Abstract
Background & aims
Liver resection (LR) and radiofrequency ablation (RFA) are commonly used for the treatment of recurrent hepatocellular carcinoma (HCC), but the optimal treatment modality remains unclear. We aimed to compare the efficacy and safety of LR vs RFA for recurrent HCC.
Methods
We searched PubMed, Embase, Web of Science, and the Cochrane Library for relevant studies. The primary outcomes were overall survival (OS) and disease-free survival (DFS). The secondary outcomes were major complications and hospital stay.
Results
Eighteen studies with 1991 patients with recurrent HCC were included. The pooled hazard ratio (HR) for OS demonstrated that LR had significantly better OS than RFA in recurrent HCC (HR, 0.81; 95% confidence interval [CI], 0.68–0.95). Specifically, LR was associated with higher 2-, 3- and 4-year OS rates compared with RFA. The pooled HR for DFS showed no significant difference between LR and RFA during the whole follow-up period (HR, 0.90; 95% CI, 0.76–1.07). However, LR was associated with significantly higher 2- to 5-year DFS rates compared to RFA. LR was also associated with more major complications (p < .001) and longer hospital stay (p < .001). Subgroup analyses demonstrated that LR and RFA had similar efficacy in patients with recurrent tumors less than 3 cm or patients presenting three or fewer recurrent nodules.
Conclusion
LR could provide better long-term survival outcomes than RFA for recurrent HCC patients, while RFA has a higher safety profile. RFA can be a good alternative to LR for patients with small-sized recurrence or patients with a limited number of recurrent nodules. However, as tumor size increases, LR tends to be more efficacious.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related mortality worldwide [Citation1]. In recent decades, although great advances have been made in the diagnosis and treatment of patients with HCC, the long-term prognosis of HCC remains unsatisfactory due to the high recurrence rate [Citation2]. The cumulative 5-year recurrence rate after curative treatment is up 70–80% [Citation3–5]. Thus, an effective therapeutic strategy for recurrence is crucial to prolong survival for HCC patients.
Strategies for treating primary HCC, including liver resection (LR), liver transplantation, radiofrequency ablation (RFA), and transcatheter arterial chemoembolization (TACE), have been widely used to treat recurrent HCC in clinical practice. However, the optimal treatment for recurrent HCC remains controversial. LR is the most commonly considered first-line therapy for recurrent HCC due to the donor shortage [Citation6,Citation7]. However, its feasibility may be limited by multifocal recurrent nodules, a small liver remnant, and inadequate liver functional reserve. RFA, as a minimally invasive and reproducible therapy, has been widely considered as an effective and safe alternative to LR in primary small HCC [Citation8,Citation9], but its role in the treatment of recurrent HCC remains unclear. A growing number of clinical studies have compared survival outcomes of recurrent HCC patients who received LR or RFA [Citation10–13]. However, most are small series and controversy exists among the results.
Although there have been several meta-analyses on this topic in the past decade, they are limited by small sample sizes of eligible studies [Citation14–17]. Besides, the results of these meta-analyses varied with the included studies. Moreover, many additional cohort studies have been published since then. Therefore, a comprehensive updated meta-analysis is warranted. This systematic review and meta-analysis aimed to compare the clinical efficacy and safety of LR and RFA in treating recurrent HCC.
Methods
Study design and literature search
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered on PROSPERO, the international prospective register of systematic reviews (CRD42020198592). A comprehensive search of PubMed, EMBASE, Web of Science, and the Cochrane Library from inception through 28 June 2020, was performed. The main keywords used for the literature search were ‘hepatocellular carcinoma’, ‘recurrent’, ‘recurrence’, ‘liver resection’, ‘hepatectomy’ and ‘radiofrequency ablation’. The detailed search strategy is included in the supplementary materials (Table S1). The reference lists of relevant articles were also searched for other eligible studies.
