Abstract
Background
Thermal ablation is a potentially curative therapy for early-stage non-small cell lung cancer (NSCLC). Early recurrence after thermal ablation necessitates our attention.
Methods
The invasion and migration abilities of NSCLC after sublethal heat stimulus were observed in vitro and in vivo. Sublethal thermal stimulus molecular changes were identified by RNA sequencing. A xenograft model of NSCLC with insufficient ablation was established to explore the epithelial-to-mesenchymal transition (EMT) and metastasis-related phenotypes alteration of residual tumors.
Results
In vitro, the invasion and migration abilities of NSCLC cells were enhanced 72 h after 44 °C and 46 °C thermal stimulus. Epithelial–mesenchymal transition (EMT) phenotypes were also upregulated under these conditions. RNA sequencing revealed that the expression of carboxypeptidase A4 (CPA4) was significantly upregulated after thermal stimulus. Significant upregulation of CPA4 and EMT phenotypes was also found in the xenograft model of insufficient NSCLC ablation. The EMT process and invasion and migration abilities can be reversed by silencing CPA4.
Conclusions
This study demonstrates that sublethal heat stimulus caused by insufficient ablation can promote EMT and enhance the metastatic capacity of NSCLC. CPA4 plays an important role in these biological processes. Inhibition of CPA4 might be of great significance for improving early-stage NSCLC survival after ablation.
Introduction
Worldwide, lung cancer is a leading cause of cancer incidence and death [Citation1]. The National Lung Screening Trial (NLST) [Citation2] and the Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON) [Citation3] demonstrated that low-dose computed tomography (CT) screening reduced the mortality of lung cancer by 20%. With the popularity of low-dose CT screening, more early-stage lung cancers were detected early.
Surgery is the recommended treatment for patients with early-stage non-small-cell lung cancer (NSCLC). Referring to National Comprehensive Cancer Network (NCCN) guidelines, for patients with clinical early-stage (Stage I, node-negative Stage IIA) NSCLC who had medical contraindications to surgical resection or who refused surgery, stereotactic body radiotherapy (SBRT) or image-guided thermal ablation was recommended [Citation4]. A large number of studies have shown no significant difference in survival between thermal ablation and SBRT for early-stage NSCLC [Citation5–8]. However, some studies have shown a correlation between tumor size and early recurrence after ablation. Tumors greater than 2 cm in size are especially likely to recur locally after radiofrequency ablation (RFA) [Citation9]. With microwave ablation (MWA), tumors larger than 3 cm in diameter have a better chance of recurrence and result in shorter survival [Citation10].
Thermal ablation is a favorable technique for the treatment of early-stage NSCLC because of its feasibility, effectiveness, and repeatability. Sufficient ablation was the great challenge of this minimally invasive treatment. How to reduce local recurrence and improve survival time are issues of concern. In hepatocellular carcinoma (HCC) [Citation11–13] and breast cancer [Citation14], local recurrence after ablation shows a more malignant phenotype with increased tumor growth and distant metastasis. However, thus far, no study has revealed whether residual tumors exhibit biological behavior changes after insufficient ablation of NSCLC.
In our study, sublethal stimulus induced by insufficient ablation was simulated in vitro to explore the biological behavior changes and molecular changes of NSCLC cells. We established a xenograft model of insufficient ablation to verify the molecular changes and mechanisms in vivo.
Materials and methods
Patients and tissue samples
NSCLC samples and matched adjacent tissues from 78 untreated patients were collected from the Characteristic Medical Center of the PLA Rocket Force from February 2012 to December 2017 with the approval of the ethics committee of this institution and written informed consent of all the participants. All protocols were in accordance with the Helsinki Declaration. The clinical characteristics of the recruited patients are presented in .
Table 1. Correlations between CPA4 expression and the clinicopathological parameters of 78 NSCLC patients.
Among these 78 patients, 44 (56.4%) patients were male, and 34 (43.6%) were female, with ages ranging from 52 to 91 (mean 74.4 ± 7.0). Squamous cell carcinoma was pathologically diagnosed in 19 patients (24.4%), and non-squamous cell carcinoma was pathologically diagnosed in 59 patients (75.6%). Based on the TNM staging system, 34 patients presented with stage I and stage II disease, followed by 44 with stage III and stage IV disease. The overall survival period was defined as the time from initial diagnosis to the date of death.
Cell culture
A549 and H1975 cells were obtained from the Cell Bank Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Two cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA) with 10% fetal bovine serum (FBS, HyClone, Logan, UT).
In vitro heat treatment
Heat treatment was simulated in vitro according to a previously reported procedure [Citation15–18]. Adherent monolayers of A549 and H1975 cells were grown to 70% confluence, and 5 × 105 cells were suspended in 1 ml DMEM, collected in 1.5 ml Boilproof Microtubes (MCT-150-B, Axygen, Corning, NY), and immediately exposed to heat stimulus using a digital dry bath incubator (CHB-100, KeHuai Instrument, Jiangsu, China) at temperature settings of 37 °C, 42 °C, 44 °C, 46 °C, 48 °C, and 50 °C for 10 min. Cells were then seeded into 60 mm dishes in 10 ml of DMEM with 10% FBS and incubated at 37 °C in a humidified atmosphere of 5% CO2. After 12 h, the cells were trypsinized and centrifuged at 800 rpm for re-inoculation in new dishes to remove debris and dead cells.
Trypan blue exclusion assay
Twenty-four hours after heat treatment, re-adherent cells were harvested by trypsinization. Then, the cells were stained with a 0.4% trypan blue solution (DingGuo Biotech, Shanghai, China) and counted using a cell counting plate.
Cell proliferation assay
Cell proliferation assays were analyzed by a CCK-8 kit (AR1160, Boster, Pleasanton, CA). After 24 h of heat treatment, cells were trypsinized and counted, with 2000 cells seeded per well in 96-well plates. The incubated cells were treated with 10 μl of CCK-8 reagent. The absorbance was recorded 24 h, 48 h, 72 h, and 96 h after thermal stimulus. The absorbance was measured at 450 nm using a Molecular Devices SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA).
Colony formation assay
Twenty-four hours after heat treatment, re-adherent cells were harvested by trypsinization. The cells were then plated in 6-well plates (500 cells/well) and cultured for 2 weeks. The resulting colonies were stained with crystal violet (DingGuo Biotech, Shanghai, China). Only colonies containing more than 50 cells were regarded as positive colonies and counted.
Apoptosis assay
Apoptosis assays were performed using an apoptosis kit (MK1028, Boster, Pleasanton, CA). A total of 1 × 105 cells/ml were trypsinized and collected 24 h after heat treatment using FACS buffer, washed and stained with 5 µl Annexin V-FITC and 5 µl PI. Each sample was analyzed with flow cytometry (BD FACSCalibur flow cytometer). Data were analyzed by FlowJo software (version 10, SPSS Inc., Chicago, IL).
