Abstract
Objective
To evaluate the clinical effects of image-guided thermal ablation for the treatment of symptomatic adenomyosis (AD).
Data sources
We searched PubMed, Web of Science, Cochrane Library, EMBASE, ClinicalTrials.gov and Google Scholar for literature from January 2000 to September 2020.
Methods of study selection
We included all studies reporting clinical outcomes of image-guided thermal ablation for AD, involving high-intensity focused ultrasound (HIFU), percutaneous microwave ablation (PMWA) and radiofrequency ablation (RFA). Two independent researchers performed study selection according to the screening criteria.
Results
A total of 38 studies representing 15,908 women were included. Compared with those at baseline, the visual analog scale scores, the symptom severity scores and the menorrhagia severity scores decreased significantly after these thermal ablation therapies. The mean ablation time was 92.18 min, 24.15 min and 31.93 min during HIFU, PMWA and RFA, respectively. The non-perfused volume ratio of AD was 68.3% for HIFU, 82.5% for PMWA and 79.2% for RFA. The reduction rates of uterine volume were 33.6% (HIFU), 46.8% (PMWA) and 44.0% (RFA). The reduction rates of AD volume were 45.1% (HIFU), 74.9% (PMWA) and 61.3% (RFA). The relief rates of dysmenorrhea were 84.2% (HIFU), 89.7% (PMWA) and 89.2% (RFA). The incidence of minor adverse events was 39.0% (HIFU), 51.3% (PMWA) and 3.6% (RFA). The re-intervention rates were 4.0% (HIFU) and 28.7% (RFA). The recurrence rate was 10.2% after HIFU. The pregnancy rates were 16.7% (HIFU), 4.93% (PMWA) and 35.8% (RFA).
Conclusion
Image-guided HIFU, PMWA and RFA may be effective and safe minimally invasive therapies for symptomatic AD.
Introduction
Adenomyosis (AD) is a common benign gynecologic condition characterized by the invasion or diffusion of endometrial glands and stroma into the myometrium in childbearing age women. With a quoted incidence ranging from 8% to 27%, the prevalence of AD increased with age reaching a peak of 32% in women aged 40–49 [Citation1,Citation2]. The main symptoms include menorrhagia, dysmenorrhea, chronic pelvic pain and even subfertility, which influence the quality of life (QoL) seriously, although some women are asymptomatic [Citation3].
Conservative medical treatments (including nonsteroidal anti-inflammatory drugs, progestogens, oral contraceptives or gonadotropin-releasing hormone agonist) are effective in relieving pain symptoms and abnormal uterine bleeding, resulting in a more acceptable option than surgical treatment, especially in diffuse forms and in those women requiring preservation of fertility [Citation4]. However, medical therapy is accompanied by side effects and frequent recurrence [Citation5]. In patients whose symptoms are refractory to medical treatment or in whom long-term medical treatment is not suitable, the usual choice for radical treatment is considered to be hysterectomy [Citation6]. As the hysterectomy is not an option for women wishing to remain their fertility, several uterine-sparing interventions, especially the minimally invasive treatments, have been advocated in recent years [Citation7].
One of the minimally invasive therapies is image-guided thermal ablation that destroys the lesions by a rapid temperature rise. Currently, there are several thermal energy sources used in clinical practice, involving high-intensity focused ultrasound (HIFU), percutaneous microwave ablation (PMWA) and radiofrequency ablation (RFA) [Citation8]. HIFU ablation is performed under the guidance of ultrasound or magnetic resonance imaging (MRI) without the use of an antenna or electrode. During treatment, patients are treated in the prone position. The concentrated ultrasound waves penetrate the tissue and induce instant coagulative necrosis of the target lesion in a well-circumscribed area based on the ability to transform sound absorption energy into heat energy [Citation9]. Routinely, patients were hospitalized for only one day and all patients were discharged after a 24-h observation period after HIFU. By comparison, during PMWA and RFA, the patients adopted the supine position, and the antenna or electrode is directly inserted into the target lesion under the guidance of ultrasound. PMWA uses electromagnetic energy to rotate adjacent polar water molecules rapidly, while RFA uses a high-frequency alternating electrical current to create ionic agitation. These procedures generate heat energy to induce subsequent tissue necrosis [Citation10,Citation11]. Compared with HIFU and RFA techniques, PMWA can achieve higher tissue temperature and larger ablation volume [Citation12]. With the advantages of significant alleviation of symptoms, rapid recovery and relatively low risk of complications, more gynecologists are considering image-guided thermal ablation as an option in the management of symptomatic AD [Citation13].
Growing researches have reported the clinical benefits of HIFU, PMWA and RFA in the management of symptomatic AD, but there is no consensus on which one is the most suitable therapy. To provide solid evidence for clinical decisions, we performed a systematic review and meta-analysis to comprehensively evaluate the efficacy and safety of these thermal ablation therapies in women with symptomatic AD.
Methods and materials
This meta-analysis was performed according to the reporting guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The review protocol was registered in the PROSPERO online database (CRD42020205489) prospectively.
Literature search
We searched databases including PubMed, Web of Science, Cochrane Library, EMBASE, ClinicalTrials.gov and Google Scholar from January 2000 to September 2020 to retrieve articles reporting the clinical effects of HIFU, PMWA or RFA for the treatment of symptomatic AD according to PRISMA guidelines. We adjusted the search strategies according to the databases. For example, our search strategy on PubMed was: ((focused ultrasound [Title/Abstract]) OR (HIFU [Title/Abstract]) OR (MRgFUS [Title/Abstract]) OR (High-Intensity Focused Ultrasound Ablation [MeSH Terms]) OR (radiofrequency [Title/Abstract]) OR (microwave [Title/Abstract])) AND ((adenomyosis [Title/Abstract]) OR (endometrioma [Title/Abstract]) OR (adenomyoma [Title/Abstract])) (). We also hand-searched the reference of the included articles and relevant reviews for more researches.
