Abstract
Treatment of metastatic colorectal carcinoma has evolved in the era of increasingly effective systemic therapies. Increasing survival rates provide opportunities for repeated focal therapies to be directed at limited metastatic disease. Surgical resection and other ablative therapies to eliminate oligometastases in the most common sites, namely liver and lung, have been proven to prolong survival. As such, patients develop additional sites of metastasis in the course of their disease, including adrenal, peritoneal, nodal, and skeletal metastases. Data supporting aggressive focal therapy for extrahepatic, extrapulmonary metastases are limited. This manuscript summarizes findings of surgical studies of cytoreduction in these patients, describes limited data from ablation case series that include these metastases, and presents a rationale for further investigation of thermal ablation within this patient population.
Introduction—mCRC as a model for local therapy
Treatment of metastases from colorectal cancer (mCRC) requires a multidisciplinary approach, particularly in the setting of recurrent oligometastatic disease where patients may require multiple treatments of new disease sites over time. A key question when faced with a patient with oligometastatic CRC is whether all known disease sites may be treated safely and effectively with a local therapy. Historically, surgical resection has been the treatment of choice in patients who are medically fit for surgery, followed by thermal ablative or radiation therapies in those who recur or are unfit for surgery. Patients or tumors that are not candidates for local therapy are treated with systemic therapy alone.
Liver and lung are the two most common sites of oligometastatic CRC. Extensive data have demonstrated that treatment of hepatic and pulmonary oligometastatic CRC with local therapies can not only improve overall survival but can be curative in some patients [Citation1]. Historically, curative intent hepatic metastasectomy has been the standard of care for hepatic CRC metastases, providing improved overall survival rates of 26 to 51% at 5 years and even resulting in cure in up to 20% of patients at 10 years [Citation1,Citation2]. Unfortunately, up to 50% of patients with hepatic CRC metastases are not surgical candidates. Similar to the liver, pulmonary metastasectomy has historically been considered the standard of care for pulmonary oligometastatic CRC with data supporting improved oncologic outcomes when all disease sites can be resected [Citation3,Citation4].
Image-guided percutaneous thermal ablation has emerged as an alternative to surgical resection in patients with limited hepatic or pulmonary oligometastatic CRC. Recent data have demonstrated the safety and efficacy of percutaneous image-guided thermal ablation for hepatic mCRC, resulting in long-term disease control and lower morbidity than surgical resection [Citation5–7]. However, more recent data have demonstrated long-term oncologic outcomes of pulmonary radiofrequency ablation (RFA) comparable to that of surgical resection [Citation8]. A panel of experts has suggested that pulmonary ablation may be more appropriate as first-line treatment in patients with limited mCRC [Citation9]. Taken together, management of hepatic and pulmonary CRC oligometastases with surgical or ablative therapies serves as a model for use of local therapies to improve oncologic outcomes.
Surgical management of extrahepatic, extrapulmonary metastatic colorectal cancer
With improvement in both local control of hepatic and pulmonary CRC oligometastatic disease as well as improved efficacy of systemic therapies, patients with mCRC are living longer and developing extrahepatic, extrapulmonary sites of metastases, including other intraabdominal sites such as the peritoneum, lymph nodes, or other solid organs as well as brain and bone metastases with varying impact on patient morbidity and mortality by site. Similar to the liver and lung, the key first question is can all known sites of disease be resected. However, fewer data exist on oncologic outcomes for surgical resection of extrahepatic, extrapulmonary CRC metastases [Citation1,Citation4,Citation10]. An important study by Lee-Ying et al. evaluated survival after R0 metastatic resection of mCRC between liver and lung, locoregional disease and other distant disease sites. The authors found that patients with oligometastatic lung and locoregional disease have a similar survival advantage from resection as patients with hepatic oligometastatic disease [Citation4]. However, patients with R0 resection of other distant oligometastatic disease had a significantly lower survival compared to resection of hepatic oligometastatic disease. European Society of Medical Oncology (ESMO) consensus guidelines for management of patients with mCRC acknowledge differences in prognosis based on metastatic site within their definition of oligometastatic disease. Patients with 1-2 liver metastases and a solitary osseous metastasis may be considered oligometastatic, per their definition, whereas patients with more than one osseous metastasis do not [Citation11].