Cell-cycle analysis
Twenty-four hours after heat treatment, re-adherent cells were harvested by trypsinization. A total of 1 × 106 cells were collected and fixed with 70% cold ethanol. After fixation, PI staining solution with RNase A (Abcam Biosciences, Cambridge, UK) was added. After 30 min of incubation, stained samples were run on flow cytometry, and data were analyzed using modfit software LT4.1 (SPSS Inc., Chicago, IL).
In vivo metastasis assay
The heat-treated cells above were cultured for 24 h, and an intravenous metastasis model was established. At 24 h after heat stimulus, 5 × 106 NSCLC cells were injected into the tail vein of each mouse. Twelve weeks after vein injection, the mice were euthanized and examined. Livers and lungs were harvested, and the numbers of gross metastatic foci were observed using a dissection microscope. Metastatic nodules were fixed with formalin, sectioned serially and stained with hematoxylin–eosin (H&E) for standard histological examination. There were three mice in each group.
Cell migration and invasion assays
Cell migration and invasion were assessed by transwell assays (Corning Incorporated Costar, Corning, NY). Briefly, in the migration assay, 5 × 104 cells suspended in 100 μl serum-free DMEM were seeded into the upper chamber of each well of 24-well plates containing 8.0-µm pore size membranes. FBS (500 μl) was added to the lower chamber of each well. After 24 h, cells that had reached the underside of the membrane were stained with crystal violet, and then 5 randomly selected areas (100× magnification) per well were counted. For the invasion assay, the upper compartment was precoated with 100 μl of Matrigel (0.8 mg/ml, BD Biosciences, Franklin Lakes, NJ), and cells were stained 48 h after cells were seeded onto the membranes.
Quantitative real-time PCR (RT-PCR)
Total mRNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA), and cDNA was synthesized using an All-in-One First-Strand cDNA Synthesis Kit (GeneCopoeia, Rockville, MD). RT-PCR was performed with BlazeTaq SYBR Green qPCR MIX 2.0 Kit (GeneCopoeia, Rockville, MD). The primer sequences used to determine the expression of the target genes were as follows:
The PCR consisted of 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 30 s, annealing for 1 min at 55 °C and primer extension for 1 min at 72 °C. The comparative cycle threshold (Ct) method was used to quantitate gene expression. β-actin was the internal control.
Immunohistochemistry (IHC) and immunofluorescence staining (IF)
For IHC, tissues embedded in paraffin were sectioned into 5-μm slides. The primary antibodies used in our study are listed below: E-cadherin (CST#3195, 1:200), N-cadherin (CST#13116, 1:200), Vimentin (Abcam#ab8978, 1:200), MMP-2 (CST#40994, 1:200), MMP-9 (CST#13667, 1:200), and CPA4 (Genetex#GTX81517, 1:100). The quantification of IHC was performed by McCarty’s H-score system [Citation19], which incorporates both the intensity of the specific staining and the percentage. The proportion score represented the estimated fraction of positively stained tumor cells (0 = none; 1 = less than 25%; 2 = 25–75%; 3 = greater than 75%). The intensity score represented the average staining intensity of positive tumor cells (0 = none; 1 = weak; 2 = intermediate; 3 = strong). The two scores were multiplied to generate the immunoreactivity score (IS) for each case. Five fields were randomly selected to add up to the total score. CPA4 expression was defined as either high expression (score ≥ 15) or low expression (score < 15). The results were reviewed by pathologists who were blinded to the research purpose.
For IF, the cells were cultured on confocal dishes and fixed, permeabilized with 0.5% Triton X-100 (Thermo Fisher Scientific, Waltham, MA), and incubated with Vimentin (Abcam#ab8978, 1:200) and CPA4 (Genetex#GTX81517, 1:100) overnight at 4 °C, followed by incubation with secondary antibodies conjugated with Alexa Fluor 488 (Abbkine#A23220, 1:500) and 594 (Abbkine#A23420, 1:500). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. Images were captured by confocal microscopy (LSM980, Zeiss, Jena, Germany).
Western blot analysis
Total cellular proteins were extracted using RIPA lysis buffer (89900, Thermo Fisher Scientific, Waltham, MA) containing protease inhibitor cocktail (78430, Thermo Fisher Scientific, Waltham, MA). Protein concentration was measured according to the instructions of the BCA kit (Thermo Scientific, Waltham, MA). The primary antibodies used in our study are listed below: E-cadherin (CST#3195,1:1000), N-cadherin (CST#13116, 1:1000),Vimentin (Abcam#ab8978, 1:1000), MMP-2 (CST#40994, 1:1000), MMP-9 (CST#13667,1:1000), CPA4 (Genetex#GTX81517,1:500), and GAPDH (Am1020b, YTHX Biotechnology, 1:2000). The signals were detected using the Western Blotting Detection System (Bio-Rad, Hercules, CA)
RNA-sequencing (RNA-seq) and data analysis
Twenty-four hours after heat treatment, re-adherent cells were harvested by trypsinization. RNA was isolated by an RNA Fast2000 Extraction kit (220011, Fastagen, Oakland, CA). Transcriptome RNA-Seq was performed using Illumina high-throughput RNA sequencing [Citation20]. In brief, the DNA libraries were sequenced according to the Illumina TruSeq v3 protocol on an Illumina HiSeq2500 sequencer (Illumina, San Diego, CA).
Gene expression was quantified using FeatureCounts software (version 1.4.6) based on the ENSEMBL gene annotation for GRCH38. RNA-Seq data were normalized by trimmed mean of M-values (TMM) using EdgeR’s normalization factor, followed by quantile normalization, and presented by log2-fold change (log2 FC) scales.
Genes with significant down- or upregulation (fold change ≥ 1.5, p < 0.001) under the indicated conditions were analyzed by the web-based functional analysis tool Ingenuity Pathway Analysis (IPA) to visualize and annotate their biological functions and pathways.
Gene set enrichment analysis (GSEA) was performed using GSEA software (http://software.broadinstitute.org/gsea/).
RNA interference
Small interfering RNA (siRNA) targeting human CPA4 was purchased from GenePharma (Shanghai, China).
The siRNA sequences were as follows:
siRNA transfections were performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA; Thermo Fisher Scientific, Waltham, MA), and the transfection medium was Opti-MEM (Gibco, Carlsbad, CA; Thermo Fisher Scientific, Waltham, MA). The final concentration of siRNA was 50 nM, the medium was replenished after 24 h, and the cells were harvested 48 h post-transfection. The efficiency of transfection was verified with qRT-PCR and Western blot.