Eligibility criteria
shows the flowchart of the study selection process following PRISMA. Two independent researchers performed study selection according to the screening criteria. If they had different opinions, a third researcher was consulted. First, the titles and abstracts of articles were screened for eligibility. Second, all relevant articles were collected for full-text evaluation. We considered all articles, of any study design, that evaluated the effectiveness and safety of HIFU, PMWA or RFA in women with symptomatic AD.
Inclusion criteria: (1) the patients of studies were women with confirmed symptomatic AD; (2) studies evaluating the clinical effectiveness and safety of HIFU, PMWA or RFA in the management of symptomatic AD; (3) if the studies compared HIFU, PMWA or RFA with other therapies, the data of single HIFU, PMWA or RFA group was included; (4) studies including at least 1 of the outcome measures of interest that we specified as follows; (5) studies published in peer-reviewed journals; and (6) studies written in English.
Exclusion criteria: (1) studies including HIFU combined with medical treatments for AD rather than HIFU therapy alone; (2) studies involving endometrial ablation rather than HIFU, PMWA or RFA; (3) overlapped or repeated studies; (4) reviews, commentaries, abstracts, unpublished studies, case reports, secondary analyses, letters or summaries of meetings; (5) non-English studies.
The outcome measures of interest were:
Visual analog scale (VAS) score: VAS score is used to evaluate the scale of dysmenorrhea before and after treatment. It provides a measure on a scale between 0 and 10 points to classify the pain intensity: no pain (0 points), slight pain (1–3 points), moderate pain that affects sleep (4–6 points) and severe pain that is hard to bear and affects sleep or appetite (7–10 points).
Symptom severity score (SSS): SSS questionnaire includes eight items: prolonged menstruation, menstrual blood clotting, menstrual disorders, increased amount of menstrual blood loss, pelvic tightness/pressure, frequent urination during day times, frequent urination during night times and fatigue. The higher scores of SSS indicate more serious symptoms.
Quality of life score: The QoL questionnaire consists of 29 health-related items in six domains: activities, concern, control, self-consciousness, energy/mood and sexual function. The higher scores indicate better QoL [Citation14].
Menorrhagia severity score: Menorrhagia severity score is measured using the number of completely soaked sanitary napkins during a menstrual period: less than five pieces (one point), 6–10 pieces (two points), 10–15 pieces (three points), 15–20 pieces (four points) and more than 20 pieces (five points).
Mean ablation time: The mean time required to ablate the AD lesion during treatment.
Non-perfused volume (NPV) ratio of AD: The NPV ratio is calculated according to the following equation: NPV% = the volume of the necrotic tissue/the volume of the targeted lesions × 100%.
Reduction rate of uterine volume and AD volume: Ultrasound examination and MRI are used to evaluate the changes in uterus volume and AD volume before and after treatment. The supra inferior diameter (D1), mediolateral diameter (D2) and anteroposterior diameter (D3) of the uterus and AD lesion are measured. This formula is used to calculate the volume (V): V = 0.5233 × D1 × D2 × D3. The reduction rate of volume = the reduced volume after treatment/the original volume before treatment × 100%.
Relief rate of dysmenorrhea: The numbers of patients who achieved clinical success with varying degrees of alleviation of their dysmenorrhea symptoms after treatment were recorded.
Incidence of minor adverse events: Adverse events are defined as major or minor adverse events according to the Society of Interventional Radiology (SIR) grading system [Citation15]. The self-limited events that require no therapy or only symptomatic treatment are classified as minor adverse events. The serious events that need therapy are classified as major adverse events.
Rate of re-intervention, recurrence and pregnancy: The performance of a new intervention owing to symptomatic recurrence of AD was considered to be a re-intervention. The numbers and outcomes of pregnancy after treatment were recorded.
Data extraction
Two researchers independently extracted clinical information from the included articles. We recorded characteristics of the included studies such as the first author, time of publication, treatments, follow-up duration, the number of participants, the age of patients, the volume of AD and uterus, and clinical outcome measures. We extracted the mean ± standard deviation (SD) or median scores of VAS, SSS, QoL and menorrhagia severity questionnaire at baseline and after treatment, mean ± SD or median minutes of ablation time, NPV ratio of AD, the reduction rate of uterine volume and AD volume, relief rate of dysmenorrhea, the incidence of minor adverse events, rate of re-intervention, recurrence and pregnancy.
Quality assessment
The risk of bias and evidence quality of included articles was evaluated according to the Cochrane Handbook for Systematic Reviews of Interventions. The following characteristics will be assessed: (1) allocation concealment; (2) random sequence generation; (3) blinding of outcome assessment; (4) blinding of personnel and participants; (5) incomplete outcome data; (6) selective reporting; (7) other bias. All researches were classified as ‘high risk of bias’, ‘low risk of bias’ or ‘unclear risk of bias’ for each characteristic.
Statistical analysis
Stata 14.0 (StataCorp, College Station, TX) was used for meta-analysis. The studies were aggregated according to the types of thermal ablation treatments (HIFU, PMWA or RFA). We calculated standardized mean difference (SMD) with a 95% confidence interval (CI) for continuous data (scores of VAS, SSS, QoL and menorrhagia severity before and after therapy and mean ablation time). If the means were not reported, we estimated them using the median, sample size and interquartile ranges [Citation16]. The dichotomous data of the outcome measures (including NPV ratio of AD, the incidence of minor adverse events, the rate of re-intervention, recurrence and pregnancy) were pooled with 95% CI. The pooled proportion was estimated using the Freeman–Tukey Double arcsine transformation.