A recent review by Stewart et al. evaluated current data on cytoreductive strategies for CRC metastases including peritoneum, lymph nodes, bone and brain [Citation1]. For limited peritoneal metastatic disease, peritoneal cytoreduction with surgical resection and heated intraperitoneal chemotherapy (HIPEC) has been shown to be safe at experienced centers with increasing evidence for efficacy [Citation12]. However, there is currently no evidence that there is a survival benefit of cytoreductive surgery for peritoneal CRC disease compared to modern systemic therapy [Citation1,Citation13]. Nonetheless, there are numerous ongoing clinical trials evaluating peritoneal cytoreductive strategies in combination with systemic therapy which may improve outcomes for patients with peritoneal disease from mCRC [Citation1].
Regarding less common sites of metastatic disease, limited data exist to suggest a survival benefit for lymphadenectomy of distant lymph node metastases from mCRC in the salvage setting [Citation1,Citation14]. Osseous metastases from mCRC are uncommon and tend to present late in the disease course. However, when they occur, osseous metastases may be a source of significant morbidity, chiefly pain. While there are no data to suggest a survival benefit to treatment of osseous metastatic disease, palliative treatment of painful bone metastases is an important consideration in the multidisciplinary, longitudinal management of patients with mCRC [Citation1,Citation15]. Finally, CRC brain metastases are uncommon but when present are associated with a poor prognosis including a lower 1-year cause-specific survival (30%) compared to patients without CRC brain metastases (90%) [Citation1]. Overall, there has been increasing survival of patients with CRC brain metastases over the past several decades with use of multimodality treatment approach including systemic therapy, radiation therapy and surgery but treatment largely remains palliative. In summary, there are limited but encouraging data supporting potential improved oncologic outcomes for surgical resection of extrahepatic, extrapulmonary CRC metastases. Nonetheless, cytoreductive strategies combined with systemic therapy may continue to improve oncologic outcomes while palliative intent treatments remain an important consideration for improving patient quality of life for patients with extrahepatic, extrapulmonary CRC metastases.
Evidence supporting ablation of extrahepatic, extrapulmonary metastatic colorectal cancer
Several series have been published to evaluate outcomes following thermal ablation of extrahepatic, extrapulmonary metastases from heterogeneous patient populations. Most of these studies include patients with a wide variety of primary tumors, often with small numbers of patients of each primary tumor type, and do not report outcomes based on tumor histology. In addition, many series do not explicitly state that the targeted patient population had oligometastatic disease even excluding series of palliative ablation to treat painful bone metastases. It is possible that patients with widely metastatic disease do not respond as favorably as those with oligometastases in terms of local control rates [Citation16,Citation17]. Moreover, some patients in these studies were treated with oligoprogression, or accelerated tumor growth of the targeted lesion compared to background quiescent or slowly progressive metastatic disease. Some publications have also included tumors located in a variety of sites, including extrahepatic, extrapulmonary viscera, bone, lymph nodes, and other soft tissue sites [Citation18–20]. These variations in reported groups make interpretation of the data challenging.
Case reports have described successful heat-based thermal ablation of splenic, sternal, and sacral CRC metastases specifically [Citation21–23]. Multiple single-center case series documenting the role of thermal ablation in the treatment of adrenal (), bone, and soft tissue metastases have included small numbers of mCRC patients [Citation18,Citation19,Citation24–30]. summarizes these outcomes, including mCRC sub analyses where possible.