Study selection
Three reviewers (YYJ, YHL, and LFY) independently assessed articles for eligibility, and discrepancies were resolved by a consensus and confirmed by another author YY. The inclusion criteria for the eligible studies were as follows: (Citation1) studies comparing LR with RFA for recurrent HCC; (Citation2) the treatment of primary HCC should be radical, including hepatectomy or local ablation with curative intent: (Citation3) randomized controlled trials (RCTs), prospective or retrospective studies, at least 10 patients were included in both groups of LR and RFA; and (Citation4) studies including at least one objective evaluation of the following outcomes: overall survival (OS), disease-free survival (DFS), complications and/or hospital stay. Studies that met any of the following criteria were excluded: (Citation4) studies on patients with multiple malignancies, or with other liver malignancies instead of HCC, such as cholangiocellular carcinomas or metastatic liver tumors; (Citation2) the treatment of primary HCC was liver transplantation or non-radical treatment; (Citation3) noncomparative studies, including abstracts, conference articles, expert opinions, editorials, case series, letter, reviews, and meta-analyses; (Citation4) replicated data reported by the same authors; and (Citation5) studies not published in English.
Data extraction and quality assessment
The same three reviewers independently extracted the following variables from studies that met the inclusion criteria: first author, year of publication, data source, country, study design, inclusion period, number of participants, participant and tumor characteristics, overall survival data, disease-free survival data, procedure-related complication, hospital stay, etc. The survival data were either reported in the studies or derived from the survival curves. We also attempted to contact the author through email if there existed any missing information which is required.
The Cochrane Collaboration’s tool was used to assess the risk of bias in RCTs [Citation18]. The modified Methodological Index for Non-Randomized Studies (MINORS) score was used to assess the quality of non-randomized controlled studies. The total score of the modified MINORS is 18. A study with a modified MINORS scores of ≥12 was considered high quality [Citation19,Citation20].
Statistical analysis
The meta-analysis was performed with STATA 16.0 according to the Cochrane Handbook for Systematic Reviews of Interventions [Citation21]. The primary outcomes of this meta-analysis were OS and DFS. The hazard ratios (HRs) and 95% CIs for OS and DFS were extracted directly from the included studies or calculated via the methods described in detail by Tierney et al. [Citation22]. The odds ratios (ORs) and 95% CIs were calculated for OS and DFS at 1 to 5 years. The 1- to 5-year OS and DFS rates for patients treated with LR and RFA were pooled. The secondary outcomes were major complications and hospital stay. The major complication was defined as an event that led to substantial morbidity and disability, increased level of care required, prolonged hospitalization, or resulted hospital readmission. The OR and 95% CI were used to compare major complications and the weighted mean difference (WMD) was used to compare hospital stay between LR and RFA groups.
Cochran’s Q test and Higgins’ I2 statistic were used to assess heterogeneity among the pooled results. Depending on the level of heterogeneity, either fixed or random-effects models were used to pool data. If p > .05 and I2 < 50%, the fixed-effects model was used to pool data. Otherwise, the random-effects model was used. The significance of pooled estimates was determined by the Z-test, and a p-value <.05 was considered statistically significant.
Subgroup analysis and sensitivity analysis
Because RFA is usually used in patients with a small-sized and limited number of tumors, we would perform the subgroup analyses in the patients with HCC tumors less than 3 cm and in the patients with three or fewer nodules. In addition, subgroup analysis would be conducted based on the different interval of time to recurrence (TTR) from initial treatment, if eligible studies were sufficient. Sensitivity analysis was used to determine whether modification of the inclusion criteria affected the final results.
Publication bias
Publication bias was investigated if the number of eligible articles was ≥10. Visual inspection of the funnel plot and Egger’s and Begg’s tests were performed to assess publication bias. If publication bias was detected, Duval and Tweedie’s nonparametric trim-and-fill procedure was performed to further assess the possible effect of publication bias.