Xenograft tumor model and insufficient ablation of NSCLC
A xenograft tumor model was employed as described [Citation21]. Briefly, 1 × 106 A549 cells were injected subcutaneously into the flanks of 3- to 4-week-old BALB/c nude mice (18–20 g), which were purchased from Yiming Bio Co., Ltd. (Beijing, China). The protocol was approved by the ethical committee on Animal Assays of the Animal Care Committee of Characteristic Medical Center of the PLA Rocket Force. Tumor volume was calculated according to the following formula: volume (mm3) = (largest diameter × shortest diameter2)/2. When the tumor volume reached 1000 mm3, the ablation was performed.
A total of 10 mice were randomly divided into two groups: the ablation group and the control group. There were five mice in each group. The ablation device was a microwave ablation device (MTC-100, Fuzhong Medical Hi-Tech Co., Ltd. Nanjing, China). Referring to several previous studies [Citation11,Citation16], insufficient ablation was performed with a lower energy protocol, in which the power was 5 W and the duration was 30 s, as shown in . This ensured the presence of residual tumors. The control group was sham-operated by inserting a needle electrode into the tumor without performing ablation. All mice were sacrificed on the 10th day. Tumors were placed in a 4% paraformaldehyde solution. Tumor sections were assessed by H&E staining and IHC.
Statistical analysis
Statistical analysis was performed by SPSS 23.0 software (SPSS, Chicago, IL) and Prism GraphPad 7.0 (GraphPad Software, La Jolla, CA). All data shown are the results of at least three independent experiments and are expressed as the mean ± standard error of the mean (SEM). The differences between groups were compared using Student’s t test. Pearson’s χ2 test was used to analyze differences between CPA4 expression and NSCLC clinicopathological parameters. Correlations of continuous variables were analyzed by the Pearson correlation test. Survival curves were constructed by the Kaplan–Meier method, and a log-rank test was used for comparison. All tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Sublethal thermal stimulus at 46 °C and 48 °C inhibited the proliferation and increased the apoptosis of NSCLC cells in vitro, and 44 °C and 46 °C stimulus enhanced the invasion and migration ability of NSCLC cells in vitro
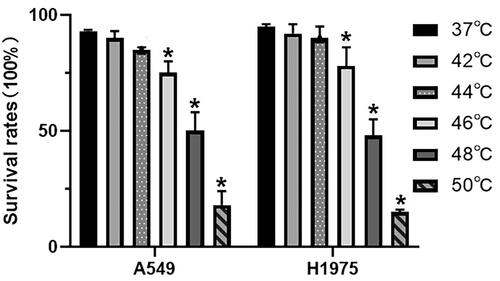
We determined the survival rate after 24 h of heat treatment. The survival rate after heat treatment decreased as the temperature increased. As shown in , 24 h after heat treatment at 42 °C, the survival rates of A549 and H1975 cells were 90.0 ± 3.5% and 92.3 ± 4.0%, respectively, while those at 44 °C were 85.3 ± 1.7% and 90.4 ± 3.2%, and the survival rates were 75.5 ± 3.5% and 78.0 ± 4.2% at 46 °C, respectively. After heat treatment at 48 °C, the survival rates of the two cell lines were 50.2 ± 5.7% and 48.5 ± 5.0%. At 50 °C, the cell survival rates were less than 20% and the cells became round and non-adherent. The cell survival rates were significantly reduced by thermal stimulus above or equal to 46 °C. Referring to other studies and trypan blue exclusion assays, 42-48 °C was the sublethal treatment temperature in vitro.
Figure 1. Survival rates of A549 and H1975 cells after different heat treatment protocols. Ten-min heat treatment at 37 °C, 42 °C, 44 °C, 46 °C, 48 °C, and 50 °C. Three independent experiments were performed. Error bars represent standard error (±SEM).
The proliferation of A549 and H1975 cells was inhibited by heat treatment at 46 °C and 48 °C, while the proliferation abilities of A549 and H1975 cells showed no significant change after treatment at 42 °C and 44 °C (). Consistent with the results of the proliferation assay, 46 °C and 48 °C resulted in cell cycle arrest in the G0/G1 phase of NSCLC cells ().
Figure 2. Proliferation, cell cycle, and apoptosis of NSCLC cells after sublethal thermal stimulus. (A) Cells proliferation was detected by CCK-8 assay at 24 h, 48 h, 72 h, and 96 h after heat treatment. (B) Colony formation assay was performed to detect the proliferation ability of the heat treatment NSCLC cells. (C) Statistical analysis of cell colony formation after heat treatment at different temperatures. (D) A549 and H1975 cells were harvested at 24 h after heat treatment. Cells cycle was determined by flow cytometry. Statistical analysis was in the lower right corner. (E) A549 and H1975 cells were harvested at 24 h after heat treatment. Cellular apoptosis was assayed by Annexin V/PI staining and detected by flow cytometry. Statistical analysis was in the lower right corner. Presented data were representative one from at least three independent experiments. Error bars represent standard error (±SEM). *p < 0.05, ** p < 0.01, *** p < 0.001.
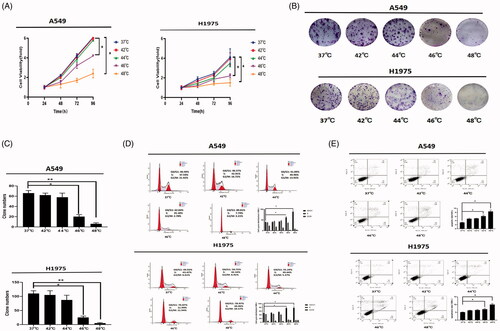
Compared to the control group, the apoptosis of cells was also significantly increased at 46 °C and 48 °C, by 6.2 ± 1.2% (p < 0.05) and 8.8 ± 0.7% (p < 0.05) in A549 cells and 8.8 ± 1.3% (p < 0.05) and 19.0 ± 2.0% (p < 0.05) in H1975 cells, respectively. In the 42 °C and 44 °C groups, there was no significant increase in apoptosis compared to the control group ().
Invasion and migration abilities were detected by transwell assay after 72 h of incubation after thermal stimulus. In both A549 and H1975 cells, the invasion and migration abilities were enhanced at 44 °C and 46 °C. Of these, the number of invaded and migrated cells was elevated more in the 44 °C group ().