Figure 1. 48-year-old male with extrahepatic, extrapulmonary oligometastatic colorectal cancer treated with multidisciplinary approach over several years. (A, C) Axial contrast-enhanced CT and (B, D) 18F-FDG PET/CT show large, FDG-avid (A, B) right common iliac lymph node (white arrows) and (C,D) left adrenal metastases (white arrows). Patient was started on immunotherapy with excellent response for the right common iliac lymph node metastasis but not for the left adrenal metastasis (not shown). (E) Patient subsequently underwent CT-guided cryoablation of the left adrenal metastasis (white arrow). Follow-up CT at (F) 3 months and (G) 5 years post ablation demonstrates evolution of the ablation zone with no recurrent disease in the left adrenal gland at long-term follow-up (white arrow). (H) During the 5-year follow-up period, the patient also developed a subcutaneous metastasis in the right abdominal wall which was surgically resected (white arrow). He is currently without evidence of disease.
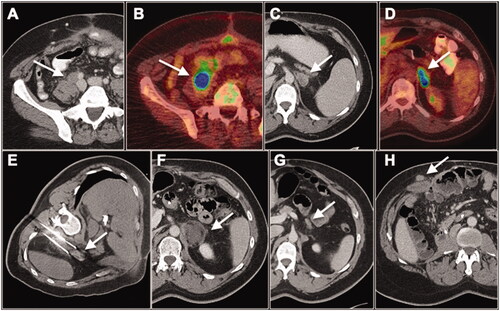
Table 1. Selected studies evaluating thermal ablation for local tumor control of extrahepatic, extrapulmonary metastases, including colorectal carcinoma.
Despite being a less common site of metastasis, adrenal metastases from CRC have been included in several adrenal ablation cohorts. Hasegawa reported successful treatment of 41 adrenal metastases with radiofrequency ablation, including 5 CRC metastases, with a local control rate of 77% and overall survival of 75%, including a median survival of 26.2 months in the CRC cohort [Citation27]. Gao reported a 93% local control rate and 83% overall survival at 12 months in a 43-lesion cohort of adrenal metastases treated with microwave ablation, including 4 CRC metastases [Citation26]. The Mayo group reported a 91% local control rate and 52% overall survival at 36 months in a cohort of 37 adrenal metastases treated with cryoablation and radiofrequency ablation, including 4 CRC metastases [Citation30].
Ablative treatment to achieve local control of soft tissue and osseous metastases, including those from CRC, has also been described [Citation18,Citation19,Citation25,Citation31]. Littrup reported a cohort of 251 metastatic soft tissue lesions, including 13 from CRC, with a 90% local control rate and very low major complication rate (2.3%) [Citation18]. Para-aortic lymph node metastases from CRC occur in 2% of patients. A systematic review including 370 patients suggested a survival benefit for patients who undergo para-aortic lymph node dissection; median overall survival was 34–40 months in the resection group versus 3–14 months for patients who did not undergo surgery [Citation14]. Our center has recently published a retrospective study of cryoablation for nodal metastases from various primary tumors [Citation31]. This study included 55 patients who underwent 61 cryoablation procedures to treat 65 lymph node metastases. Six of the treated lymph nodes were from mCRC with only 50% local control in this small subpopulation. A number of publications have detailed successful local control of limited nodal metastases from gynecologic malignancies achieved by thermal ablation [Citation32].
