Abstract
Background
High-intensity focused ultrasound (HIFU) is a promising and non-invasive therapy for symptomatic uterine fibroids. Currently, the main image-guided methods for HIFU include magnetic resonance-guided (MR-HIFU) and ultrasound-guided (US-HIFU). However, there are few comparative studies on the therapeutic efficacy and safety of MR-HIFU and US-HIFU in treating symptomatic uterine fibroids with a volume <300 cm3.
Objective
We performed this meta-analysis to evaluate the efficacy and safety of MR-HIFU and US-HIFU in treating symptomatic uterine fibroids with a volume <300 cm3.
Methods
We searched relevant literature in PubMed, EMBASE, Cochrane Library CNKI from inception until 2021. The mean value, the proportion, and their 95% confidence intervals (CIs) were measured by random-effects models. Publication bias was assessed using funnel plots.
Results
48 studies met our inclusion criteria—28 describing MR-HIFU and 20 describing US-HIFU. The mean non-perfused volume rate (NPVR) was 81.07% in the US-HIFU group and 58.92% in the MR-HIFU group, respectively. The mean volume reduction rates at month-3, month-6, and month-12 were 42.42, 58.72, and 65.55% in the US-HIFU group, while 34.79, 37.39, and 36.44% in the MR-HIFU group. The incidence of post-operative abdominal pain and abnormal vaginal discharge in the US-HIFU group was lower than that of MRI-HIFU. However, post-operative skin burn and sciatic nerve pain were more common in the US-HIFU group compared with MRI-HIFU. The one-year reintervention rate after MR-HIFU was 13.4%, which was higher than 5.2% in the US-HIFU group.
Conclusion
US-HIFU may show better efficiency and safety than MR-HIFU in treating symptomatic fibroids with a volume <300 cm3.
Introduction
Uterine fibroids are the most common benign reproductive tract tumors in women of childbearing age and the leading indication for hysterectomy in the clinic [Citation1]. Treatment of uterine fibroids depends on the location, number, and size of fibroids, the patient's symptoms, age, and desire to maintain fertility [Citation2]. Surgical treatments are the main treatment for symptomatic fibroids, which generally include myomectomy and hysterectomy [Citation3,Citation4]. However, many women refuse to take surgeries for fibroids, especially among young women. High-intensity focused ultrasound (HIFU) is a non-invasive thermal ablation therapy recommended for symptomatic fibroids, showing several proven advantages, such as fewer complications, no general anesthesia, and shorter recovery time [Citation5,Citation6]. Currently, the HIFU systems are guided by either US or MRI to treat fibroids at a precise focal point, without damaging overlying and adjacent structures [Citation7,Citation8]. US-HIFU treatments ensure therapeutic accuracy and effectiveness via changing echogenicity in the targeted regions [Citation9]. In comparison, MR-HIFU treatments guarantee safety and effectiveness through monitoring temperature variation and providing feedback [Citation10].
To date, there are few comparative publications on the efficacy and safety of MR-HIFU and US-HIFU in treating uterine fibroids. Therefore, we performed this meta-analysis to evaluate the safety and efficacy of MR-HIFU and US-HIFU in treating symptomatic fibroids via comparing the NPVR, the reduction rates in different sessions, the incidence of related adverse events, and the one-year reintervention rate. Meanwhile, focused ultrasound ablation is limited by large uterine fibroids, which often result in the failure of treatment [Citation7,Citation8]. A previous study indicated that the clinical benefit of HIFU came under observation in treating fibroids with volume <500 cm3 [Citation11]. Hence, we inveterately focused our attention on fibroids with the volume <300 cm3 treated by HIFU to explore more appropriate reference intervals.
Methods
Search strategy
We searched the relevant literature in PubMed, EMBASE, Cochrane Library, and CNKI database from inception until 2021. The search strategy included the following domains of Medical Subject Heading terms: ‘uterine fibroid’, ‘ultrasonic focused ablation’. These terms were combined with ‘AND’ or ‘OR’. The search strategy of PubMed was shown in Supplemental Material S1 as an example.
