Abstract
Purpose
To evaluate the efficacy and safety of radiofrequency ablation for low-risk papillary thyroid microcarcinoma (PTMC) in patients aged 55 years or older.
Methods
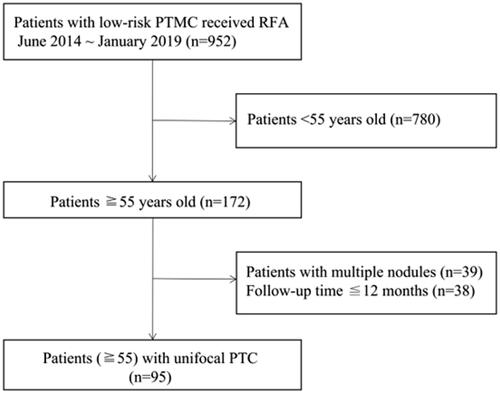
This retrospective study included 95 patients aged 55 years or older who underwent radiofrequency ablation (RFA) for PTMCs between June 2014 and January 2019. Incidence and duration of postoperative complications were recorded and evaluated. Tumor volume and volume reduction rate (VRR) changes were calculated. Patients were also closely monitored for tumor recurrence, regrowth, and lymph node metastasis.
Results
All nodules were completely ablated. The mean initial volume of the ablated thyroid nodules was 107.27 ± 99.10 mm3, and the volume decreased significantly during the follow-up time. The VRR in 1st, 3rd, 6th, 12th, 18th, 24th and 36th month were −591.64 ± 623.65%, −170.89 ± 319.51%, 9.74 ± 128.43%, 77.99 ± 45.26%, 99.35 ± 3.61%, 99.45 ± 3.05% and 99.78 ± 1.54%, respectively. No patient had any life-threatening complications. One patient had lymph node metastasis and one had a recurrence; both underwent a second radiofrequency ablation treatment and achieved satisfactory treatment results.
Conclusions
Our study suggests that radiofrequency ablation is a safe and effective option for low-risk PTMC in patients aged 55 years or older who are at a high risk of general anesthesia and postoperative complications or those who refuse surgery.
Introduction
Since the early 1980s, the incidence of thyroid cancer has been increasing significantly, and it ranks ninth in cancer incidence worldwide [Citation1]. According to Global Cancer Statistics 2018, the global thyroid cancer incidence rate is 10.2 per 100,000 person-years in women and 3.4 per 100,000 in men. However, the causes of the increased incidence are not well understood. A complex interplay in diagnostic changes, ionizing radiation, obesity, hormonal exposures, and certain environmental pollutants may have resulted in such a drastic change [Citation2,Citation3].
Papillary thyroid carcinoma (PTC) is a well-differentiated malignant thyroid tumor. PTC accounts for the majority of thyroid cancers, but it is an indolent tumor [Citation4,Citation5] with a good prognosis [Citation6–8]. More than 50% of PTCs are less than 1 cm in size and are referred to as papillary thyroid microcarcinoma (PTMC) [Citation5]. Low-risk PTMC has been defined as a PTMC without extrathyroidal extension, vascular invasion, or metastases [Citation4]. It has a 99% survival rate at 20 years [Citation9]. Active surveillance has been mentioned as a safe and effective management strategy for some of these very low-risk PTMC by the 2015 American Thyroid Association (ATA) guidelines [Citation4]. However, the identification of patients with a tendency for tumor progression among the majority of those with indolent and stable PTMC is still an unsolved issue. In addition, according to the data provided by Hye-Seon Oh et al. [Citation10], nearly half of the patients with PTMC (48.3%) who accepted active surveillance experienced anxiety during their follow-up and underwent delayed thyroid surgery thereafter.
Thyroidectomy has been used as the first-line treatment for the most low-risk PTMC [Citation11]. However, because of the distinct anatomical position of the thyroid gland, surgery may result in complications such as permanent recurrent laryngeal nerve paralysis, hypoparathyroidism, and a life-long dependency on levothyroxine, which may cause osteoporosis and cardiac disease [Citation12,Citation13]. Therefore, there is a controversy regarding overtreatment by surgery for such low-risk but high-incidence disease.