Heterogeneity between articles reflects variance from each study and may be attributable to differences in the study design, study population, analysis methods, location or other characteristics. We tested the heterogeneity among studies using the I2 statistic and its 95% CI (I2 value >50% indicates significant heterogeneity). We used a random-effect model if there was significant heterogeneity, and used a fixed-effect model if there was no considerable heterogeneity. If I2 values showed significant heterogeneity, the sensitivity analysis and subgroup analysis were considered to be performed. Visual inspection of the funnel plot was used to test publication bias, and the statistical significance was further evaluated by Egger’s linear regression asymmetry test.
Results
Literature search
After removing duplicates, the literature search resulted in 276 records. The titles and abstracts of these records were screened, and 183 records were further evaluated for eligibility. The full text of 63 studies fulfilled the criteria for inclusion. We excluded 22 articles that did not include any outcome measure of interest and excluded three repeated articles. Finally, 38 articles [Citation17–54], met all inclusion criteria and were included in this meta-analysis. The types of ablation were HIFU in 28 studies [Citation17–44], PMWA in five studies [Citation46–50] and RFA in four studies [Citation51–54]. Besides, one study [Citation45] compared PMWA with RFA in the management of AD.
Study characteristics and risk of bias
A total of 38 studies representing 15,908 women with symptomatic AD were included in this meta-analysis, 15,123 (95.1%) of whom underwent HIFU, 513 (3.2%) of whom underwent PMWA and 272 (1.7%) of whom underwent RFA. Most of the studies were single-arm clinical researches without a control group, and a few studies compared HIFU, PMWA or RFA with other treatments. summarizes the details on the author, time of publication, follow-up duration, treatments, the number of participants, the age of patients, the volume of AD and uterus, and outcome measures of included articles. The median age ranged between 36.0 years and 43.1 years in the HIFU group, between 36.0 years and 43.1 years in the PMWA group and between 37.0 years and 44.4 years in the RFA group. The median AD volume ranged between 44.70 cm3 and 222.56 cm3 in the HIFU group, between 71.6 cm3 and 124.3 cm3 in the PMWA group and between 25.5 cm3 and 71.1 cm3 in the RFA group. The median uterus volume ranged between 150.65 cm3 and 445.0 cm3 in the HIFU group, between 269.9 cm3 and 297.6 cm3 in the PMWA group and between 238.30 cm3 and 260.85 cm3 in the RFA group.
Table 1. Characteristics of the studies included in this meta-analysis (N = 38).
As most included articles were single-arm clinical studies and there were no randomized controlled trials (RCTs), a high risk of selection bias was observed in these studies. None of the studies blinded the personnel and participants as all the patients were fully informed about the therapies they received, so a high risk of performance bias was observed in all studies. All the included articles had a high risk of bias in at least two domains, indicating a high risk of bias within these researches.
Meta-analysis
present the details of the synthesized results to assess the effectiveness and safety of HIFU, PMWA and RFA for the treatment of symptomatic AD. are the forest plots for the outcome measures.
Table 2. Meta-analysis results of the VAS, SSS, QoL and menorrhagia severity scores of HIFU, PMWA and RFA for symptomatic adenomyosis.
Table 3. Meta-analysis results of the change in VAS and SSS scores and mean ablation time of HIFU, PMWA and RFA for symptomatic adenomyosis.
Table 4. Meta-analysis results of the NPV ratio of adenomyosis, the incidence of minor adverse events, the rate of re-intervention, recurrence and pregnancy of HIFU, PMWA and RFA for symptomatic adenomyosis.
Scores of VAS, SSS, QoL and menorrhagia severity
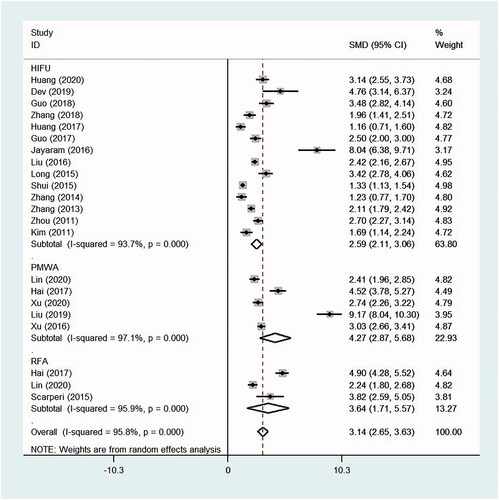
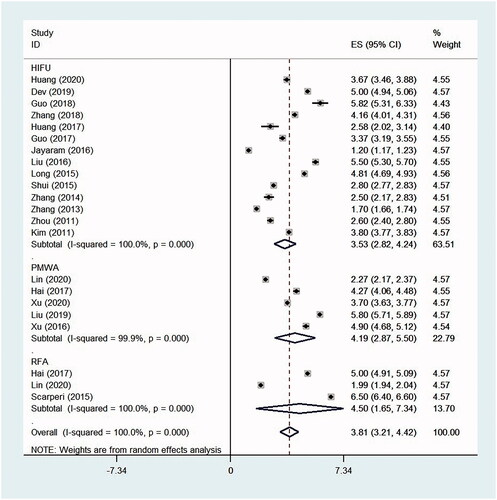
Compared with those at baseline, the VAS scores decreased significantly at the time of follow-up after HIFU, PMWA and RFA treatment, with the SMD 2.59 (95% CI, 2.11–3.06, p < .0001, I2 = 93.7%; 14 studies), 4.27 (95% CI, 2.87–5.68, p < .0001, I2 = 97.1%; five studies) and 3.64 (95% CI, 1.71–5.57, p < .0001, I2 = 95.9%; three studies), respectively. The mean decrease (absolute value) in the VAS scores from baseline to the time of follow-up were 3.53 (95% CI, 2.82–4.24, p < .0001, I2 = 100%) after HIFU, 4.19 (95% CI, 2.88–5.50, p < .0001, I2 = 99.9%) after PMWA and 4.50 (95% CI, 1.65–7.34, p = .002, I2 = 100%) after RFA, respectively ( and ).