Study screening
After the systematic search, the total literature was screened manually for eligible studies. Two reviewers (YL and ZS) independently reviewed the title and abstract to find out articles eligible for full-text screening. After the full-text screening, the literature that met the inclusion criteria was enrolled. Any disagreements were discussed and solved by consensus or third-party arbitration (WXL). The inclusion criteria were as follows: (1) Exposure to MR-HIFU or US-HIFU; (2) The mean volume of treated uterine fibroids was <300 cm3; (3) Non-perfusion volume percentage was evaluated immediately after surgery; (4) the incidence of post-operative adverse events was reported; (5) The reduction rate of uterine fibroids volume and one-year reintervention rate after HIFU were reported; (6) The treatment time of HIFU was reported. The exclusion criteria were as follows: (1) non-English articles or non-Chinese articles; (2) not a human study; (3) no outcomes that we needed; (4) document literature not available or duplicated.
Data extraction
The data of bibliography (year and author), study design (samples size, guiding modes of HIFU), and outcome measures (types of fibroids, location of fibroids, NPVR, short-term reduction rate of uterine fibroids, the incidence of related adverse events, and the one-year reintervention rate after HIFU) were extracted from eligible articles included in the final meta-analysis. Any disagreements were solved by consensus or third-party arbitration (WXL) when the disagreements appeared.
Quality evaluation
We used the MINORS (methodological index for non-randomized studies) to evaluate the quality of the included literature [Citation12]. The general section of the scale contains eight items, including the purpose of the study, the selection of target patients, calculation of sample size, data collection, endpoints of studies, objective evaluation of endpoints, follow-up time, loss of follow-up <5% and. Two reviewers (YL and ZS) assessed the quality of the literature independently and the disagreements were resolved by discussion or third-party arbitration (WXL). A total score of more than eight was considered to be of adequate quality [Citation12].
Statistical analysis
Meta-analysis of continuous variables and dichotomous variables was performed using R 4.0.2 software. p < 0.05 was regarded as statistically significant. According to the heterogeneity test results, the appropriate model (random or fixed) was then selected to merge the outcome indicators [Citation13]. The I2 value <50% was considered as low heterogeneity, 51–75% was considered as moderate heterogeneity, and more than 75% was considered as high heterogeneity [Citation14]. If the I2 value more than 50%, the fixed-effect model was chosen. Otherwise, the random-effect model was selected [Citation15]. A Funnel plot was used to judge the publication bias [Citation16].
Results
Characteristics of studies and quality evaluation
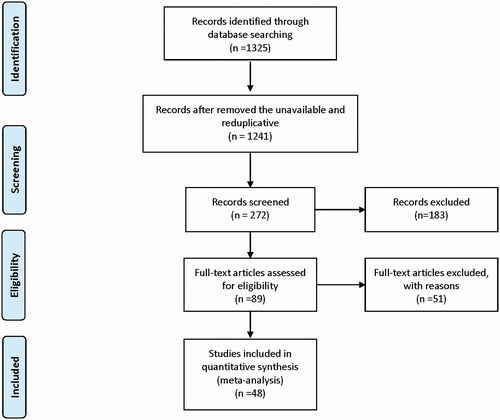
The flowchart of the systematic search and screening process was shown in . One thousand three hundred and twenty-five literatures were included in the preliminary screening phase. In these articles, 89 articles met the criteria for full-text review. Finally, a total of 48 articles were included in the meta-analysis. According to the image-guided methods, these studies were divided into the MR-HIFU group (n = 28) and the US-HIFU group (n = 20). A total of 6247 patients were included. Among them, the MR-HIFU group contained 2179 patients and the US-HIFU group contained the remaining 4068 patients. The basic information of the included studies was shown in Supplemental Material Table S2. The quality evaluation of included literature was presented in Supplemental Material Table S3. All of the included studies were of adequate quality.