Results
Study characteristics
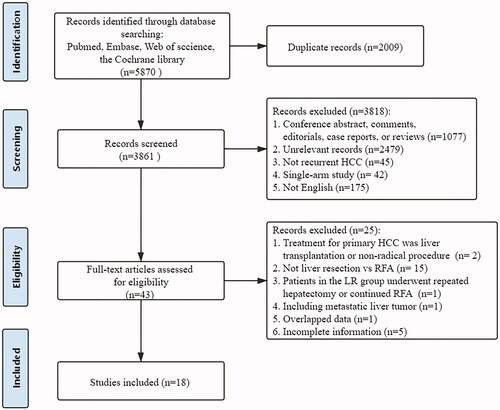
After the initial search and removal of duplicates, a total of 3861 articles were identified for screening. The study selection process is shown in . Ultimately, 18 studies (1 RCT, 3 propensity score matching-non RCTs [PSM-NRCTs], 14 NRCTs) compared LR with RFA for RHCC were included in our meta-analysis [Citation10–13,Citation23–36]. The included studies evaluated a total of 1991 patients with recurrence of HCC; of these, 862 patients underwent LR, and 1129 patients underwent RFA. There were 7 studies that mentioned the types of LR, both anatomic and non-anatomic hepatectomy were used, but the number of participants receiving anatomic and non-anatomic hepatectomy was barely specified. The detailed characteristics of the included studies are shown in .
Table 1. Baseline characteristics of included studies and patients.
The quality assessment results of these studies are shown in and Figure S1. The RCT was an open-label trial and assessed as having a moderate risk of bias. Concerning PSM-NRCTs and NRCTs, most studies were assessed as high quality.
Meta-analysis of outcomes
Overall survival
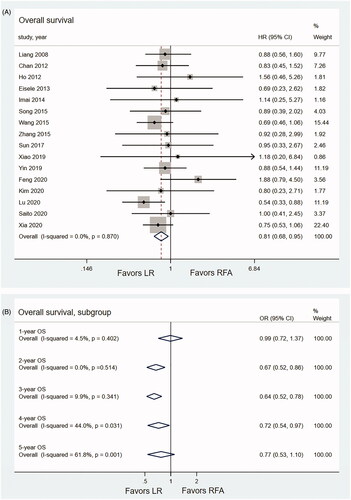
The pooled OS was compared based on 16 studies which incorporated 1902 patients (817 patients for LR, 1085 patients for RFA) [Citation10–13,Citation24–28,Citation30–36]. The pooled HR for OS demonstrated that LR had significantly better OS than RFA in recurrent HCC (HR, 0.81; 95% CI, 0.68–0.95; p < .001; I2 = 0.0%) (). The pooled analysis demonstrated that the recurrent HCC patients that underwent LR had significantly higher 2-, 3- and 4-year OS rates than those of RFA (OR2-year OS, 0.67, 95% CI, 0.52–0.85; p = .001; I2 = 0.0%; OR3-year OS, 0.64, 95% CI, 0.52–0.78; p < .001; I2 = 9.9%; OR4-year OS, 0.72, 95% CI, 0.54–0.97; p = .033; I2 = 44.0%). No significant difference was observed between LR group and RFA group at the first year (OR1-year OS, 0.99, 95% CI, 0.72–1.38; p < .001; I2 = 4.5%) and the fifth year (OR5-year OS=0.77, 95% CI, 0.53–1.10; p = .148; I2 = 61.8%) ().
Figure 2. Forest plots comparing OS of LR and RFA. (A) Pooled HR for OS in recurrent HCC patients. (B) Pooled ORs for 1-, 2-, 3-, 4- and 5-year OS rates.
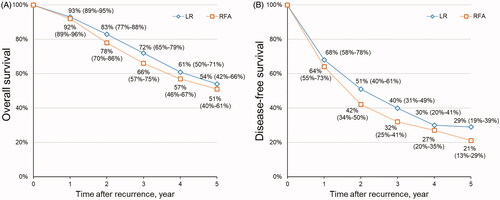
The pooled 1-, 2-, 3-, 4- and 5-year OS rates for patients with recurrent HCC after LR were 92%, 83%, 72%, 61% and 54%, respectively, and those after RFA were 93%, 78%, 66%, 57% and 51%, respectively ().