Several single-center series have reported outcomes from thermal ablation of oligometastases in multiple locations, including extrahepatic, extrapulmonary sites and breast carcinoma, prostate carcinoma, non-small cell lung carcinoma, renal cell carcinoma, sarcoma, and melanoma oligometastaes [Citation33–38]. In a retrospective, single-center study of 122 bone metastases treated with curative intent, Deschamps et al. found that local tumor control correlated with lesion size less than 2 cm, metastases that were metachronous, and absence of cortical bone erosion [Citation25]. In a similar study of 46 patients with oligometastatic disease to bone, Cazzato et al. observed that local progression-free survival, disease-free survival, and overall survival did not differ by tumor histology, but size greater than 2 cm predicted local tumor progression [Citation39]. Barral et al. reported 114 breast cancer oligometastases in 79 patients, including 18 patients with bone metastases, showing 1- and 2-year local control rates of 83.0 and 76.1%, respectively [Citation34]. Erie et al. demonstrated local tumor control in 83% of prostate carcinoma metastases, including one perirectal lymph node and 17 bone metastases, with median follow up of 27 months [Citation33]. A series of non-small cell lung carcinoma metastases treated with cryoablation and followed for 11 months (mean) by Bang et al. included 31 extrahepatic, extrapulmonary metastases (7 adrenal, 12 superficial, 2 para-aortic/isolated, and 10 bone), which showed 96.8% local control and 8% major complication rate in this subset [Citation35]. In a series of 64 sarcoma metastases to the musculoskeletal system in 41 patients, Vaswani et al. showed 1-year local control rates of 70% in all patients but 100% among the 10 patients with oligometastatic disease [Citation17]. These series demonstrate that ablation for local control of limited extrahepatic, extrapulmonary metastases is achievable for a variety of primary tumors. Specific, dedicated series of patients with mCRC in these locations treated with ablation are absent from the published literature, however.
In short, percutaneous image guided thermal ablative therapies offer several potential treatment and oncologic advantages for patients with extrahepatic, extrapulmonary CRC metastases. Ablative therapies can provide local tumor control at disease sites not responding to or progressing on systemic therapy [Citation40]. Consequently, the ability to provide local oligometastasis control may offer patients the option of a ‘systemic therapy holiday’ to minimize or obviate the side effects of systemic therapy [Citation41–44]. Moreover, different ablative technologies can be used to tailor treatment to different anatomic locations such as near hollow viscous, nerves or blood vessels. For example, newer non-thermal ablative therapies such as irreversible electroporation (IRE) may allow for treatment of soft tissue oligometastatic disease near critical structure such as lymph nodes in the porta hepatis near the common bile duct or axilla or pelvis near the brachial plexus or lumbosacral plexus [Citation45,Citation46]. Cryoablation can be utilized in treatment of adrenal, soft tissue, and bone metastases from CRC. Precise iceball visualization can allow added intraprocedural confidence and mitigate risk to important adjacent structures such as nerves or bowel [Citation30]. Similarly, microwave ablation can be successful in treatment of adrenal and soft tissue metastases while bipolar radiofrequency ablation can offer important reproducibility and precision in osseous applications such as the spine [Citation47]. While specific data do not exist for partial versus complete ablation of extrahepatic, extrapulmonary CRC metastases, existing surgical and ablation data for hepatic and pulmonary CRC metastases suggest that the best oncologic outcomes occur when all sites of disease can be resected and/or ablated [Citation1]. As such, complete ablation should be the goal when treating extrahepatic, extrapulmonary CRC metastases with ablation. Multiple adjuvant techniques can be employed to increase the ability to achieve a complete ablation zone around the oligometastatic site such as hydrodissection, intraoperative nerve monitoring or use of ureteral warming catheter or pyeloperfusion [Citation48–53]. Taken together, alternating treatment approaches including ablation for local tumor control may result in prolonged progression free-survival while minimizing potential dose-limiting side effects of systemic therapy.
Future directions
Historical standards of improved outcomes after surgical metastasectomy have laid the groundwork for developing interventional oncology treatments to control metastatic disease. Survival benefits of aggressive local therapy for mCRC established by surgical resection of hepatic and pulmonary metastases were used to establish trials to assess the role of thermal ablation in these patients. These studies have shown similarly improved outcomes following thermal ablation of oligometastases in liver and lung. These precedents may be more difficult to establish in the treatment of extrahepatic, extrapulmonary mCRC. Following resection of the primary tumor and/or hepatic CRC metastases, repeated intra-abdominal surgeries carry greater risk of complications, which may limit tolerance for resection of metachronous peritoneal, nodal, or adrenal metastases. Likewise, surgical morbidity often precludes curative resection of many musculoskeletal tumors compared to other common sites of metastases. Importantly, postoperative complications typically delay or may even preclude chemotherapy. In these locations, thermal ablation and stereotactic body radiotherapy are more appropriate local therapies, and trials of these treatment modalities for oligometastases of various primary tumors are ongoing. These modalities can offer repeatable treatment with limited morbidity for patients likely to develop serial metastases over several years. Studies of ablation for hepatic mCRC show the value of repeatability that allows for similar survival outcomes compared to surgery [Citation54,Citation55].