Ultrasound-guided radiofrequency ablation (RFA) has attained satisfactory outcomes in thyroid disease in recent years [Citation14–18]. Long-term observation of RFA in the treatment of PTMC showed a good volume reduction rate (VRR), low tumor recurrence, and low lymph node metastasis rate [Citation12,Citation14]. In terms of safety, complications associated with general anesthesia can be avoided, and a lower trauma rate and more rapid recovery can be achieved [Citation12,Citation19]. For patients who are at high risk for surgery or those who refuse surgery, RFA may be a good alternative treatment method. However, to date, investigators have not evaluated the effectiveness and safety of RFA in patients aged 55 years or older who may have a lower tumor progression rate but higher mortality [Citation20,Citation21]. Therefore, our aim was to assess the efficacy and safety of RFA in individuals aged 55 years or older with low-risk PTMC, to provide an additional reference for clinical treatment strategies for such patients.
Material and methods
Study population
The ethics committee of the Chinese People’s Liberation Army General Hospital approved this retrospective study. All patients provided written informed consent before the RFA procedure. They requested RFA treatment because of contraindications to surgery or because they refused thyroidectomy and active surveillance.
Ninety-five patients from June 2014 to January 2019 were enrolled (). The inclusion criteria were as follows: (1) aged 55 years or older; (2) core-needle biopsy-confirmed PTC; (3) underwent RFA therapy; (4) maximum tumor diameter less than 1 cm; (5) no local, lymph node, or distant metastasis; and (6) refusal of, or ineligibility for, surgery. Patients were excluded if they met one of the following conditions: (1) received other therapy before or after RFA for their PTMCs; (2) contraindications for RFA such as coagulation abnormalities and a severe heart condition; (3) tumor close to the dangerous triangle (within 5 mm from the trachea-esophageal groove); or (4) incomplete follow-up information.
Pre-RFA evaluation
Before RFA, all patients underwent detailed imaging examination, including routine ultrasound with an S2000 scanner (Siemens, Mountain View, CA) or a Mindray Reaona 7 scanner (Mindray, Shenzhen, China), color Doppler flow imaging (CDFI), and contrast-enhanced ultrasound (CEUS) with 2.4 ml SonoVue (Bracco International, Milan, Italy). Laboratory tests (routine blood analysis, coagulation function, and thyroid function tests), electrocardiogram (ECG), and chest radiography were performed for a comprehensive evaluation to exclude patients with contraindication for RFA.
The formula for the calculation of the tumor volume was V = πabc/6 [Citation22,Citation23] (V was the volume; a was the longest diameter, and b and c were the other two diameters of the tumor). The tumor locations were recorded as left and right lobes and cephalocaudal part (upper, middle and lower) of the thyroid. Tumor’s sonographic features with high suspicion (median > 70 ∼ 90%) for malignancy were microcalcifications, hypoechoic composition, irregular margins, and tall shape. In addition, CDFI was used to assess the blood supply, divided into four levels according to the Adler blood flow grading [Citation24]: no blood flow, 1–2 punctate or short rod blood flow, 3–4 punctate or 1 vessel blood flow, and multiple colored blood flow. Tumors were confirmed by histological pathology.
RFA equipment
Tumors were ablated using a bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18-gauge internal-cooled radiofrequency applicator with a 0.9 cm active tip (CelonProSurgemicro 100-T09; Olympus Surgical Technologies Europe) in this study.