Disease-free survival
Data on DFS were available in 10 studies which incorporated 1075 patients (435 patients for LR, 640 patients for RFA) [Citation11,Citation12,Citation24,Citation26,Citation30,Citation32–36]. The pooled HR showed no significant difference in DFS between the LR and RFA during the whole follow-up period (HR, 0.90; 95% CI, 0.76–1.07, p = .217, I2 = 0.0%) (). The results of meta-analysis showed that there was no significant difference in terms of 1-year DFS rate between the LR and RFA (OR1-year DFS, 0.82, 95% CI, 0.62–1.08, p = .161, I2 = 14.9%). The LR was associated with significantly higher 2-year (OR2-year DFS, 0.68, 95% CI, 0.52–0.88, p = .003, I2 = 0.0%), 3-year (OR3-year DFS, 0.70, 95% CI, 0.54–0.91, p = .009, I2 = 0.0%), 4-year (OR4-year DFS, 0.75, 95% CI, 0.57–0.99, p = .019, I2 = 15.4%) and 5-year (OR5-year DFS, 0.59, 95% CI, 0.44–0.79, p < .001, I2 = 0.0%) DFS rates compared with the RFA ().
Figure 4. Forest plots comparing DFS of LR and RFA. (A) Pooled HR for DFS in recurrent HCC patients. (B) Pooled ORs for 1-, 2-, 3-, 4- and 5-year DFS rates.
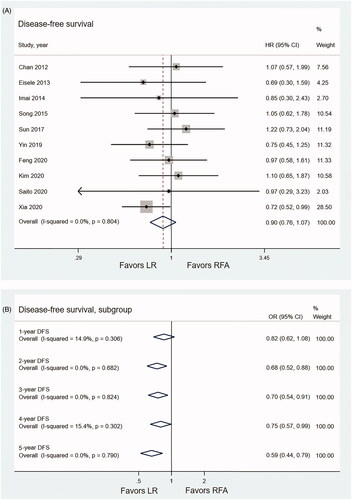
The pooled 1-, 2-, 3-, 4- and 5-year DFS rates for patients with recurrent HCC after LR were 68%, 51%, 40%, 30% and 29%, respectively, and those after RFA were 64%, 42%, 32%, 27% and 21%, respectively ().
Treatment-related complications and mortality
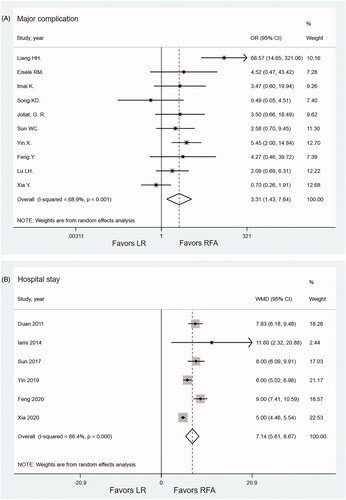
For treatment complications, 10 studies with 1120 patients (518 patients for LR, 602 patients for RFA) were included [Citation10–13,Citation26,Citation29,Citation30,Citation32,Citation33,Citation36]. The meta-analysis showed that the major complications in the RFA group were significantly fewer than in the LR group (OR 3.31; 95% CI 1.43–7.64; p = .005, I2 = 68.9%) (). Only three studies reported procedure-related mortality. Three patients in the LR group had procedure-related death during hospitalization; one patient died because of hepatic failure after the LR; one patient died because of bile duct injury with bile leakage and septic shock; in the remaining one patient, the exact cause of death was not reported. None of the patients in the RFA group had procedure-related death during hospitalization.
Hospital stay
A total of six studies with 632 patients reported the length of hospital stay [Citation23,Citation26,Citation30,Citation32,Citation33,Citation36]. The pooled mean length of hospital stays were 12.45 days (95% CI, 7.95–15.16) and 5.33 days (95% CI, 3.78–6.87) for LH and RFA groups, respectively. The length of hospital stay was significantly shorter in the RFA group than that in the LR group (WMD = 7.14; 95% CI, 5.61–8.68; p < .001, I2 = 86.4%) ().