Patient selection for aggressive local therapy in mCRC remains an open question. Recent research has attempted to differentiate the true oligometastatic phenotype from patients with occult diffuse metastases on a molecular and genotypic basis, primarily based on expression of specific microRNAs [Citation56]. In 134 patients with colorectal carcinoma liver oligometastases resected for cure, Pitroda et al. used an integrative analysis of multiple molecular studies to characterize and stratify distinct groups based on risk of disease progression and death [Citation57]. Our center has examined whether molecular biomarkers of poor prognosis, specifically defects in the DNA mismatch repair system that produce microsatellite instability (MSI), should promote or exclude patients from local therapy to treat CRC oligometastases. One such study found that an aggressive approach including metastasectomy and/or thermal ablation was associated with improved survival, regardless of MSI status [Citation58]. Future developments in molecular biomarkers will hopefully allow appropriate triage of patients with mCRC between systemic and local therapies.
Conclusion
In conclusion, while evidence supporting thermal ablation of hepatic and pulmonary metastases from colorectal cancer are extensive, published reports regarding treatment of extrahepatic, extrapulmonary oligometastases are lacking. Surgical experience suggests outcomes improve for select patients treated with cytoreductive resection at expert centers. Case series reporting ablation of adrenal, non-visceral soft tissue, nodal, and osseous metastases within mixed populations or non-colorectal tumor histology also provide hints that the mCRC population may benefit from an aggressive multidisciplinary approach to their extrahepatic, extrapulmonary metastatic disease. This remains an area for further investigation, potentially including biomarkers to select patients most appropriate for ablative therapies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Stewart CL, Warner S, Ito K, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55(9):330–379.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580.
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20(2):572–579.
- Lee-Ying R, Bernard B, Gresham G, et al. A comparison of survival by site of metastatic resection in metastatic colorectal cancer. Clin Colorectal Cancer. 2017;16(2):e23–e8.
- Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204.
- Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014;50(5):912–919.
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015. DOI:10.1093/jnci/djx015.
- Zhong J, Palkhi E, Ng H, et al. Long-term outcomes in percutaneous radiofrequency ablation for histologically proven colorectal lung metastasis. Cardiovasc Intervent Radiol. 2020;43(12):1900–1907.
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur Radiol. 2015;25(12):3438–3454.
- Phelip JM, Tougeron D, Leonard D, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51(10):1357–1363.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422.
- Cashin PH, Mahteme H, Spang N, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur J Cancer. 2016;53:155–162.
- Quenet F, Elias D, Roca L, UNICANCER-GI Group and BIG Renape Group, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266.
- Wong JS, Tan GH, Teo MC. Management of para-aortic lymph node metastasis in colorectal patients: A systemic review. Surg Oncol. 2016;25(4):411–418.
- Callstrom MR, Dupuy DE, Solomon SB, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer. 2013;119(5):1033–1041.
- Gardner CS, Ensor JE, Ahrar K, et al. Cryoablation of bone metastases from renal cell carcinoma for local tumor control. J Bone Joint Surg Am. 2017;99(22):1916–1926.
- Vaswani D, Wallace AN, Eiswirth PS, et al. Radiographic local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41(8):1223–1232.
- Littrup PJ, Bang HJ, Currier BP, et al. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol. 2013;24(12):1817–1825.
- McMenomy BP, Kurup AN, Johnson GB, et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J Vasc Interv Radiol. 2013;24(2):207–213.