Therapeutic efficiency of MR-HIFU and US-HIFU
Immediate post-operative non-perfusion volume rate (NPVR)
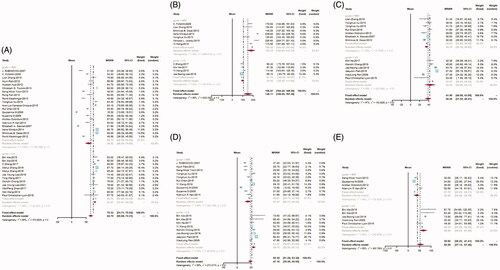
A total of 30 articles reported data on the NPVR post-operative. There were 20 articles in the MR-HIFU group and 10 in the US-HIFU group. The results of the meta-analysis revealed that the mean NPVR was 58.92% (95%CI: 46.94–70.89) and 81.07% (95%CI: 77.61–84.53) in the MRI-HIFU group and US-HIFU group, respectively ().
Total treatment time
Eleven studies reported the data on the time of treatment: seven studies in the MR-HIFU group and four in the US-HIFU group. The mean treatment time in the MR-HIFU group was 178.19 min (95%CI: 140.24–216.15) and 96.9 min (95%CI: 82.20–111.60) in the US-HIFU group, respectively ().
The reduction rate of uterine fibroids volume at month-3, month-6, and month-12
In total, 21 studies reported data on the short-term reduction rate of uterine fibroids. There were 13 studies included in the MR-HIFU group and eight studies included in the US-HIFU group. While in the US-HIFU group, the mean reduction rates at month-3, month-6, and month-12 were 42.42% (95%CI: 30.66–54.17), 58.72% (95%CI: 52.26–65.17), and 65.55% (95%CI: 49.54–81.56). The mean reduction rates of the MR-HIFU group were 34.79% (95%CI: 30.76–38.83), 37.79% (95%CI: 26.71–49.23), and 36.44% (95%CI: 24.49–48.38). at month-3, month-6, and month-12, respectively ().
One-year reintervention rate after HIFU
A total number of 11 articles reported the data on the one-year reintervention rate after HIFU. There were seven articles contained in the MR-HIFU group and four articles in the US-HIFU group. After preliminary analysis of the data, we found that the incidence of adverse events was low. To avoid the occurrence of extreme values, the data were corrected by performing the inverse hyperbolic sine transformation. After that, we re-performed the meta-analysis. Then, the result indicated that the reintervention rate in the MR-HIFU group was 13.4% (95%CI: 5.4–21.4) and 5.2% (95%CI: 2.0–8.4) in the US-HIFU group ().
Therapeutic safety of MR-HIFU and US-HIFU
There were 34 studies that reported the data on related adverse events in total. Among these, 21 articles reported post-operative skin thermal injury, 18 articles reported post-operative sciatic nerve pain, 27 articles reported post-operative abdominal pain and 20 articles reported post-operative abnormal vaginal discharge. Here, we also corrected the data by performing the inverse hyperbolic sine transformation to avoid the appearance. And then, we re-perform the meta-analysis.
The rate of post-operative skin thermal injury in the MR-HIFU group reached 4.5% (95%CI: 2.5–6.4) and 14.4% (95%CI: 7.3–21.5) in the US-HIFU group, respectively (). The prevalence of post-operative sciatic nerve pain was 8.9% (95%CI: 3.8–12.3) and 15.7% (95%CI: 8.2–23.3) in the MR-HIFU group and US-HIFU group (). The mean incidence of post-operative abdominal pain in the MR-HIFU group was 37.0% (95%CI: 21.9–52.2) and 31.2% (95%CI: 21.2–41.1) in US-HIFU (). The proportion of post-operative abnormal vaginal discharge was 20.3% (95%CI: 9.2–31.3) in the MR-HIFU group and 11.3% (95%CI: 7.7–14.9) in the US-HIFU group ().
Publication bias
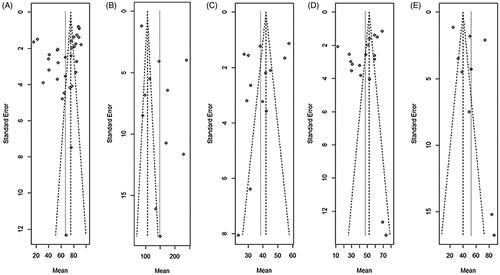
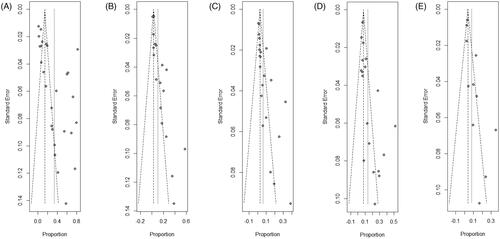
The funnel plots of each meta-analysis were roughly symmetric, indicating that there was no significant publication bias in our analysis ().