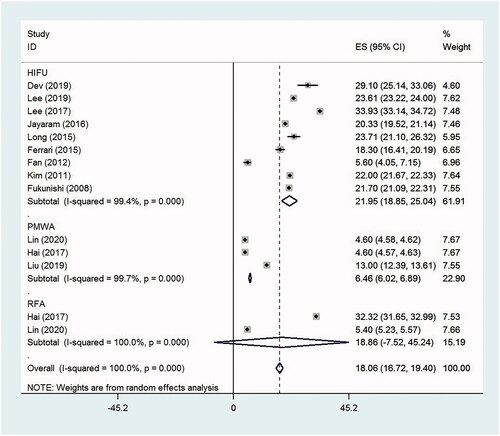
The SSS scores decreased significantly from baseline to the time of follow-up in women undergoing HIFU and PMWA, with the SMD 2.14 (95% CI, 1.56–2.72, p < .0001, I2 = 90.2%; nine studies) and 1.45 (95% CI, 0.47–2.44, p = .004, I2 = 94.4%; three studies), respectively. However, the difference in SSS scores before and after RFA treatment was not statistically significant, with the SMD 2.15 (95% CI, −0.03 to 4.33, p = .053, I2 = 98.1%; two studies). The mean decrease (absolute value) in the SSS scores from baseline to the time of follow-up was 21.95 (95% CI, 18.86–25.04, p < .0001, I2 = 99.4%) after HIFU and 6.46 (95% CI, 6.02–6.89, p < .0001, I2 = 99.7%) after PMWA, respectively ( and ).
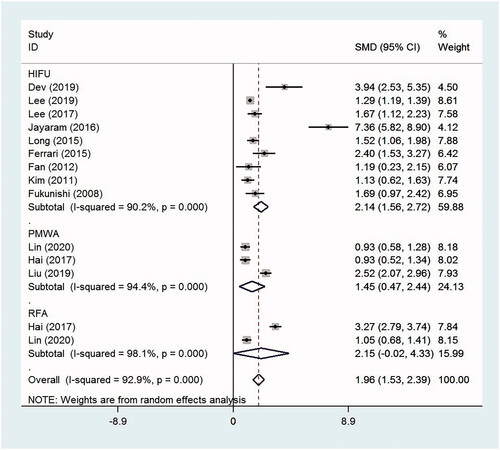
The QoL scores and menorrhagia severity scores were only reported in studies regarding HIFU treatment. The QoL scores increased at the time of follow-up compared with those at baseline after HIFU, with the overall SMD −2.44 (95% CI, −4.89 to 0.04, p = .05, I2 = 99.6%; three studies). However, the result was not statistically different. The menorrhagia severity scores decreased significantly from baseline to the time of follow-up, with the overall SMD 2.46 (95% CI, 1.48–3.45, p < .0001, I2 = 97.3%; six studies).
Mean ablation time
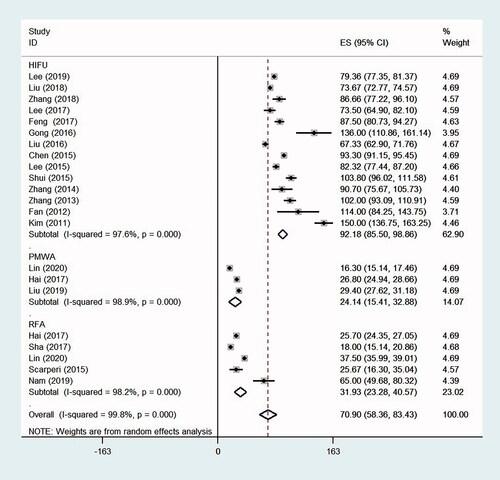
The mean ablation time was 92.18 min (95% CI, 85.50–98.86, p < .0001, I2 = 97.6%; 14 studies) during HIFU, 24.15 min (95% CI, 15.41–32.88, p < .0001, I2 = 98.9%; three studies) during PMWA and 31.93 min (95% CI, 23.28–40.57, p < .0001, I2 = 98.2%; five studies) during RFA. The overall mean ablation time of these thermal ablation therapies was 70.90 min (95% CI, 58.36–83.43, p < .0001, I2 = 99.8%; 22 studies) ().