- Filippiadis D, Charalampopoulos G, Tsochatzis A, et al. Feasibility and safety of percutaneous computed tomography guided radiofrequency ablation of lymph nodes in oligometastatic patients: a single center's experience. Br J Radiol. 2021;94(1121):20200445.
- Maiettini D, De Angelis V, Graziosi L, et al. Sacrum colon-rectal cancer metastasis: microwave ablation for palliative pain treatment. Recenti Prog Med. 2016;107(12):673–676.
- Marangio A, Prati U, Luinetti O, et al. Radiofrequency ablation of colorectal splenic metastasis. AJR Am J Roentgenol. 2002;178(6):1481–1482.
- Wessel BE, Coldwell D. Colon cancer metastasis to the sternum: palliative treatment with radiofrequency ablation and cement injection. Radiol Case Rep. 2016;11(4):357–360.
- Cazzato, RL, De, Marini P, Leonard-Lorant, I, Dalili, D, Koch, G, Autrusseau, PA, et al. Percutaneous thermal ablation of sacral metastases: Assessment of pain relief and local tumor control. Diagn Interv Imaging. 2021;102(6):355–361.
- Deschamps F, Farouil G, Ternes N, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24(8):1971–1980.
- Gao Y, Zheng L, Liang P, et al. Evaluating the efficacy and safety of ultrasound-guided percutaneous microwave ablation for the treatment of adrenal metastasis. J Cancer Res Ther. 2020;16(5):1088–1092.
- Hasegawa T, Yamakado K, Nakatsuka A, et al. Unresectable adrenal metastases: clinical outcomes of radiofrequency ablation. Radiology. 2015;277(2):584–593.
- Levy J, Hopkins T, Morris J, et al. Radiofrequency ablation for the palliative treatment of bone metastases: outcomes from the multicenter Osteocool tumor ablation post-market study (OPuS One Study) in 100 patients. J Vasc Interv Radiol. 2020;31(11):1745–1752.
- Wallace AN, McWilliams SR, Connolly SE, et al. Percutaneous image-guided cryoablation of musculoskeletal metastases: pain palliation and local tumor control. J Vasc Interv Radiol. 2016;27(12):1788–1796.
- Welch BT, Callstrom MR, Carpenter PC, et al. A single-institution experience in image-guided thermal ablation of adrenal gland metastases. J Vasc Interv Radiol. 2014;25(4):593–598.
- Parvinian A. A single-institution experience in percutaneous image-guided cryoablation of lymph node metastases. AJR. 2021;217(1):152–156.
- Butros SR, DelCarmen MG, Uppot RN, et al. Image-guided percutaneous thermal ablation of metastatic pelvic tumor from gynecologic malignancies. Obstet Gynecol. 2014;123(3):500–505.
- Erie AJ, Morris JM, Welch BT, Kurup AN, et al. Retrospective review of percutaneous image-guided ablation of oligometastatic prostate cancer: a single-institution experience. J Vasc Interv Radiol. 2017;28(7):987–992.
- Barral M, Auperin A, Hakime A, et al. Percutaneous thermal ablation of breast cancer metastases in oligometastatic patients. Cardiovasc Intervent Radiol. 2016;39(6):885–893.
- Bang HJ, Littrup PJ, Goodrich DJ, et al. Percutaneous cryoablation of metastatic renal cell carcinoma for local tumor control: feasibility, outcomes, and estimated cost-effectiveness for palliation. J Vasc Interv Radiol. 2012;23(6):770–777.
- Welch BT, Callstrom MR, Morris JM, et al. Feasibility and oncologic control after percutaneous image guided ablation of metastatic renal cell carcinoma. J Urol. 2014;192(2):357–363.
- Hirbe AC, Jennings J, Saad N, et al. A phase II study of tumor ablation in patients with metastatic sarcoma stable on chemotherapy. Oncologist. 2018;23(7):760–e76.
- White ML, Atwell TD, Kurup AN, et al. Recurrence and survival outcomes after percutaneous thermal ablation of oligometastatic melanoma. Mayo Clin Proc. 2016;91(3):288–296.