Discussion
According to current guidelines, the majority recommended fibroid size as the reference to establish screening criteria and fibroids with 3–10 cm were recommended for HIFU treatment [Citation7,Citation8,Citation17,Citation18]. Divya holds the view that good outcomes of HIFU came under observation in treating patients with fibroid volumes <500 cm3 [Citation11]. With the advancement of current technology, there is no clear limitation on the size of fibroids. However, for large fibroids, multiple HIFU ablation may be required, or drugs, such as GnRH-a may be needed before ablation. Meanwhile, long-term pretreatment might increase the risk of deep vein thrombosis [Citation19]. Hence, treating fibroid with suitable size using HIFU might maximize the benefit of patients and reduce the risk of adverse reactions.
There were few publications that compared the efficacy and safety of MR-HIFU and US-HIFU in treating uterine fibroids so far. Therefore, we performed this meta-analysis to evaluate the safety and efficacy of MR-HIFU and US-HIFU in treating fibroids with the volume <300 cm3 via comparing the NPVR, treatment time, the reduction rates of fibroid volume, the incidence of related adverse events, and the one-year reintervention rate after HIFU.
Clinical efficacy assessment
NPVR is considered a significant technical parameter positively connected with clinical success in HIFU [Citation7,Citation19]. NPV of more than 80% resulted in a high success rate among patients [Citation20]. In our analysis, the mean NPVR in the MR-HIFU group was 58.92% and was lower than 81.07% in the US-HIFU group. The summarized result indicated that US-HIFU could get more chance of being successful in treating fibroids with the volume <300 cm3.
Treatment time of HIFU was a crucial indicator for the clinical efficacy of HIFU. In our analysis, the mean treatment time in the MR-HIFU group was 178.19 min and 96.9 min in the US-HIFU group, respectively. Combined with the result of NPVR, US-HIFU might take less time to achieve therapeutic results, which was consistent with previous studies [Citation21].
Short-term reduction rates of uterine fibroid volume are also an important criterion for evaluating therapeutic efficacy [Citation22]. Previous guidelines suggested that a 3-months reduction rate of more than 20–50% revealed significant efficacy and favorable prognosis [Citation18]. MR-HIFU and US-HIFU were both effective methods for the fibroids with the volume <300 cm3 according to our analysis. Additionally, the reduction rates in the MR-HIFU group were similar to those reported by Inez [Citation23] and lower than those of US-HIFU at the same periods in our analysis. The ablation effect depends on the focused performance of the system and the selection of treatment regimens. The physical focal threshold of the US-guide focusing system was less than that of the MR-guide, which indicated greater focused power and higher accuracy [Citation24,Citation25]. Moreover, real-time ultrasound monitoring could reach the edge of fibroids and increase the ablation coverage [Citation25].
Simultaneously, The reintervention rate of the MR-HIFU group was 13.4%, and higher than the US-HIFU group. Noteworthily, the reintervention rate was consistent with the ablation ratio. Then, in our analysis, the total reintervention rate of HIFU was similar with uterine artery embolization and myomectomy [Citation26–30]. However, the summarized reintervention rate in our analysis was 8.1%, and lower than Evelien’s (9.7%) [Citation30]. The difference might be caused by the relevant literature included. Previous studies suggested that the high reintervention ratio of HIFU might be caused by inadequate patient selection [Citation31–33].
Clinical safety assessment
The most common adverse reactions after HIFU therapy were post-operative abdominal pain, sciatic nerve pain, abnormal vaginal discharge, and skin thermal injury according to previous studies [Citation34,Citation35]. The prevalence of abdominal pain and abnormal vaginal discharge in MR-HIFU was higher than US-HIFU in our analysis. Ultrasound guidance has good controllability, which could stop immediately according to patients’ status and make timely adjustments according to patients’ feedback. Post-operative abnormal vaginal discharge was considered to be associated with relative position between fibroids and endometrial. The ultrasound-guided ablation process could be monitored in real-time to reduce the thermal damage to the endometrium [Citation24,Citation25].