Figure 3. Sublethal thermal stimulus promoted NSCLC cells invasion and migration in vivo and in vitro. (A) Representative images and quantification of cell invasion and migration results. A549 and H1975 cells were treated at sublethal temperature during 10 min, invasion and migration detected by transwell assays at 72 h after thermal stimulus. Scale bars, 100 µm. (B) Representative images of lung metastases under stereoscope. Arrows indicate metastatic foci. Scale bars, 200 µm. (C) The statistical graph of lung metastases nodules in different groups was presented. (D) Representative images of liver metastases under stereoscope. Arrows indicate metastatic foci. The outline of the metastatic foci was marked with dotted lines. Scale bars, 200 µm. (E) The statistical graph of liver metastases nodules in different groups was presented. Presented data were representative one from at least three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
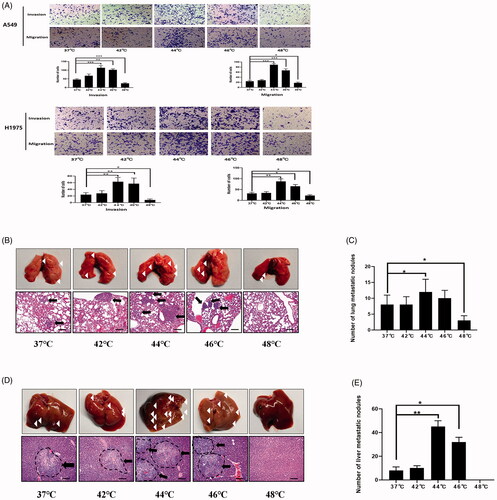
Taken together, the sublethal temperatures of 46 °C and 48 °C can inhibit the proliferation of NSCLC cells. At 44 °C and 46 °C, cell invasion and migration were enhanced, and this enhancement was most obvious at 72 h. About 44 °C is the trigger temperature to stimulate cell invasion and migration, and this temperature does not affect cell proliferation.
Sublethal thermal stimulus at 44 °C and 46 °C increased the metastatic capacity of A549 cells in tail vein tumor models
To assess metastatic capacity in vivo, A549 cells treated at different temperatures were injected into the tail veins of nude mice. As shown in , in the 44 °C and 46 °C groups, the numbers of metastases on the lung surface and liver were significantly greater than those in the control group. Of these, the number of metastases was greater in the 44 °C group.
Increases in the invasion and migration abilities of NSCLC cells at 44 °C and 46 °C were confirmed in vivo.
Thermal stimulus at 44 °C and 46 °C led to epithelial-mesenchymal transition (EMT) in NSCLC in vitro
Snai1 and Twist1 are well-known transcription factors that determine EMT. The expression levels of Snai1 and Twist1 were detected by RT-PCR. In A549 cells, the mRNA expression of Snai1 and Twist1 peaked 72 h after 44 °C heat treatment. The same result was also obtained in H1975 (). EMT markers were detected by Western blot after incubation for 72 h after thermal stimulus, showing that E-cadherin expression decreased and N-cadherin and Vimentin expression increased in the 44 °C and 46 °C groups compared with the control group, indicating the occurrence of the EMT process (). Immunofluorescence also indicated that vimentin fluorescence was significantly increased in the 44 °C and 46 °C groups (). Between them, the changes in EMT markers were more significant in the 44 °C group.
Figure 4. Sublethal thermal stimulus promoted NSCLC cell EMT in vitro. (A) EMT transcription factors (Snai1 and Twist1) expression of NSCLC cells detected by RT-PCR at 24 h, 48 h, and 72 h after heat treatment. (B) After 72 h of NSCLC cells were heat-treated, the protein levels of EMT markers were detected by western blot. (C) Immunofluorescence analysis of Vimentin expression in A549 and H1975 cells after 72 h of different thermal temperatures treatment. Scale bars, 10 µm. (D) The relative fluorescence intensity statistical graph of Vimentin. (E) After heat treatment at 44 °C, EMT markers expression of NSCLC cells was detected at different time by western blot. Presented data were representative one from at least three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
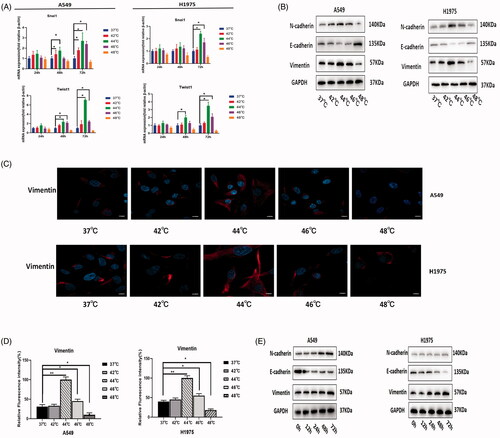
Furthermore, we observed changes in EMT markers over time under thermal stimulus at 44 °C. At 72 h, NSCLC cells expressed the lowest amount of E-cadherin and the highest amounts of N-cadherin and Vimentin (). This result suggested that EMT was most obvious 72 h after thermal stimulus.
The above results suggested that 44 °C thermal stimulus for 72 h most significantly promoted EMT characteristics in NSCLC.
Differentially expressed genes (DEGs) after 44 °C heat treatment in A549 cells
About 44 °C was the trigger temperature for inducing malignant transition of NSCLC in vitro as determined through the above studies. The mechanism might be that thermal stimulus initiated the EMT process in NSCLC. To explore which molecule plays the key role in this process, RNA sequencing was performed after 24 h of thermal stimulus at 44 °C.
Seventy-four upregulated and 20 downregulated differentially expressed genes were identified by RNA sequencing (). The top 13 upregulated genes and top 5 downregulated genes are shown in the heatmap by order of fold changes. The expression level of CPA4 mRNA in the thermal stimulus group was significantly higher than that in the control group (fold change = 2.17, p < 0.001) ().
Figure 5. A549 cells treated by 44 ° during10 min, detecting differentially expressed genes (DEGs) and functions through RNA-sequencing assay. (A) The number of up-regulated and down-regulated genes (fold change ≥1.5 and p < 0.001). Red column represents upregulated genes; blue column represents downregulated genes. (B) Heatmap showed the TOP 18 DEGs. Red indicates upregulated, whereas green indicates downregulated. (C) Scatter plot of the KEGG pathway enrichment of DEGs. The longitudinal axis represents pathway name; the horizontal axis represents rich factor; the dot size represents the number of DEGs in this pathway; and the color of the dot corresponds to different Q-value ranges. (D) and (E) GSEA revealed a significant enrichment of cell adhesion molecules and EMT signature after sublethal heat treatment.