Subgroup analysis
Subgroup-analysis showed that there was no significant difference in all OS and DFS outcomes among the two treatments in patients with tumor size ≤3 cm (). For patients with three or fewer recurrent nodules, most OS and DFS outcomes were not significantly different between the LR and RFA groups, while 5-year DFS showed a significance (OR5-year DFS, 0.55, 95% CI, 0.36–0.84, p = .006, I2 = 0.0%) ().
Table 2. Subgroup analyses.
There were four studies that reported the comparison of LR vs RFA in recurrent HCC patients with different TTR. The studies by Liang et al. [Citation10], Wang et al. [Citation27] and Xia et al. [Citation36] showed that there was no significant difference in OS between LR and RFA for patients with a TTR ≤ 1 year or > 1 year. Lu et al. [Citation13] reported that, for patients who presented tumor recurrence within 2 years, the OS of the LR group was better than those of the RFA group (log-rank test, p = .004). For patients with a TTR >2 years, there was no significant difference in the OS between patients who were treated by LR or RFA (log-rank test, p = .718). Detailed data are shown in Table S2. We were not able to conduct a planned subgroup analysis because of the relatively small number of eligible studies.
Sensitivity analysis and publication bias
Sensitivity analysis was performed for all outcomes by excluding studies with sample size <20 in any treatment group [Citation31,Citation35], studies including extrahepatic recurrence [Citation10,Citation26], studies that included other radical procedures for primary HCC than surgical resection [Citation11,Citation26], and studies with inadequate methodological quality [Citation28,Citation35]. All results did not change significantly.
Funnel plots of the study results were shown in Figures S2–S4. The funnel plots on OS and 3-year OS following LR or RFA treatment of recurrent HCC showed asymmetries. The meta-analysis of OS and 3-year OS showed potential publication biases with the p-values lower than .05 given by Egger’s test. Therefore, the trim and fill method was conducted and this did not substantively change the results. The adjusted HR for OS was 0.75 (95% CI, 0.64–0.87), and the adjusted OR for 3-year OS was 0.52 (95% CI, 0.42–0.61). There was no evidence of publication bias for other results, as the funnel plot analysis showed a symmetrical appearance and all Begg’s and Egger’s test p-values >.05.
Discussion
We performed the newest and largest systematic review and meta-analysis to date, to compare the clinical efficacy and safety of LR and RFA for patients with recurrent HCC. The meta-analysis provides evidence that LR could provide better long-term survival outcomes for recurrent HCC patients, while it was associated with an increased risk of major complications and required longer hospital stay when compared with RFA.
Our meta-analysis indicated that LR was associated with better 2- to 5-year DFS rates for patients with recurrent HCC compared with RFA, although the pooled HR of DFS for the entire follow-up period did not reach a statistical significance. HCC is highly likely to microdisseminate into the intrahepatic vascular structures and spreads via neighboring portal branches. These characteristics have been considered as the most significant independent risk factor for HCC recurrence and patient survival [Citation37,Citation38]. LR can provide complete removal of the tumor-bearing portal territory where micrometastases and microscopic vascular invasion can be detected, contributing to better tumor control and longer survival [Citation8]. However, it is often impossible to detect micrometastases by external ultrasonography, so they usually cannot be dealt with by RFA. Besides, there have been concerns about the potential for tumor seeding via a puncture or intrahepatic dissemination of tumor cells after RFA. Therefore, RFA tends to have poor performance in controlling tumor progression [Citation39–41]. The similar superiority of LR over RFA in terms of DFS for recurrent HCC was also observed by Cai et al. and Chen et al. [Citation14,Citation16]. However, given the advantage of minimal invasiveness and high repeatability of RFA, repeat ablation may be a valid strategy to achieve better local tumor control. The feasibility of this strategy needs further investigation.