RFA procedure
An experienced ultrasound physician (YK. L) with 20 years’ experience in clinical work in thyroid diagnosis and treatment conducted all the examinations and surgery to minimize bias. Patients were in a supine position to fully expose the neck area. They were routinely sterilized and draped in sterile towels in the operating area. Before the operation, CEUS was performed again to confirm the enhancement mode of the tumor and the surgery route. One percent lidocaine was injected with an 18PTC needle for local anesthesia, and 20–30 ml saline was injected along with the thyroid capsule into the area between the thyroid gland and surrounding critical structures, such as the trachea and vessels, to keep them away from the ablation area and to prevent heat injury. Then, an RF needle was inserted into the tumor and moved from deep to shallow (i.e., the ‘moving shot technique’) with an output power of 3–6 W. Ablation was completed when the transient hyperechoic echotexture completely covered the tumor. CEUS was used immediately after RFA to evaluate the ablation [Citation25]. Each diameter of the ablation zone must have been larger than the tumor diameter by more than 2 mm [Citation14,Citation26], to ensure complete tumor ablation.
Post-RFA evaluation
Discomfort and complications were closely monitored in the first 2 h after RFA. Patients were followed up at 1, 3, 6 and 12 months in the first year, every 6 months in the second year and every 12 months thereafter. Two-dimensional ultrasound and laboratory tests were performed to assess changes in the ablation focus, lymph node status, and the basic condition of the patients. VRR was used to reflect the recovery of the ablation zone and calculated by the following equation: VRR = [(Initial volume − Final volume) × 100]/Initial volume [Citation27–29].
Statistical analysis
SPSS 24.0 (IBM Corp.) and R software, version 3.5.3 (The R Foundation for Statistical Computing) were used to analyze the data. Means ± standard deviations were expressed for continuous data and frequencies and percentages were used for categorical data. Wilcoxon signed rank-sum test was used in the comparisons of volume changes before and after RFA. p < 0.05 indicated statistical significance.
Results
Pre-RFA assessment
shows all the patient demographic data. Twenty-four men (25.3%) and 71 women (74.7%) were included in this study. The mean age was 66 years (range 55–74) and the mean follow-up period was 36.6 ± 16.6 (range 15–74) months. The locations of the 95 PTMCs were distributed as follows: 8 (8.4%) in the isthmus, 45 (47.4%) in the left lobe, and 42 (44.2%) in the right lobe; 13 (14.8%) in the upper area, 49 (55.7%) in the middle, and 26 (27.4%) in the lower part of the thyroid gland. In terms of shape, there were 49 (51.6%) tumors with a ratio of tall to wide greater than 1, and 63 (66.3%) tumors with unclear borders. The mean length of the maximum diameters of the tumors was 0.607 ± 0.196 cm (range, 0.2–1.0), and the mean initial tumor volume was 107.27 ± 99.10 mm3 (range, 6.29–424.44). Among these PTMCs, 42 (45.7%) tumors had punctate calcifications, 8 (8.5%) had plaque calcifications, and only 1 (1.1%) had large calcifications. According to Adler blood flow classification method, 65 (68.4%) PTMCs belonged to grade 0, 25 (26.3%) PTMCs to grade 1, and 5 (5.3%) to grade 2. The BRAFV600E mutation was present in 59 (77.6%) patients.
Table 1. Demographics and characteristics of the nodules.
RFA assessment
summarizes the RFA parameters. The mean output power, energy, time, and unit energy (formula: Energy/Post-RFA volume) were 4.89 ± 1.433 W (range, 3–8), 0.749 ± 0.497 J (range, 0.17–2.54), 177.74 ± 128.091 s (range, 43–767), and 0.849 ± 0.474 J/ml (range, 0.274–2.867), respectively. The mean volume of post-RFA immediately was 1.043 ± 0.752 ml (range, 0.1–4.842), and the ratio of post-RFA volume to initial volume was 16.745 ± 14.529 mm3 (range 0.864–96.333). summarizes the prevalence of complications, which was 2% and included one mild episode of pain and one paroxysmal arrhythmia. Both complications were relieved in a short period.
Table 2. RFA information of the 95 nodules.
Table 3. Complications after RFA.