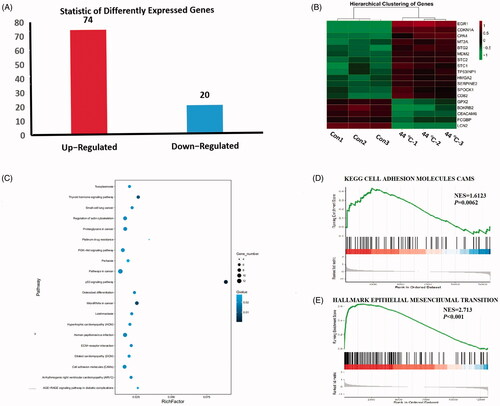
The top 20 most significantly enriched pathways were selected to produce the Kyoto Encyclopedia of Genes and Genomes (KEGG) scatter plot. The rich factor was the ratio of the number of DEGs to the number of genes annotated in a given pathway ().
GSEA demonstrated a significant enrichment of gene signature-associated cell adhesion molecules (normalized enrichment score, NES = 1.61, p < 0.01) and EMT (normalized enrichment score, NES = 2.73, p < 0.01) ().
RNA sequencing revealed that the mRNA expression of EMT-related molecules was upregulated after 44 °C heat treatment, and the expression level of CPA4 was significantly upregulated. We hypothesized that CPA4 played an important role in the malignant transition and EMT of NSCLC after thermal stimulus.
Cpa4 was highly expressed in NSCLC tissues and associated with patient prognosis
To determine CPA4 expression in NSCLC, IHC was performed in 78 primary human NSCLC tissues and 40 paracarcinoma tissues. As presented in , CPA4 was highly expressed in NSCLC specimens and showed stronger staining, whereas CPA4 staining was weaker in paracarcinoma tissues (p = 0.029).
Figure 6. CPA4 expression up-regulated in NSCLC predicted poor prognosis. (A) Representative images of expression of CPA4 in NSCLC tissues and para-carcinoma tissues. Scale bars, 100 µm. (B) The expression of CPA4 in NSCLC tissues was significantly increased compared with that of para-carcinoma tissues. (C) Kaplan–Meier survival analysis of CPA4 expression in patients with NSCLC subdivided with two groups. NSCLC with high expression of CPA4 has shorter survival. (D) The RNA expression of N-cadherin and Vimentin is positively correlated with CPA4 from TCGA datasets.
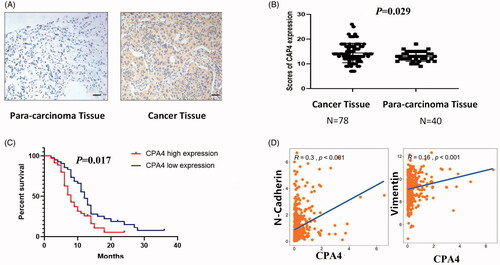
We further analyzed the association between CPA4 expression and clinicopathological parameters. As presented in , high expression of CPA4 was observed more often in patients with poorly differentiated (65.7% versus 25.0%, p < 0.01) positive lymph node status (59.4% versus 31.7%, p = 0.014) and in advanced-stage patients (56.8% versus 29.4%, p = 0.02).
Moreover, the Kaplan–Meier survival curve showed that high CPA4 expression was correlated with shorter overall survival (p = 0.017) (). From the Cancer Genome Atlas (TCGA) datasets, the expression of CPA4 was positively correlated with the EMT markers N-cadherin (R = 0.30, p < 0.01) and Vimentin (R = 0.16, p < 0.01) ().
The expression of CPA4 can be induced by sublethal thermal stimulus
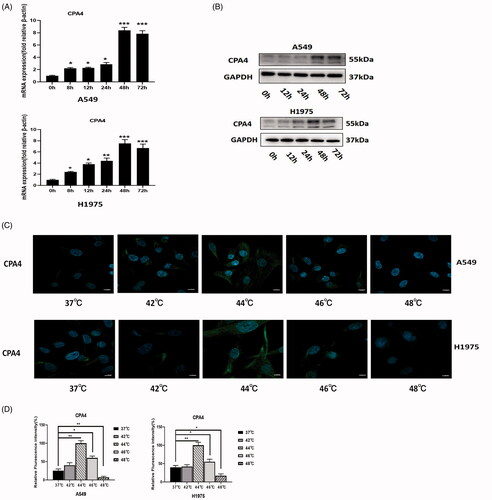
In both A549 and H1975 cells, after heat treatment at 44 °C, the mRNA of CPA4 was upregulated gradually and peaked at 48 h. It decreased slightly at 72 h (). The protein expression of CPA4 was consistent with the mRNA expression trend, and the expression level peaked at 48 h (). We confirmed this conclusion by IF assay. The expression of CPA4 in NSCLC cells was significantly upregulated 48 h after 44 °C thermal stimulus ().
Figure 7. Relationship between CPA4 expression and sublethal thermal stimulus. (A) The RNA expression level of CPA4 at different time after thermal stimulus at 44 °C was detected by RT-PCR. (B) The protein expression level of CPA4 at different time after thermal stimulus at 44 °C was detected by western blot. (C) IF analysis of CPA4 expression in A549 and H1975 cells after 48 h of different thermal temperatures treatment. Scale bars, 10 µm. (D) The relative fluorescence intensity statistical graph of CPA4 in A549 and H1975 cells after 48 h of different thermal temperatures treatment. All groups were compared with the 0 h group. Experiments were independently repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001.
Silencing CPA4 reversed the malignant biological behavior and EMT of NSCLC caused by sublethal thermal stimulus
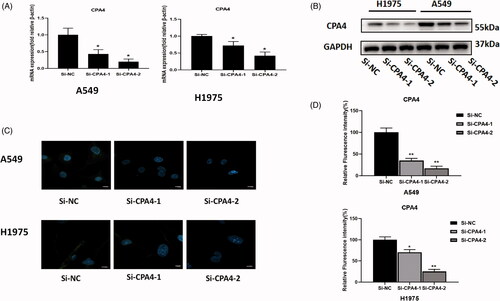
The interference efficiency of the siRNA was detected by RT-PCR, Western blot, and IF to confirm the feasibility of the siRNA knockdown. In A549 cells, compared with si-NC cells, the relative mRNA expression levels of the si-CPA4-1 group and si-CPA4-2 groups were 67.0 ± 17.2% and 33.3 ± 10.0%, respectively (p < 0.05). In H1975 cells, compared with si-NC cells, the relative mRNA expression levels of the si-CPA4-1 group and si-CPA4-2 groups were 76.0 ± 12.2% and 45.5 ± 14.3%, respectively (p < 0.05) (). Western blotting and IF also confirmed a significant decrease in protein levels after silencing CPA4 ().
Figure 8. RNA interference efficiency was measured by RT-PCR: (A) western blot. (B) and IF (C and D). Scale bars, 10 µm. Experiments were independently repeated three times. * p < 0.05, ** p < 0.01, *** p < 0.001.