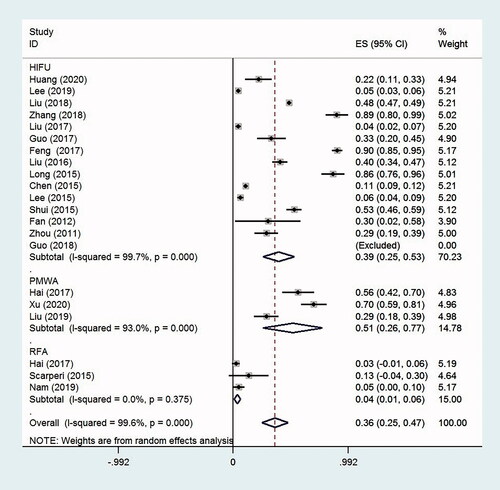
NPV ratio of adenomyosis
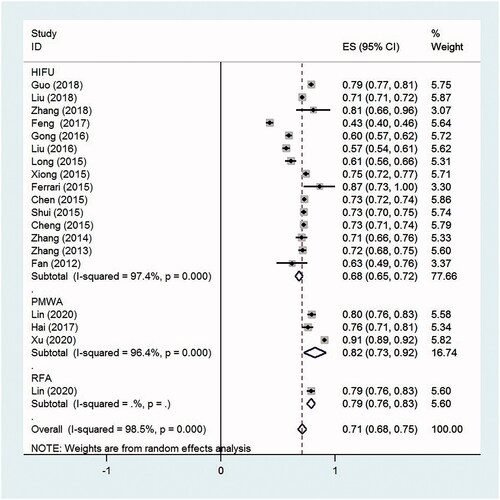
The NPV ratio of AD was 68.3% (95% CI, 65.0–71.6%, p < .0001, I2 = 97.4%; 15 studies) for HIFU, 82.5% (95% CI, 72.7–92.2%, p < .0001, I2 = 96.4%; three studies) for PMWA and 79.2% (95% CI, 75.7–82.7%, p < .0001; one study) for RFA. The overall NPV ratio was 71.4% (95% CI, 67.7–75.2%, p < .0001, I2 = 98.5%; 19 studies) for these thermal ablation therapies ().
The reduction rate of uterine volume and adenomyosis volume
The reduction rate of uterine volume after HIFU, PMWA and RFA was 33.6% (95% CI, 18.0–49.1%, p < .0001, I2 = 95.9%; seven studies), 46.8% (95% CI, 17.9–75.8%, p = .002, I2 = 96.0%; three studies) and 44.0% (95% CI, 36.0–52.0%, p < .0001, I2 = 0%; two studies), respectively. The overall reduction rate of uterine volume for all therapies was 38.8% (95% CI, 27.4–50.1%, p < .0001, I2 = 95.2%; 12 studies).
The reduction rate of AD volume was 45.1% (95% CI, 35.4–54.9%, p < .0001, I2 = 84.4%; seven studies), 74.9% (95% CI, 67.7–82.1%, p < .0001, I2 = 0%; two studies) and 61.3% (95% CI, 52.5–70.2%, p < .0001, I2 = 22.8%; three studies) after HIFU, PMWA and RFA, respectively. The overall reduction rate of AD volume for all therapies was 54.6% (95% CI, 47.2–62.0%, p < .0001, I2 = 83.4%; 11 studies).
Relief rate of dysmenorrhea
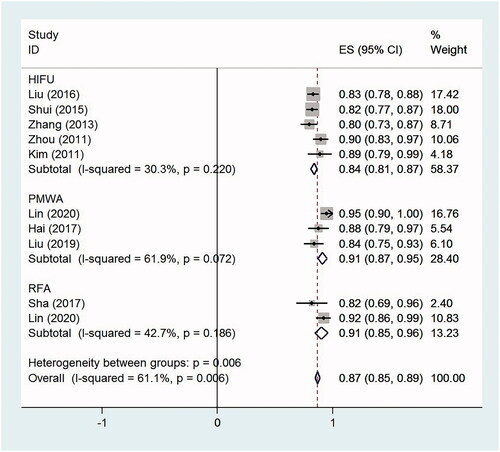
The relief rate of dysmenorrhea in women undergoing HIFU, PMWA and RFA was 84.2% (95% CI, 80.8–87.6%, p < .0001, I2 = 30.3%; five studies), 89.7% (95% CI, 82.7–96.7%, p < .0001, I2 = 61.9%; three studies) and 89.2% (95% CI, 79.9–98.5%, p < .0001, I2 = 42.7%; two studies), respectively. The overall relief rate of dysmenorrhea was 86.8% (95% CI, 83.3–90.4%, p < .0001, I2 = 61.1%; 10 studies) for all ablation therapies ().
Incidence of minor adverse events
There was no major adverse event occurred in the included studies, while the incidence of minor adverse events was 39.0% (95% CI, 25.4–52.5%, p < .0001, I2 = 99.7%; 14 studies) after HIFU, 51.3% (95% CI, 25.8–76.9%, p < .0001, I2 = 93.0%; three studies) after PMWA and 3.6% (95% CI, 0.9–6.3%, p = .01, I2 = 0%; three studies) after RFA. The overall incidence of minor adverse events was 36.0% (95% CI, 24.8–47.1%, p < .0001, I2 = 99.6%; 21 studies) after all ablation therapies ().
Rate of re-intervention, recurrence and pregnancy
The re-intervention rate was 4.0% (95% CI, 3.0–5.1%, p < .0001, I2 = 0%; three studies) in the HIFU group and 28.7% (95% CI, 8.2–49.3%, p = .006, I2 = 89.1%; two studies) in the RFA group. The overall re-intervention rate after these therapies was 11.6% (95% CI, 6.0–17.1%, p < .0001, I2 = 92.5%; five studies). There were no studies that reported the re-intervention rate after PMWA treatment. The recurrence rate which was only reported in women undergoing HIFU was 10.2% (95% CI, 4.8–15.5%, p < .0001, I2 = 92.5%; five studies). The pregnancy rate was 16.7% (95% CI, 1.3–32.2%, p = .034, I2 = 94.4%; four studies) after HIFU, 4.93% (one study) after PMWA and 35.8% (one study) after RFA, respectively.