Follow-up assessment
The mean volumes of ablated lesions at 1-month, 3-month, 6-month, 12-month, 18-month, 24-month and 36-month follow up were 484.36 ± 556.08 mm3 (range, 0–4401.60), 213.25 ± 318.80 mm3 (range, 0–2554.50), 84.41 ± 162.69 mm3 (range, 0–1383.36), 24.17 ± 69.81 mm3 (range, 0–603.65),1.45 ± 10.70 mm3 (range, 0–88.00), 0.89 ± 5.07 mm3 (range, 0–31.40) and 0.39 ± 2.79 mm3 (range, 0–23.60), respectively. The volume at each follow-up was significantly smaller than that measured at the prior follow-up. The mean VRR was −591.6 ± 623.7%, −170.9 ± 319.5%, 9.7 ± 128.4%, 78.0 ± 45.3%, 99.4 ± 3.6%, 99.5 ± 3.1%, and 99.8 ± 1.5% at each follow-up point () and significant differences were also observed between every two follow-up points before 18 months (p < 0.01). Forty-four (46.3%) tumors completely disappeared within 12 months during their follow-up period ( and ). Ultimately, only one patient had a glandular recurrence, and this patient underwent a successful RFA retreatment.
Figure 2. A 60-year old woman with a papillary thyroid carcinoma in the middle of the left thyroid lobe (A) A hypoechoic nodule (white arrow) with an irregular margin was detected in the middle of the left thyroid lobe. (B) Immediately after radiofrequency ablation (RFA), a non-enhanced area measuring 1.4 × 1.4 × 1.4 cm was observed in the ablation zone. (C–E) Follow-up images showed that the ablation zone decreased gradually to 1.1 × 1.1 × 0.8, 0.9 × 1.0 × 0.8, and 0.6 × 0.7 × 0.5 cm at 1, 3 and 6 months, respectively. (F) At 12 months after RFA, the ablation area had disappeared on ultrasonography.
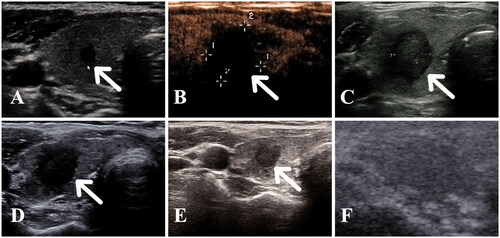
Figure 3. Tumor disappearance time curve after ablation. 46.3% of tumors completely disappeared within 12 months during their follow-up period.
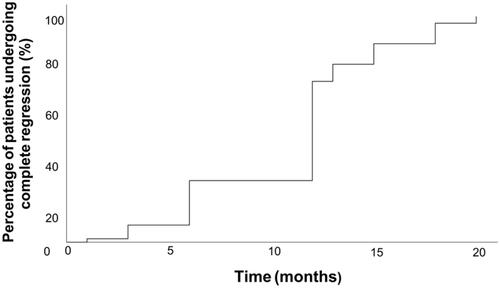
Table 4. Volume changes of the nodules in the 95 patients.
Discussion
Thyroid cancer ranks ninth in cancer incidence worldwide [Citation1]. The incidence rate of PTMC, a type of PTC smaller than 1 cm that is often found by chance during examination for other head or neck problems [Citation30], has been increasing rapidly in recent years [Citation31]. There is widespread controversy over the clinical treatment strategy for PTMC. Surgery is still the first-line treatment for most low-risk PTMCs. However, postoperative complications, including permanent recurrent laryngeal nerve paralysis, hypoparathyroidism, osteoporosis, and cardiac events, decrease patients’ quality of life [Citation32–34]. According to some studies, surgery can be considered as an overtreatment clinical strategy for such a low-risk but high-incidence disease [Citation35]. Active surveillance has been mentioned as a safe and effective management strategy for some of these very low-risk PTMC in the 2015 ATA guidelines [Citation4]. However, the indications and contraindications for this clinical strategy are not very clear.