CPA4 knockdown by small interfering RNA reduced the invasion and migration abilities promoted by sublethal thermal stimulus (). Consistent with the cell migration and invasion assays, si-CPA4 not only downregulated the background levels of MMP protein but also reversed the upregulation of MMP protein expression caused by thermal stimulus (). Western blot and IF showed that the expression of EMT markers was downregulated after silencing CPA4 ().
Figure 9. CPA4 altered biological behavior and EMT process of NSCLC. (A) Representative images and quantification of cell invasion and migration results. Silencing CPA4 reversed the enhanced invasion and migration abilities of NSCLC cells caused by sublethal thermal stimulus. Scale bars, 50 µm. (B) Silencing CPA4 reversed the up-regulated expression of MMP-2, MMP-9 caused by sublethal thermal stimulus in NSCLC cells. (C) Silencing CPA4 decreased the expression of the phenotypes associated with EMT in NSCLC cells. It also blocks the EMT process activated by sublethal thermal stimulus. (D) Representative images of Vimentin expression detected by IF assay. Silencing CPA4 reversed the expression of Vimentin caused by thermal stimulus using IF assay. Scale bars, 10 µm. (E) The relative fluorescence intensity statistical graph of vimentin in A549 and H1975 cells after different thermal temperatures treatment and silencing CPA4. (F) Cellular apoptosis was assayed by Annexin V/PI staining and detected by flow cytometry. The apoptosis rates of A549 and H1975 were not significantly increased after CPA4 knockdown. A549 and H1975 with silenced CPA4 stimulated at 44 °C, the apoptosis rates increased significantly. Presented data were representative one from three independent experiments. * p < 0.05, ** p < 0.01,*** p < 0.001.
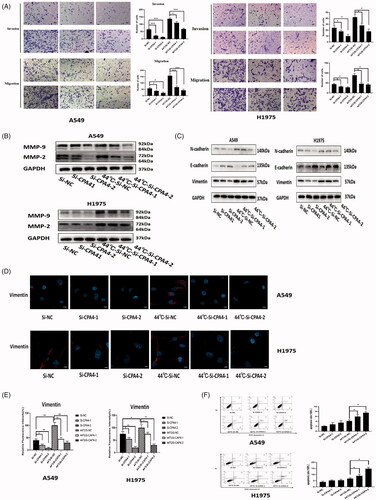
Silencing CPA4 can increase apoptosis after thermal stimulus in A549 and H1975 cells
After CPA4 knockdown, A549 and H1975 cells were more sensitive to heat damage and showed increased apoptosis rates. After 44 °C heat treatment, in A549 cells, the apoptosis rates were 6.0 ± 0.5% in the si-CPA4-1 group and 7.5 ± 0.3% in the si-CPA4-2 group, while the apoptosis rate was 4.2 ± 0.3% in the negative control group (p < 0.05). In H1975 cells, the apoptosis rates were 9.2 ± 0.9% in the si-CPA4-1 group and 14.8 ± 1.2% in the si-CPA4-2 group, while the apoptosis rate was 6.7 ± 0.7% in the negative control group (p < 0.05) ().
Insufficient ablation increased metastatic potential and induced EMT in NSCLC in vivo
After 10 days of insufficient thermal ablation, the mice were sacrificed, and the tumors were dissected. The tumor volume of the insufficient ablation group was significantly smaller than that of the sham ablation group (246.0 ± 81.2 mm3 versus 1206.0 ± 32.2 mm3, p < 0.01) ().
Figure 10. Expression of EMT markers, metastasis-associated proteins and CPA4 in insufficient ablation of xenograft tumor model. (A) Implementation of insufficient microwave ablation on xenograft mice. Ablation power was 5 W with a 30 s duration. Ten days after ablation, the representative images of the isolated tumors from sacrificed mice were presented. (B) Representative images of ablation of different sites, HE staining, Scale bars, 50 µm. In the sufficient ablation zone, the nucleus were pyknosis and fragmentation, the cytoplasm was eosinophilic. Cells in the insufficient ablation zone showed spindle changes, which might result in EMT process. (C) Representative images of E-cadherin, N-cadherin, and Vimentin staining in the control and ablation groups. Compared with the control group, the expression of E-cadherin was lower in residual tumors, while the expression of N-cadherin and Vimentin was higher in residual tumors. Scale bars, 50 µm. (D) Representative images of MMP-2 and MMP-9 staining in the control and ablation groups. Compared with the control group, the expression of MMP-2 and MMP-9 was higher in residual tumors. Scale bars, 50 µm. (E) Representative images of CPA4 staining in the control and ablation groups. Compared with the control group, the expression of CPA4 was higher in residual tumors. Scale bars, 50 µm. Presented data were representative one from three independent experiments.
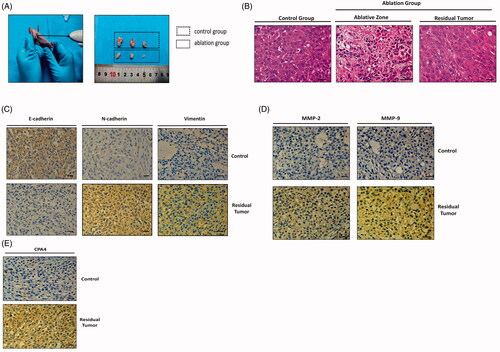
At the sufficient ablation zone, the cells appeared to be necrotic. Compared with the control group, cells in the insufficient ablation zone showed spindle changes, which might result in the EMT process ().
Compared with the sham ablation group, the expression of E-cadherin decreased significantly in residual tumors, while N-cadherin and vimentin were upregulated (). This indicates that EMT occurred in the sublethal ablation zone. The expression of MMP-2 and MMP-9 was also detected and showed significant upregulation in the residual tumor tissues (). The expression of CPA4 was also significantly higher in residual tumors than in the control group ().
Discussion
Some recent studies reported local recurrence rates of 30–40% for early-stage NSCLC after ablation [Citation22–25]. Tumor size is widely recognized as an important factor related to the efficacy of lung tumor ablation [Citation26,Citation27], and tumor morphology is also an important risk factor for recurrence [Citation28]. Sufficient inactivation of some early-stage NSCLC was difficult to achieve due to these risk factors. We hypothesize that residual tumor cells from insufficient ablation are the reason for the high relapse rate and shorter 5-year survival rate for early-stage NSCLC.
In HCC, several studies have found that insufficient ablation enhances the invasion and migration abilities of residual tumors. Some have found that sublethal thermal stimulus induced by insufficient ablation can induce EMT in residual HCC cells and breast cancer cells [Citation15,Citation16,Citation18,Citation29]. Other studies have also found that sublethal thermal stimulus lead to the differentiation of viable HCC cells into cancer stem cells [Citation11,Citation30–32].