Discussion
Main findings and comparison with existing literature
This meta-analysis revealed that HIFU, PMWA and RFA were effective in alleviating the dysmenorrhea, menorrhagia and other AD-related symptoms, which was consistent with the reduction of uterus volume and AD volume. Compared to those at baseline, the VAS, SSS and menorrhagia severity scores decreased significantly and the relief rates of dysmenorrhea were all above 80% at the time of follow-up after these treatments. The result also demonstrated that there was a continuous remission in the symptoms throughout follow-up. A previous study [Citation55] showed that the improvement in dysmenorrhea symptom has a strong correlation with the extent of the endometrial penetration. Generally, the larger the range of coagulative necrosis, the better the clinical symptomatic relief of the treatment [Citation42].
All these thermal ablative therapies led to significant reduction in the uterine volume and AD volume. During the operation, real-time ultrasound imaging was used to monitor the area of target lesions and detect the response to the treatment. Contrast-enhanced ultrasound was used to assess the range and vascular perfusion of the lesion. Contrast-enhanced MRI was performed 1–3 days after the operation to evaluate the NPV ratio of AD. We noticed that the mean ablation time during HIFU was significantly longer than during PMWA and RFA, while the NPV ratio of AD as well as the reduction rates of uterine volume and AD volume were higher in the PMWA and RFA groups than in the HIFU group. This result may be due to the difference in ablative mechanism and procedure between HIFU and PMWA/RFA. Although HIFU is noninvasive without an ablative electrode, the effect of HIFU is often limited to the location of the AD lesion. The energy attenuation occurs gradually during transmission of the ultrasonic wave along its propagation path [Citation10]. If the AD lesion is too deep, for example on the posterior uterine wall, the ultrasound wave cannot penetrate deep enough and the lesion may not be completely ablated. However, the posterior uterine wall tends to be the most severely involved in women with AD. Gong et al. [Citation30] reported that compared to patients with AD at the posterior uterine wall, the efficiency of HIFU was significantly higher in patients with AD at the anterior uterine wall. In comparison, PMWA and RFA are performed by percutaneous or transcervical insertion of an electrode into the lesion, and the generated heat induces necrosis directly on the target lesion, which could overcome the above disadvantage of energy attenuation during HIFU and improve the efficiency of ablation to some extent. RFA is only effective in fibroids with a relatively small size because increasing impedance limits further deposition of electricity into the tissue as the temperature rises. MWA does not rely on the conduction of electricity into the lesion and it is not limited by impedance. As a result, the advantages of MWA over the other techniques include consistently higher tissue temperature, larger ablation volume, shorter operation time, less sensitivity to tissue type with more consistent results, and less procedural pain.
In these studies, no thermal ablation-related major adverse events were reported and the treatments for the minor adverse events were well tolerated by patients, indicating that thermal ablation is a safe therapy for AD. The incidence of minor adverse events was 39.0%, 51.3% and 3.6% after HIFU, PMWA and RFA, respectively. The main minor adverse events were transient sciatic nerve pain, transient unilateral leg weakness, transient hematuria, skin burn, vaginal bleeding and lower abdominal pain in the HIFU group [Citation19–21,Citation24,Citation26,Citation28,Citation35–37], and transient macroscopic hematuria, vaginal discharge and lower abdominal pain in the PMWA and RFA groups [Citation46–48,Citation51,Citation52,Citation54]. Although all cases were minor and recovered without any permanent adverse effects, these events revealed thermal injury to the surrounding tissues, such as the sciatic nerve, bladder, endometrium, bowel and abdominal skin. In order to optimize the effect of treatments and reduce the potential thermal injury, standardization of ablative margin, ablation time, accurate temperature monitoring, and deployment of the electrode needle should be established in the future.
Adenomyosis is prone to relapse, and the pooled re-intervention rates were relatively low after HIFU (4.0%) and RFA (28.7%). Usually, the recognition of the AD lesion and separating the normal myometrium from myometrial tissue invaded by the lesion can be difficult, especially in diffuse AD [Citation52]. Therefore, adenomyomectomy has proven to be less effective because of the high recurrence rate [Citation13]. During the thermal ablation, most of the AD tissue was ablated while leaving at least 3–5 mm myometrium from both the serosa and endometrial layer respectively to maintain the integrity of serosa and endometrium. The incompletely ablated AD lesion may continue to grow over time, and the symptoms may recur or even become aggravated. Hence, various types of additional treatments, such as hysterectomy, additional thermal ablation and medical therapy were required to relieve the symptoms [Citation51]. We noticed that in the studies reporting the re-intervention rate, the follow-up time was longer in the RFA group than in the HIFU group. The different length of follow-up time is the main factor that accounts for the difference in re-intervention rate. Generally, the longer the follow-up time, the higher the recurrence rate and re-intervention rate. Therefore, we cannot conclude that the women who received the RFA treatment had a higher re-intervention rate than those who received the HIFU treatment. The long-term re-intervention risks of these procedures are inconclusive, and more long-term comparative studies are needed in the future.