With advances in ultrasound technology, many new minimally invasive treatment methods have emerged for thyroid therapy, such as thermal ablation, radiation therapy, and alcohol sclerotherapy [Citation36,Citation37]. Thermal ablation is the most common treatment for PTC, especially RFA, which is used for recurrent PTC, lymph node metastasis, and low-risk PTMCs [Citation12,Citation15,Citation38,Citation39]. High temperatures (>60 °C) produced by RFA induce rapid protein denaturation, which immediately causes coagulative necrosis in tumor cells [Citation40]. After RFA, the ablation zone gradually shrinks and disappears over time. According to a large cohort and long-term observation after RFA for PTMC in our previous study, the VRR was 98.8% and the recurrence rate was 2.42% [Citation41]. In this study, the VRR and recurrence rate have reached the same level in patients aged 55 years or older.
RFA is more active compared to active surveillance but has gentler intervention than surgery. To date, there is no strategy that allows to accurately predict which patients with low-risk PTMC may develop tumor progression or which ones are suitable for active surveillance. According to Nilubol and Kebebew [Citation21], 28.8% patients with classic PTC experienced thyroid cancer-related mortality, and age ≥45 years was the only independent prognostic factor for this observed mortality. In another study of nonoperative management of elderly PTMC patients aged >65 years, the overall 5-year survival rate was lower, 23% [Citation42]. In addition, in a recent study, Pitoia et al. [Citation8] summarized the clinical outcomes of active surveillance around the world, and the rate of tumor growth larger than 3 mm and lymph node metastasis could reach 22% and 3.8%, respectively. All these results suggested that even small-sized PTC tumors might require active therapeutic intervention, in particular for aged patients.
In studies that compared the outcomes of surgical resection with those of RFA treatment in low-risk PTMCs, investigators found that RFA was not inferior to surgical resection, potentially also granting a higher quality of life [Citation12,Citation32,Citation41,Citation43]. In surgery, aged patients often experience more challenges such as the risk of general anesthesia, surgical trauma, and postoperative complications due to their additional diseases associated with age. By comparison, RFA can be performed using local anesthesia causing minimal trauma, which could help avoid or reduce the challenges mentioned above. In our study, four patients were older than 70 years. They were evaluated using imaging and laboratory tests before RFA. None of the four patients had complications and all have recovered well after RFA without recurrence or lymph node metastasis.
In our study, only 1 patient (1%) had a recurrence, which was a lower frequency than that found in other studies [Citation19,Citation29]. This difference was probably related to the fact that the patients aged 55 years or older in our study were not prone to tumor progression, as reported by Koshinma et al. and Ito et al. [Citation13,Citation20]. Lymph node metastasis after surgery was an important reason for a second operation; however, in our study, there was no lymph node metastasis, which indirectly confirmed the complete effectiveness of RFA. The patient who had recurrence within the gland underwent a second ablation and achieved a satisfactory result.
There were limitations in this study. First, this was a single-center retrospective study and the follow-up period was not sufficiently long to assess the long-term effects. Second, because there were not enough patients with PTMC who voluntarily accepted active monitoring in our medical center, we could not perform comparative studies. Third, only four patients older than 70 years old were included in our study, a larger sample size is needed to confirm our findings. However, this study was the first one to reveal the effect of RFA treatment in patients aged 55 years or older with low-risk PTMC. Compared with aggressive surgical procedures and conservative active monitoring strategies, RFA can also achieve complete elimination of tumor cells and be effective and safe for patients aged 55 years or older.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Kitahara CS, Brenner AV. Thyroid cancer. New York (NY): Oxford University Press, 2018.
- Sanabria A, Kowalski LP, Shah JP, et al. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 2018;40(4):855–866.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322.
- Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020;30(7):985–991.
- Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31(2):183–192.
- Pitoia F, Smulever A. Active surveillance in low risk papillary thyroid carcinoma. World J Clin Oncol. 2020;11(6):320–336.
- Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ. 2014;348:g3045.
- Oh HS, Ha J, Kim HI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid. 2018;28(12):1587–1594.
- Wrenn SM, Wang TS, Toumi A, et al. Practice patterns for surgical management of low-risk papillary thyroid cancer from 2014 to 2019: a CESQIP analysis. Am J Surg. 2021;221(2):448–454.
- Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years’ follow-up. Thyroid. 2020;30(3):408–417.