Moreover, the shortcomings of RFA mentioned above might be overcome by MWA or applying track ablation. It has been proven that MWA can produce significantly larger areas of necrosis, deeper tissue penetration, higher intratumor temperature, less tumor seeding risk, and less susceptibility to heat-sink effect over RFA [Citation42–45]. These advantages of MWA might help to clear micrometastases and microvascular invasion around the tumor, or at least prevent them from growing. However, there have been few studies on this topic and further studies are necessary to validate this possibility. Moreover, there has been evidence indicating that track ablation can effectively inactivate the viable cells attached to the needle applicator, which are correlated with local tumor progression [Citation46]. Thus, applying track ablation is a good way to overcome the risk of tumor seeding and lead to better tumor control.
The advantage of LR in the complete elimination of tumor tissue and better tumor control ability might also explain its superiority to RFA in terms of OS for recurrent HCC patients. In our study, the pooled estimate for the HR of OS for the entire follow-up period showed that LR was superior to RFA in treating recurrent HCC. The meta-analysis of 1- to 5-year OS rates confirmed again the superiority of LR to RFA, although the ORs of 1- and 5-year OS rates failed to reach statistical significance. This might be because the relatively short observation duration in the first year and small effective sample size at the fifth year may limit statistical analysis results. Nevertheless, the main findings of our meta-analysis differed from those of previous meta-analyses [Citation14–17]. No significant difference between LR and RFA in terms of OS for recurrent HCC was observed in these meta-analyses. This might be because the number of included studies and patients in these meta-analyses was too small to show any clear differences. Besides, this discrepancy may also be attributable to the difference in the inclusion criteria of studies. Thus, more multi-center, large-sample, high-quality RCTs are required.
However, it is noteworthy that the application of LR in clinical practice is often restricted by poor hepatic functional reserve, less liver remnant, and/or widespread intrahepatic recurrence [Citation47]. In contrast, the highly target-selective characteristic of RFA can conserve more nontumorous liver parenchyma and cause limited injury to the small or cirrhotic liver remnant. Furthermore, our meta-analysis showed that RFA was associated with significantly fewer major complications and a shorter length of hospital stay. Therefore, for recurrent HCC patients with well-preserved liver function and resectable tumor lesions, LR will undoubtedly remain the first choice. For those who are not suitable to undergo LR, RFA would be a good option for its advantage of less invasiveness and improved safety. In this scenario, however, increased surveillance with respect to preventing or controlling the tumor’s further progression needs to be carried out.
In general, the extent of tumor necrosis by RFA is negatively correlated with tumor size. As reported, RFA can achieve complete ablation of tumor tissues in a small HCC nodule which is less than 3 cm in diameter [Citation48]. In our subgroup analysis of evaluating recurrent HCC ≤3 cm, RFA showed equivalent efficacy in terms of OS and DFS compared with LR. This is in accordance with the findings in primary HCC [Citation49,Citation50]. Therefore, RFA also can be an alternative to LR for small HCC. Unfortunately, the subgroup analysis of evaluating recurrent HCC >3 cm was unable to carry out due to limited reported data. However, from the results of our present study, the efficacy of LR for recurrent HCC seems to increase as tumor size increases. This is in agreement with the results reported by previous meta-analyses [Citation15,Citation16,Citation51]. Another subgroup analysis showed that RFA and LR obtained very similar results in treating patients with 3 or fewer recurrent HCC nodules, which further demonstrated the equal efficacy of RFA and SR in the treatment of patients with a limited number of recurrent HCC. However, due to the relatively small sample in the subgroup analyses, high-quality RCTs stratified by tumor size and tumor number of recurrent HCC are needed to confirm the reliability of these conclusions.
In clinical practice, recurrent HCC is often simply categorized into early recurrence and late recurrence, according to the TTR, because TTR can partially reflect the cellular origins of recurrence [Citation52,Citation53]. Early recurrence (TTR ≤1 or 2 years) is generally considered to be related to intrahepatic metastases, microvascular invasion and microsatellite lesions generated by primary HCC, while late recurrence (TTR >1 or 2 years) is mainly related to de novo occurrence due to a carcinogenic cirrhotic environment [Citation53]. Due to these different characteristics, early and late recurrence might need different treatment strategies. We found four studies reported the comparison of LR vs RFA in early and late recurrence. A significant difference in OS was only observed in the study by Lu et al. [Citation13]. They found LR could provide a survival benefit for patients with early recurrence which relapsed within 2 years compared with RFA. However, the other three studies suggested no significant difference in OS between LR and RFA in early recurrence (TTR ≤1 year) [Citation10,Citation27,Citation36]. For late recurrence, all these four studies did not observe a difference in OS between LR and RFA. Due to the limited number of eligible studies and different cutoff values of early and late recurrence among studies, we did not perform the meta-analysis. Clearly, more research is needed to explore the best treatment for different patterns of recurrence.