The results showed that the pregnancy rates were 16.7%, 4.93% and 35.8% in women undergoing HIFU, PMWA and RFA, respectively, but only six studies were available (HIFU, four studies; PMWA, one study; RFA, one study). All of these studies reported successful pregnancy and delivery cases after the thermal ablation therapies. The study of Huang et al. [Citation17] revealed that HIFU treatment led to significantly better pregnancy rates and natural conception rates compared to laparoscopic excision surgery. Yang et al. [Citation50] reported that PMWA had no significant impact on ovarian function and fertility. Nam [Citation51] demonstrated that RFA had the advantages of improving fertility in women with AD without the need for abdominal incisions or punctures, bleeding, and long-term hospitalization. These results indicated the thermal ablation therapy could be considered as a minimally invasive treatment option for women who desire to maintain fertility. However, the inclusion criteria for patients’ fertility desire and fertility potential (such as the differences in age and volume of AD between these therapies) varied significantly in these studies. For example, Nam [Citation51] included patients who were under the age of 44 years and had tried one or more pregnancy attempts after RFA. By comparison, Huang et al. [Citation17] only included patients with defined infertility. Yang et al. [Citation50] reported patients who had a desire for children were not eligible for PMWA and most of the patients used birth control measures after treatment. Besides, some studies that reported the pregnancy rate did not describe the volume of AD tissue or even the patients’ age, so we cannot compare these confounding factors between the included studies. As good-quality comparative data are lacking and the fertility desire as well as fertility potential may be different in these studies, we cannot conclude that RFA ablation is more favorable for desired pregnancy than the others. Although the current pregnancy outcomes after thermal ablation are encouraging, more studies with a larger population are needed to ascertain the safety and efficacy [Citation36].
Strengths
To the best of our knowledge, this is the first meta-analysis that focused on evaluating the efficacy and safety of the thermal ablation therapies (including HIFU, PMWA and RFA) in women with symptomatic AD. The advantages and disadvantages of each treatment should be considered when choosing a therapy, but a direct comparison of these therapies for treating AD has not been reported. Therefore, this meta-analysis synthesized the currently available literature and it will provide valuable evidence for clinical decisions.
Limitations
There were several limitations in our meta-analysis: (1) the main limitation was that most included studies were single-arm studies rather than comparative studies, which might affect the accuracy of the results and we cannot provide conclusive evidence. (2) The small amount of evidence regarding PMWA and RFA led to a large discrepancy in the number of patients in the three groups with 95.1% patients in the HIFU group, which could lead to unreliable results and limit the applicability of our findings. (3) There was a high risk of bias within the included articles. (4) Substantial heterogeneity was observed in the outcome measures. (5) We excluded non-English articles.
Conclusion
This systematic review and meta-analysis revealed that image-guided HIFU, PMWA and RFA resulted in significant alleviation of dysmenorrhea, menorrhagia and other AD-related symptoms, obvious reduction in the uterine volume and AD volume, relatively low re-intervention rates, and encouraging pregnancy outcomes after treatments. Few major adverse events were reported, and all minor adverse events recovered without any permanent adverse effects, indicating the safety of thermal ablation. Besides, PMWA and RFA seemed to be superior to HIFU in terms of the mean ablation time, NPV ratio of AD, and reduction rate of uterine volume and AD volume.
Despite the limitations, our study provided valuable information on the clinical outcomes after HIFU, PMWA and RFA treatments for AD. In conclusion, as minimally invasive alternatives to surgical interventions, these thermal ablative therapies may be effective and safe treatments in the management of symptomatic AD, especially for women who want to preserve fertility and avoid surgery.
Author contributions
Responsible for the initial concept, data acquisition, and final review of the manuscript (Lu Liu, Baiying Lei). Data acquisition, analysis and interpretation, drafting the article (Lu Liu, Baiying Lei, Tianfu Wang). Final approval of the version to be published (Baiying Lei).
Ethics approval: The ethics approval was not necessary because this article was a systematic review and meta-analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Maheshwari A, Gurunath S, Fatima F, et al. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18(4):374–392.
- Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27(12):3432–3439.
- Farquhar C, Brosens I. Medical and surgical management of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006;20(4):603–616.
- Vannuccini S, Luisi S, Tosti C, et al. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018;109(3):398–405.
- Mikos T, Lioupis M, Anthoulakis C, et al. The outcome of fertility-sparing and nonfertility-sparing surgery for the treatment of adenomyosis. A systematic review and meta-analysis. J Minim Invasive Gynecol. 2020;27(2):309–331.e3.
- Younes G, Tulandi T. Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol. 2018;25(2):265–276.
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril. 2014;101(2):472–487.
- Chen J, Porter AE, Kho KA. Current and future surgical and interventional management options for adenomyosis. Semin Reprod Med. 2020;38(2/3):157–167.
- Zhang L, Rao F, Setzen R. High intensity focused ultrasound for the treatment of adenomyosis: selection criteria, efficacy, safety and fertility. Acta Obstet Gynecol Scand. 2017;96(6):707–714.
- Meng X, He G, Zhang J, et al. A comparative study of fibroid ablation rates using radio frequency or high-intensity focused ultrasound. Cardiovasc Intervent Radiol. 2010;33(4):794–799.
- Zhao WP, Han ZY, Zhang J, et al. A retrospective comparison of microwave ablation and high intensity focused ultrasound for treating symptomatic uterine fibroids. Eur J Radiol. 2015;84(3):413–417.
- Wang Y, Liang P, Yu X, et al. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25(6):455–461.
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia. 2015;31(3):280–284.
- Harding G, Coyne KS, Thompson CL, et al. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes. 2008;6(1):99.
- Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20(7 Suppl.):S189–S191.
- Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805.
- Huang YF, Deng J, Wei XL, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with high-intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia. 2020;37(1):301–307.
- Dev B, Gadddam S, Kumar M, et al. MR-guided focused ultrasound surgery: a novel non-invasive technique in the treatment of adenomyosis – 18 month's follow-up of 12 cases. Indian J Radiol Imaging. 2019;29(3):284–288.
- Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221.
- Guo Q, Xu F, Ding Z, et al. High intensity focused ultrasound treatment of adenomyosis: a comparative study. Int J Hyperthermia. 2018;35(1):505–509.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61.
- Zhang X, Guo Y, Chen J, et al. Effect of pre-treatment with gonadotropin-releasing hormone analogue GnRH- on high-intensity focussed ultrasound ablation for diffuse adenomyosis: a preliminary study. Int J Hyperthermia. 2018;34:1289–1297.