However, no study is currently available that addresses the biological behavior changes and mechanisms of NSCLC after insufficient ablation.
In vitro, 44 °C and 46 °C could enhanced invasion and migration abilities and induced EMT of NSCLC. Between the two temperatures, the induction ability was more obvious at 44 °C. Interestingly, CCK-8 assay and clone formation assay showed that 46 °C inhibit cell proliferation significantly, however, 44 °C did not inhibit proliferation. We believed that 44 °C is the temperature with the most malignant characteristic. In HCC, the malignant behavior of HCC cells could be enhanced at 47 °C [Citation17,Citation29,Citation31–35]. It has been proved that temperatures which induced an increase in malignant behavior were difference in different tumors.
EMT is a process with particular phenotypic changes, through which epithelial cells lose their cell-cell adhesions and cell polarity, increase the expression of mesenchymal cell markers and migratory capacity, resist apoptosis, and produce extracellular matrix components [Citation36,Citation37]. Recent cancer studies have revealed that EMT, which is essential for the transformation of early-stage tumors into aggressive malignancies, is an early event in tumor metastasis [Citation38]. In our study, EMT occurred in residual tumors after insufficient ablation. We inferred that EMT occurred after triggering temperature stimulation of NSCLC cells, leading to enhanced invasion and migration abilities. Then, the tumors recurred and metastasized.
To determine which biological process is regulated, RNA-seq was conducted. By RNA-seq, we detected that the expression of CPA4 was significantly increased after 44 °C treatment. By insufficient ablation in a xenograft model, we found that the expression of CPA4 in residual tumors was upregulated. In vitro, the expression of CPA4 was also upregulated by sublethal thermal stimulus, which was verified by PCR and Western blot.
CPA4 is a member of the metallocarboxypeptidase (MCP) family. CPA4 functions in neuropeptide processing and regulation in the extracellular environment, which are closely related to cancer progression [Citation39]. CPA4 is highly expressed in a variety of solid cancers and is closely related to poor prognosis [Citation40–46]. We found that CPA4 expression in NSCLC was significantly higher than that in para-cancerous tissues, and patients with high expression of CPA4 had shorter survival. Patient samples collected in our study showed that tumor tissue with high CPA4 expression exhibited poor differentiation, positive lymph node status and advanced stage, which predicted worse prognosis. Our findings were consistent with those of another study [Citation40].
In TCGA datasets, there was a positive correlation between CPA4 and EMT marker expression. The expression of CPA4 was also positively correlated with the expression of N-cadherin and Vimentin and negatively correlated with the expression of E-cadherin in the xenograft tumor model of insufficient ablation. In pancreatic cancer, a study showed that CPA4 overexpression promoted EMT [Citation47]. To explore whether CPA4 can initiate EMT in NSCLC, we knocked down CPA4 with siRNA and found that silencing CPA4 could reverse EMT induced by sublethal heat treatment. Overall, we confirmed that upregulated CPA4 can induce EMT. To the best of our knowledge, we were the first to demonstrate that CPA4 overexpression can induce EMT in NSCLC. Moreover, the invasion and migration abilities of NSCLC cells were also decreased after silencing CPA4. The enhancement of invasion and migration caused by sublethal heat treatment can also be reversed. This indicated that CPA4 initiated EMT and malignant transition.
In addition, silencing CPA4 can also increase the apoptosis of NSCLC cells caused by sublethal heat treatment, so CPA4 may also have a protective effect against heat damage.
Our findings show that sublethal thermal stimulus induced by insufficient ablation can lead to upregulation of CPA4 expression in NSCLC cells, initiation of EMT, and an increase in malignant characteristics, which were specifically manifested as enhanced invasion and migration abilities and enhanced adaptability to heat damage.
The process and mechanism by which insufficient ablation leads to malignant transition through CPA4 has great significance in the ablation of early-stage NSCLC. First, complete ablation is the key to reducing the recurrence of early-stage NSCLC. Tumors less than 2 cm in diameter and relatively regular in shape might be more appropriate for thermal ablation. Second, CPA4 is a secreted protein, and studies have shown that serum CPA4 levels could be used for the early detection of NSCLC [Citation48], breast cancer [Citation41], pancreatic cancer [Citation45], and liver metastasis in colorectal carcinoma [Citation44]. We can monitor recurrence after ablation by the level of CPA4 in the serum. Third, the carboxypeptidase inhibitor sabellastarte magnifica (SmCI) has been reported to suppress the metallocarboxypeptidase activity of CPA4 by forming a complex with CPA4 [Citation49]. SmCI could be used in adjuvant therapy and to prevent tumor recurrence after NSCLC ablation with high recurrence factors. In addition, one study indicated the synergistic effect of the chemotherapy combine with hyperthermia. This treatment pattern might be more effective than hyperthermia or chemotherapy alone [Citation29].
Our data suggest CPA4 as a potential and promising predictor and therapeutic target for preventing recurrence after thermal ablation.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/02656736.2022.2099786)
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513.
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15(12):1332–1341.
- NCCN guidelines: NCCN Guidelines Version 8; 2020. Non-Small Cell Lung Cancer; http://www.nccn.org.
- Iguchi T, Hiraki T, Matsui Y, et al. Survival outcomes of treatment with radiofrequency ablation, stereotactic body radiotherapy, or sublobar resection for patients with clinical stage I non-small-cell lung cancer: a single-center evaluation. J Vasc Interv Radiol. 2020;31(7):1044–1051.
- Liang L, Li G, Xie S, et al. Choice of treatment for stage IA non-small cell lung cancer patients ineligible for surgery: ablation or stereotactic body radiotherapy. J Cancer. 2020;11(6):1634–1640.
- Watson RA, Tol I, Gunawardana S, et al. Is microwave ablation an alternative to stereotactic ablative body radiotherapy in patients with inoperable early-stage primary lung cancer. Interact Cardiovasc Thorac Surg. 2019;29(4):539–543.
- Lam A, Yoshida EJ, Bui K, et al. A national cancer database analysis of radiofrequency ablation versus stereotactic body radiotherapy in early-stage non-small cell lung cancer. J Vasc Interv Radiol. 2018;29(9):1211–1217.e1.
- Ager BJ, Wells SM, Gruhl JD, et al. Stereotactic body radiotherapy versus percutaneous local tumor ablation for early-stage non-small cell lung cancer. Lung Cancer. 2019;138:6–12.
- Healey TT, March BT, Baird G, et al. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28(2):206–211.
- Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40.
- Cheng J, Li M, Lv Y. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2014;59(4):1650.
- Ouyang Y, Liu K, Hao M, et al. Radiofrequency ablation-increased CXCL10 is associated with earlier recurrence of hepatocellular carcinoma by promoting stemness. Tumour Biol. 2016;37(3):3697–3704.