- Cazzato RL, Garnon J, Caudrelier J, et al. Percutaneous radiofrequency ablation of painful spinal metastasis: a systematic literature assessment of analgesia and safety. Int J Hyperthermia. 2018;34(8):1272–1281.
- Molla M, Fernandez-Plana J, Albiol S, et al. Limited liver or lung colorectal cancer metastases. Systemic treatment, surgery, ablation or SBRT. JCM. 2021;10(10):2131.
- Petre EN, Jia X, Thornton RH, et al. Treatment of pulmonary colorectal metastases by radiofrequency ablation. Clin Colorectal Cancer. 2013;12(1):37–44.
- Sag AA, Selcukbiricik F, Mandel NM. Evidence-based medical oncology and interventional radiology paradigms for liver-dominant colorectal cancer metastases. World J Gastroenterol. 2016;22(11):3127–3149.
- Kurilova I, Gonzalez-Aguirre A, Beets-Tan RG, et al. Microwave ablation in the management of colorectal cancer pulmonary metastases. Cardiovasc Intervent Radiol. 2018;41(10):1530–1544.
- Fonck M, Perez JT, Catena V, et al. Pulmonary thermal ablation enables long chemotherapy-free survival in metastatic colorectal cancer patients. Cardiovasc Intervent Radiol. 2018;41(11):1727–1734.
- Hosein PJ, Echenique A, Loaiza-Bonilla A, et al. Percutaneous irreversible electroporation for the treatment of colorectal cancer liver metastases with a proposal for a new response evaluation system. J Vasc Interv Radiol. 2014;25(8):1233–1239 e2.
- Silk MT, Wimmer T, Lee KS, et al. Percutaneous ablation of peribiliary tumors with irreversible electroporation. J Vasc Interv Radiol. 2014;25(1):112–118.
- Pan S, Baal JD, Chen WC, et al. Image-guided percutaneous ablation of adrenal metastases: a meta-analysis of efficacy and safety. J Vasc Interv Radiol. 2021;32(4):527–535 e1.
- Kurup AN, Schmit GD, Morris JM, et al. Avoiding complications in bone and soft tissue ablation. Cardiovasc Intervent Radiol. 2017;40(2):166–176.
- Kitchin D, Lubner M, Ziemlewicz T, et al. Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia. 2014;30(5):299–305.
- Kurup AN, Morris JM, Schmit GD, et al. Neuroanatomic considerations in percutaneous tumor ablation. Radiographics. 2013;33(4):1195–1215.
- Kurup AN, Morris JM, Boon AJ, et al. Motor evoked potential monitoring during cryoablation of musculoskeletal tumors. J Vasc Interv Radiol. 2014;25(11):1657–1664.
- Favazza CP, Gorny KR, King DM, et al. An investigation of the effects from a urethral warming system on temperature distributions during cryoablation treatment of the prostate: a phantom study. Cryobiology. 2014;69(1):128–133.
- Marion JT, Schmitz JJ, Schmit GD, et al. Safety and efficacy of retrograde pyeloperfusion for ureteral protection during renal tumor cryoablation. J Vasc Interv Radiol. 2020;31(8):1249–1255.
- Otto G, Duber C, Hoppe-Lotichius M, et al. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251(5):796–803.
- Vigano L, Pedicini V, Comito T, et al. Aggressive and multidisciplinary local approach to iterative recurrences of colorectal liver metastases. World J Surg. 2018;42(8):2651–2659.
- Weichselbaum RR. The 46th David A. Karnofsky memorial award lecture: oligometastasis-from conception to treatment. J Clin Oncol. 2018;36(32):3240–3250.
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9(1):1793.
- Jin Z, Sanhueza CT, Johnson B, et al. Outcome of mismatch repair-deficient metastatic colorectal cancer: the Mayo Clinic experience. The Oncol. 2018;23(9):1083–1091.
