectal liver metastases following radiofrequency ablation. Int J Hyperthermia. 2019;36:211–219.
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768.
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28(7):2727–2734.
- Han K, Kim JH, Yang SG, et al. A single-center retrospective analysis of periprocedural variables affecting local tumor progression after radiofrequency ablation of colorectal cancer liver metastases. Radiology. 2021;298(1):212–218.
- Kaye EA, Cornelis FH, Petre EN, et al. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur Radiol. 2019;29(5):2698–2705.
- Yamashita S, Odisio BC, Huang SY, et al. Embryonic origin of primary colon cancer predicts survival in patients undergoing ablation for colorectal liver metastases. Eur J Surg Oncol. 2017;43(6):1040–1049.
- Kurilova I, Bendet A, Petre EN, et al. Factors associated with local tumor control and complications after thermal ablation of colorectal cancer liver metastases: a 15-year retrospective cohort study. Clin Colorectal Cancer. 2020;S1533-0028(20)30134-1.
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol. 2012;23(10):2619–2626.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109:djx015.
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968.
- Litiere S, Collette S, de Vries EG, et al. RECIST - learning from the past to build the future. Nat Rev Clin Oncol. 2017;14(3):187–192.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273(1):241–260.
- Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31(5):948–956.
- Yamashita S, Brudvik KW, Kopetz SE, et al. Embryonic origin of primary colon cancer predicts pathologic response and survival in patients undergoing resection for colon cancer liver metastases. Ann Surg. 2018;267(3):514–520.
- Milanovic M, Fan DNY, Belenki D, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553(7686):96–100.
- Touil Y, Igoudjil W, Corvaisier M, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20(4):837–846.
- Kaiser J. The cancer stem cell gamble. Science. 2015;347(6219):226–229.
- Marx J. Molecular biology. Cancer’s perpetual source? Science. 2007;317(5841):1029–1031.
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964.
- Pages F, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–1102.
- Halama N, Michel S, Kloor M, et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71(17):5670–5677.
- Zimmitti G, Shindoh J, Mise Y, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol. 2015;22(3):834–842.
- Brudvik KW, Mise Y, Chung MH, et al. RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23(8):2635–2643.
- Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19(13):4262–4269.
- Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249(1):364–374.
- Sotirchos VS, Fujisawa S, Vakiani E, et al. Fluorescent tissue assessment of colorectal cancer liver metastases ablation zone: a potential real-time biomarker of complete tumor ablation. Ann Surg Oncol. 2019;26(6):1833–1840.
- Tanis E, Spliethoff JW, Evers DJ, et al. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur J Surg Oncol. 2016;42(2):251–259.