- Lee JS, Hong GY, Lee KH, et al. Changes in anti-müllerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG. 2017;124(Suppl. 3):18–22.
- Liu XF, Huang LH, Zhang C, et al. A comparison of the cost-utility of ultrasound-guided high-intensity focused ultrasound and hysterectomy for adenomyosis: a retrospective study. BJOG. 2017;124(Suppl. 3):40–45.
- Huang X, Yu D, Zou M, et al. The effect of exercise on high-intensity focused ultrasound treatment efficacy in uterine fibroids and adenomyosis: a retrospective study. BJOG. 2017;124(Suppl. 3):46–52.
- Guo Y, Duan H, Cheng J, et al. Gonadotrophin-releasing hormone agonist combined with high-intensity focused ultrasound ablation for adenomyosis: a clinical study. BJOG. 2017;124(Suppl. 3):7–11.
- Jayaram R, Subbarayan K, Mithraprabhu S, et al. Heavy menstrual bleeding and dysmenorrhea are improved by magnetic resonance guided focused ultrasound surgery (MRgFUS) of adenomyosis. Fertil Res Pract. 2016;2:8.
- Feng Y, Hu L, Chen W, et al. Safety of ultrasound-guided high-intensity focused ultrasound ablation for diffuse adenomyosis: a retrospective cohort study. Ultrason Sonochem. 2017;36:139–145.
- Park J, Lee JS, Cho JH, et al. Effects of high-intensity-focused ultrasound treatment on benign uterine tumor. J Korean Med Sci. 2016;31(8):1279–1283.
- Gong C, Yang B, Shi Y, et al. Factors influencing the ablative efficiency of high intensity focused ultrasound (HIFU) treatment for adenomyosis: a retrospective study. Int J Hyperthermia. 2016;32(5):496–503.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine (Baltimore). 2016;95(3):e2443.
- Long L, Chen J, Xiong Y, et al. Efficacy of high-intensity focused ultrasound ablation for adenomyosis therapy and sexual life quality. Int J Clin Exp Med. 2015;8:11701–11707.
- Xiong Y, Yue Y, Shui L, et al. Ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia. 2015;31(7):777–783.
- Ferrari F, Arrigoni F, Miccoli A, et al. Effectiveness of magnetic resonance-guided focused ultrasound surgery (MRgFUS) in the uterine adenomyosis treatment: technical approach and MRI evaluation. Radiol Med. 2016;121(2):153–161.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676.
- Lee JS, Hong GY, Park BJ, et al. Ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroid & adenomyosis: a single center experience from the Republic of Korea. Ultrason Sonochem. 2015;27:682–687.
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: two-year follow-up results. Ultrason Sonochem. 2015;27:677–681.
- Cheng CQ, Zhang RT, Xiong Y, et al. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine (Baltimore). 2015;94(16):e729.
- Zhang X, Zou M, Zhang C, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomyosis: a prospective study. Eur J Radiol. 2014;83(9):1607–1611.
- Zhang X, Li K, Xie B, et al. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet. 2014;124(3):207–211.
- Fan TY, Zhang L, Chen W, et al. Feasibility of MRI-guided high intensity focused ultrasound treatment for adenomyosis. Eur J Radiol. 2012;81(11):3624–3630.
- Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril. 2011;95(3):900–905.
- Kim KA, Yoon SW, Lee C, et al. Short-term results of magnetic resonance imaging-guided focused ultrasound surgery for patients with adenomyosis: symptomatic relief and pain reduction. Fertil Steril. 2011;95(3):1152–1155.
- Fukunishi H, Funaki K, Sawada K, et al. Early results of magnetic resonance-guided focused ultrasound surgery of adenomyosis: analysis of 20 cases. J Minim Invasive Gynecol. 2008;15(5):571–579.
- Lin XL, Hai N, Zhang J, et al. Comparison between microwave ablation and radiofrequency ablation for treating symptomatic uterine adenomyosis. Int J Hyperthermia. 2020;37(1):151–156.
- Xu C, Tang Y, Zhao Y, et al. Use of contrast-enhanced ultrasound in evaluating the efficacy and application value of microwave ablation for adenomyosis. J Can Res Ther. 2020;16(2):365–371.
- Liu JX, Li JY, Zhao XY, et al. Transvaginal ultrasound- and laparoscopy-guided percutaneous microwave ablation for adenomyosis: preliminary results. Int J Hyperthermia. 2019;36:1233–1238.
- Hai N, Zhang J, Xu R, et al. Percutaneous microwave ablation with artificial ascites for symptomatic uterine adenomyosis: initial experience. Int J Hyperthermia. 2017;33(6):646–652.
- Xu RF, Zhang J, Han ZY, et al. Variables associated with vaginal discharge after ultrasound-guided percutaneous microwave ablation for adenomyosis. Int J Hyperthermia. 2016;32(5):504–510.
- Yang Y, Zhang J, Han ZY, et al. Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep. 2015;5:10034.
- Nam JH. Pregnancy and symptomatic relief following ultrasound-guided transvaginal radiofrequency ablation in patients with adenomyosis. J Obstet Gynaecol Res. 2020;46(1):124–132.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90(1069):20160119.
- Ti SAD, Er WF, Lei Z, et al. Clinical evaluation of three methods in the treatment of adenomyosis. Clin Exp Obstet Gynecol. 2018;45:508–512.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19(4):e2015.00071.
- McCausland AM, McCausland VM. Depth of endometrial penetration in adenomyosis helps determine outcome of rollerball ablation. Am J Obstet Gynecol. 1996;174(6):1786–1793; 1793–1794.