- Koshkina A, Fazelzad R, Sugitani I, et al. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(6):552–560.
- Cho SJ, Baek SM, Lim HK, et al. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. 2020;30(12):1745–1751.
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897–4903.
- Ha EJ, Baek JH, Lee JH, et al. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid. 2013;23(3):289–293.
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of radiofrequency ablation therapy for large benign thyroid nodules: a mayo clinic case series. Mayo Clin Proc. 2018;93(8):1018–1025.
- Lee GM, You JY, Kim HY, et al. Successful radiofrequency ablation strategies for benign thyroid nodules. Endocrine. 2019;64(2):316–321.
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36:359–367.
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34.
- Nilubol N, Kebebew E. Should small papillary thyroid cancer be observed? A population-based study. Cancer. 2015;121(7):1017–1024.
- Lin W-C, Kan N-N, Chen H-L, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43.
- Kong QF, Lv B, Wang B, et al. Association of von Willebrand factor (vWF) expression with lymph node metastasis and hemodynamics in papillary thyroid carcinoma. Eur Rev Med Pharmacol Sci. 2020;24(5):2564–2571.
- Yan L, Luo Y, Xiao J, et al. Non-enhanced ultrasound is not a satisfactory modality for measuring necrotic ablated volume after radiofrequency ablation of benign thyroid nodules: a comparison with contrast-enhanced ultrasound. Euro Radiol. 2020. DOI:https://doi.org/10.1007/s00330-020-07398-0
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.
- Cesareo R, Pacella CM, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for benign non-functioning thyroid nodules: six-month results of a randomized, parallel, open-label trial (Lara Trial). Thyroid. 2020;30(6):847–856.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Wilhelm S. Evaluation of thyroid incidentaloma. Surg Clin North Am. 2014;94(3):485–497.
- Miyauchi A, Ito Y, Oda H. Insights into the management of papillary microcarcinoma of the thyroid. Thyroid. 2018;28(1):23–31.
- Lan Y, Luo Y, Zhang M, et al. Quality of life in papillary thyroid microcarcinoma patients undergoing radiofrequency ablation or surgery: a comparative study. Front Endocrinol. 2020;11:249.
- Yuan J, Li J, Chen X, et al. Identification of risk factors of central lymph node metastasis and evaluation of the effect of prophylactic central neck dissection on migration of staging and risk stratification in patients with clinically node-negative papillary thyroid microcarcinoma. Bull Cancer. 2017;104(6):516–523.
- Ahmadi S, Gonzalez JM, Talbott M, et al. Patient preferences around extent of surgery in low-risk thyroid cancer: a discrete choice experiment. Thyroid. 2020;30(7):1044–1052.
- Krajewska J, Kukulska A, Oczko-Wojciechowska M, et al. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol. 2020;11:571421.
- Alobuia W, Gillis A, Kebebew E. Contemporary management of anaplastic thyroid cancer. Curr Treat Options Oncol. 2020;21(10):78.
- Hegedus L, Frasoldati A, Negro R, et al. European Thyroid Association survey on use of minimally invasive techniques for thyroid nodules. Eur Thyroid J. 2020;9(4):194–204.
- Han ZY, Dou JP, Cheng ZG, et al. Efficacy and safety of percutaneous ultrasound-guided microwave ablation for cervical metastatic lymph nodes from papillary thyroid carcinoma. Int J Hyperthermia. 2020;37(1):971–975.
- Wang L, Ge M, Xu D, et al. Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Can Res Ther. 2014;10(7):144–149.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208.
- Yan L, Lan Y, Xiao J, et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol. 2021;31(2):685–694.
- Megwalu UC. Observation versus thyroidectomy for papillary thyroid microcarcinoma in the elderly. J Laryngol Otol. 2017;131(2):173–176.
- Lan Y, Jin Z, Zhang Y, et al. Factors associated with health-related quality of life in papillary thyroid microcarcinoma patients undergoing radiofrequency ablation: a cross-sectional prevalence study. Int J Hyperthermia. 2020;37(1):1174–1181.