- Kong P, Pan H, Yu M, et al. Insufficient microwave ablation-induced promotion of distant metastasis is suppressed by β-catenin pathway inhibition in breast cancer. Oncotarget. 2017;8(70):115089–115101.
- Zaimoku R, Miyashita T, Tajima H, et al. Monitoring of heat shock response and phenotypic changes in hepatocellular carcinoma after heat treatment. Anticancer Res. 2019;39(10):5393–5401.
- Zhang N, Li H, Qin C, et al. Insufficient radiofrequency ablation promotes the metastasis of residual hepatocellular carcinoma cells via upregulating flotillin proteins. J Cancer Res Clin Oncol. 2019;145(4):895–907.
- Xu WL, Wang SH, Sun WB, et al. Insufficient radiofrequency ablation-induced autophagy contributes to the rapid progression of residual hepatocellular carcinoma through the HIF-1α/BNIP3 signaling pathway. BMB Rep. 2019;52(4):277–282.
- Yoshida S, Kornek M, Ikenaga N, et al. Sublethal heat treatment promotes epithelial-mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology. 2013;58(5):1667–1680.
- Yang Y, Xiao M, Song Y, et al. H-score of 11β-hydroxylase and aldosterone synthase in the histopathological diagnosis of adrenocortical tumors. Endocrine. 2019;65(3):683–691.
- Shu C, Zha H, Long H, et al. C3a-C3aR signaling promotes breast cancer lung metastasis via modulating carcinoma associated fibroblasts. J Exp Clin Cancer Res. 2020;39(1):11.
- Wang H, Li M, Rinehart JJ, et al. Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res. 2004;10(5):1633–1644.
- Hiraki T, Gobara H, Mimura H, et al. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142(1):24–30.
- Donington JS. Radiofrequency ablation in high-risk stage I non-small cell lung cancer. Cancer. 2015;121(19):3393–3394.
- Hiraki T, Gobara H, Iguchi T, et al. Radiofrequency ablation for early-stage nonsmall cell lung cancer. Biomed Res Int. 2014;2014:152087.
- Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg. 2012;93(3):921–927. discussion 927-88.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243(1):268–275.
- Hiraki T, Gobara H, Iguchi T, et al. Radiofrequency ablation as treatment for pulmonary metastasis of colorectal cancer. World J Gastroenterol. 2014;20(4):988–996.
- Yang Q, Qi H, Zhang R, et al. Risk factors for local progression after percutaneous radiofrequency ablation of lung tumors: evaluation based on a review of 147 tumors. J Vasc Interv Radiol. 2017;28(4):481–489.
- Lee TH, Bu J, Kim BH, et al. Sub-lethal hyperthermia promotes epithelial-to mesenchymal-like transition of breast cancer cells: implication of the synergy between hyperthermia and chemotherapy. RSC Adv. 2019;9(1):52–57.
- Tong Y, Yang H, Xu X, et al. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017;108(4):753–762.
- Wang X, Deng Q, Feng K, et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma cell progression via autophagy and the CD133 feedback loop. Oncol Rep. 2018;40(1):241–251.
- Zhang R, Ma M, Lin XH, et al. Extracellular matrix collagen I promotes the tumor progression of residual hepatocellular carcinoma after heat treatment. BMC Cancer. 2018;18(1):901.
- Liu Z, Dai H, Jia G, et al. Insufficient radiofrequency ablation promotes human hepatoma SMMC7721 cell proliferation by stimulating vascular endothelial growth factor overexpression. Oncol Lett. 2015;9(4):1893–1896.
- Duan XH, Li H, Han XW, et al. Upregulation of IL-6 is involved in moderate hyperthermia induced proliferation and invasion of hepatocellular carcinoma cells. Eur J Pharmacol. 2018;833:230–236.
- Zhang N, Ma D, Wang L, et al. Insufficient radiofrequency ablation treated hepatocellular carcinoma cells promote metastasis by up-regulation ITGB3. J Cancer. 2017;8(18):3742–3754.
- Jiang K, Zhao T, Shen M, et al. MiR-940 inhibits TGF-β-induced epithelial-mesenchymal transition and cell invasion by targeting Snail in non-small cell lung cancer. J Cancer. 2019;10(12):2735–2744.
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890.
- Kang Y, Massagué J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–279.
- Tanco S, Zhang X, Morano C, et al. Characterization of the substrate specificity of human carboxypeptidase A4 and implications for a role in extracellular peptide processing. J Biol Chem. 2010;285(24):18385–18396.
- Fu Y, Su L, Cai M, et al. Downregulation of CPA4 inhibits non small-cell lung cancer growth by suppressing the AKT/c-MYC pathway. Mol Carcinog. 2019;58(11):2026–2039.
- Bademler S, Ucuncu MZ, Tilgen Vatansever C, et al. Diagnostic and prognostic significance of carboxypeptidase A4 (CPA4) in breast cancer. Biomolecules. 2019;9(3):103.
- Sun L, Guo C, Burnett J, et al. Association between expression of Carboxypeptidase 4 and stem cell markers and their clinical significance in liver cancer development. J Cancer. 2017;8(1):111–116.
- Sun L, Guo C, Yuan H, et al. Overexpression of carboxypeptidase A4 (CPA4) is associated with poor prognosis in patients with gastric cancer. Am J Transl Res. 2016;8(11):5071–5075.
- Sun L, Guo C, Burnett J, et al. Serum carboxypeptidaseA4 levels predict liver metastasis in colorectal carcinoma. Oncotarget. 2016;7(48):78688–78697.
- Sun L, Burnett J, Guo C, et al. CPA4 is a promising diagnostic serum biomarker for pancreatic cancer. Am J Cancer Res. 2016;6(1):91–96.
- Ross PL, Cheng I, Liu X, et al. Carboxypeptidase 4 gene variants and early-onset intermediate-to-high risk prostate cancer. BMC Cancer. 2009;9(1):69.
- Shao Q, Zhang Z, Cao R, et al. CPA4 promotes EMT in pancreatic cancer via stimulating PI3K-AKT-mTOR signaling. OTT. 2020; 13:8567–8580.
- Sun L, Wang Y, Yuan H, et al. CPA4 is a novel diagnostic and prognostic marker for human non-small-cell lung cancer. J Cancer. 2016;7(10):1197–1204.
- Alonso del Rivero M, Reytor ML, Trejo SA, et al. A noncanonical mechanism of carboxypeptidase inhibition revealed by the crystal structure of the Tri-Kunitz SmCI in complex with human CPA4. Structure. 2013;21(7):1118–1126.
- Su T, Liao J, Dai Z, et al. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37(26):3514–3527.