There are several limitations to our study. Firstly, most evidence for this meta-analysis was largely drawn from retrospective observational studies which might have potential bias, such as selection bias that patients with more comorbidities and worse conditions, or patients with lesions located deeply in the liver parenchyma which are hard to be resected are more likely to undergo RFA. Several studies used propensity score-matched to minimize selection biases [Citation12,Citation13,Citation33], but more high-quality low-biased RCTs are still needed. Secondly, the vast majority of the included studies in the meta-analysis were from Asian populations and most studies were performed in China. Considering the heterogeneity of the etiology of HCC across different regions around the world, caution should be exercised when generalizing our results to the population in other regions. Finally, the numbers of studies in some of the subgroup analyses were rather small. Further high-quality, multicenter RCTs with long-term follow-up are required for more evidence.
Conclusion
The current study shows that LR can provide significantly better long-term survival outcomes than RFA for recurrent HCC patients, while RFA can offer benefits in safety and shorter hospital stays. In addition, RFA exhibits similar efficacy of LR for patients with recurrent HCC tumors less than 3 cm or patients with three or fewer recurrent nodules. However, as tumor size increases, LR tends to be more efficacious. More well-designed RCTs are needed for further validation.
Author contributions
Yao Yang performed literature search; Hongli Yu, Yajing You and Fangyuan Liu performed study selection and data extraction; Yao Yang and Yuemin Feng performed data analysis and wrote the manuscript; Tong Zhao, and Jianni Qi contributed to the design of the study; Jie Li, Xu Tan and Yuemin Feng contributed to interpreting the data and revised the manuscript critically for important contents; Qiang Zhu designed the study, contributed to interpreting the data, critically reviewed the manuscript. All authors approved the final version of the manuscript, including the authorship list.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462.
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314.
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729.
- Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967.
- Sugimachi K, Maehara S, Tanaka S, et al. Repeat hepatectomy is the most useful treatment for recurrent hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2001;8(5):410–416.
- Wu C-C, Cheng S-B, Yeh D-C, et al. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96(9):1049–1057.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Marrero JA, Kulik LM, Sirlin C, et al. Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750.
- Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15(12):3484–3493.
- Eisele RM, Chopra SS, Lock JF, et al. Treatment of recurrent hepatocellular carcinoma confined to the liver with repeated resection and radiofrequency ablation: a single center experience. Technol Health Care. 2013;21(1):9–18.
- Song KD, Lim HK, Rhim H, et al. Repeated hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma after hepatic resection: a propensity score matching study. Radiology. 2015;275(2):599–608.
- Lu LH, Mei J, Kan A, et al. Treatment optimization for recurrent hepatocellular carcinoma: repeat hepatic resection versus radiofrequency ablation. Cancer Med. 2020;9(9):2997–3005.
- Cai H, Kong W, Zhou T, et al. Radiofrequency ablation versus reresection in treating recurrent hepatocellular carcinoma: a meta-analysis. Medicine. 2014;93(22):e122.
- Zhang C, Zhang J, Li X, et al. Is radiofrequency ablation equal to surgical re-resection for recurrent hepatocellular carcinoma meeting the Milan criteria? A meta-analysis. J Buon. 2015;20(1):223–230.
- Chen X, Chen Y, Li Q, et al. Radiofrequency ablation versus surgical resection for intrahepatic hepatocellular carcinoma recurrence: a meta-analysis. J Surg Res. 2015;195(1):166–174.
- Gavriilidis P, Askari A, Azoulay D. Survival following redo hepatectomy vs radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. HPB. 2017;19(1):3–9.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255(3):446–456.
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Oxford (UK): Cochrane Collaboration.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Duan J, Yue H, Liu K, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: retrospective randomized control study. J Med Coll PLA. 2011;26(6):316–323.
- Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36(1):151–156.
- Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151(5):700–709.
- Imai K, Beppu T, Chikamoto A, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014;44(14):E335–E345.
- Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Euro J Surgical Oncol. 2015;41(2):236–242.
- Zhang X, Li C, Wen T, et al. Appropriate treatment strategies for intrahepatic recurrence after curative resection of hepatocellular carcinoma initially within the Milan criteria: according to the recurrence pattern. Eur J Gastroenterol Hepatol. 2015;27(8):933–940.
- Joliat GR, Allemann P, Labgaa I, et al. Treatment and outcomes of recurrent hepatocellular carcinomas. Langenbecks Arch Surg. 2017;402(5):737–744.
- Sun WC, Chen IS, Liang HL, et al. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget. 2017;8(61):104571–104581.
- Xiao H, Chen ZB, Jin HL, et al. Treatment selection of recurrent hepatocellular carcinoma with microvascular invasion at the initial hepatectomy. Am J Transl Res. 2019;11(3):1864–1875.
- Yin X, Hua T, Liang C, et al. Efficacy of re-resection versus radiofrequency ablation for recurrent Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma (HCC) after resection for primary HCC. Transl Cancer Res. 2019;8(4):1035–1045.
- Feng Y, Wu H, Huang DQ, et al. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤5 cm) after initial curative resection. Eur Radiol. 2020; 30(11):6357–6368.
- Kim JM, Joh JW, Yi NJ, et al. Living donor liver transplantation should be cautiously considered as initial treatment in recurrent hepatocellular carcinoma within the Milan criteria after curative liver resection. Ann Transl Med. 2020;8(6):288.
- Saito R, Amemiya H, Hosomura N, et al. Prognostic significance of treatment strategies for the recurrent hepatocellular carcinomas after radical resection. In Vivo. 2020;34(3):1265–1270.
- Xia Y, Li J, Liu G, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol. 2020;6(2):255–263.
- Nakashima T, Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis. 1986;6(3):259–266.
- Sasaki A, Kai S, Iwashita Y, et al. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103(2):299–306.
- Stigliano R, Marelli L, Yu D, et al. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33(5):437–447.
- Masuda T, Beppu T, Ishiko T, et al. Intrahepatic dissemination of hepatocellular carcinoma after local ablation therapy. J Hepatobiliary Pancreat Surg. 2008;15(6):589–595.
- Obara K, Matsumoto N, Okamoto M, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int. 2008;2(1):116–123.
- Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys. 2010;37(6):2967–2973.
- Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005.
- Tan W, Deng Q, Lin S, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):263–271.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8):S192–S203.
- Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18(13):3702–3710.
- Kaibori M, Matsui Y, Hijikawa T, et al. Comparison of limited and anatomic hepatic resection for hepatocellular carcinoma with hepatitis C. Surgery. 2006;139(3):385–394.
- Ruzzenente A, de Manzoni G, Molfetta M, et al. Rapid progression of hepatocellular carcinoma after radiofrequency ablation. World J Gastroenterol. 2004;10(8):1137–1140.
- Song J, Wang Y, Ma K, et al. Laparoscopic hepatectomy versus radiofrequency ablation for minimally invasive treatment of single, small hepatocellular carcinomas. Surg Endosc. 2016;30(10):4249–4257.
- Si M-B, Yan P-J, Hao X-Y, et al. Efficacy and safety of radiofrequency ablation versus minimally invasive liver surgery for small hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33(8):2419–2429.
- Yang D, Zhuang B, Wang Y, et al. Radiofrequency ablation versus hepatic resection for recurrent hepatocellular carcinoma: an updated meta-analysis. BMC Gastroenterol. 2020;20(1):402.
- Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89(3):500–507.
- Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235.