Nevertheless, the prevalence of sciatic nerve pain and skin thermal injury in the MR-HIFU group was lower than that of the US-HIFU group. These adverse events were mainly associated with the reflection of the focused ultrasound within the pelvis and the location of the uterus and fibroids. On the one hand, the reflection of the focused ultrasound within the pelvic could focus energy on the abdominal skin in front of the surrounding nerve tissues. On the other hand, sciatic nerve pain was mainly related to the posterior uterus and fibroids in the posterior wall [Citation36]. Compared with ultrasound, MRI was known to be more accurate in assessing deep tissue and the thickness of the abdominal wall [Citation37]. Bone, nerve, and fat could be imaged, which was conducive to establish a safe acoustic channel to reduce unnecessary ultrasound reflection and nerve injury [Citation24,Citation25].
Limitations of the study
First of all, loss of follow-up existed in the majority of researches included in our study. Indeed, incomprehensive results could not reflect the overall situation. Secondly, some objective factors, such as the number and location of fibroids were not well-documented in many kinds of literature. Hence, we were unable to undertake subgroup analysis according to these factors and these might be the source of heterogeneity in our meta-analysis. Thirdly, long-term results of HIFU were not included in the analysis because of the lack of long-term follow-up, such as two-year trial, three-year trial, and five-year trial. Hence, future research should focus on high-quality long-term follow-up studies.
Conclusions
US-HIFU may show better clinical practicality than MR-HIFU in treating fibroids with the volume <300 cm3 according to our meta-analysis. Additionally, choosing the most appropriate treatment for patients with fibroids according to the fully preoperative evaluation will help maximize the benefit of patients and to mitigate the risk factors amenable to intervention.
Author contributions
YL, ZS, and ZHY performed the database search and collected the data. YL performed the analysis and wrote the manuscript. ZHY and WAQ supplemented the database search. YL and WXL made revisions and finalization of the paper jobs. SGD and LJL polished the language of the article. WXL provided guidance on research directions. All the authors participated in this analysis and approved the final version of the manuscript.
Supplemental Material
Download PDF (291.2 KB)Supplemental Material
Download PDF (257.4 KB)Supplemental Material
Download PDF (101.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592.
- Stewart EA. Uterine fibroids. Lancet. 2001;357(9252):293–298.
- Uterine fibroids: medical treatment or surgery? Lancet. 1986;2:1197.
- Aarts JW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;2015:Cd003677.
- Lee SM, Choi ES, Ha E, et al. Gyejibongnyeong-Hwan (Gui Zhi Fu Ling Wan) ameliorates human uterine myomas via apoptosis. Front Pharmacol. 2019;10:1105.
- The Lancet. Therapeutics in The Lancet. Lancet. 2019;394:360.
- Kröncke T, David M. MR-guided focused ultrasound in fibroid treatment – results of the 4th radiological-gynecological expert meeting. Rofo. 2019;191(7):626–629.
- Pron G. Magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU) treatment of symptomatic uterine fibroids: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(4):1–86.
- Yu T, Luo J. Adverse events of extracorporeal ultrasound-guided high intensity focused ultrasound therapy. PLoS One. 2011;6(12):e26110.
- McDannold N, Tempany CM, Fennessy FM, et al. Uterine leiomyomas: MR imaging-based thermometry and thermal dosimetry during focused ultrasound thermal ablation. Radiology. 2006;240(1):263–272.
- Sridhar D, Kohi MP. Updates on MR-guided focused ultrasound for symptomatic uterine fibroids. Semin Intervent Radiol. 2018;35(1):17–22.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
- Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019;70:747–770.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Stuck AE, Rubenstein LZ, Wieland D, et al. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ. 1998;316(7129):469–469.
- Yoon S-W, Lee C, Cha SH, et al. Patient selection guidelines in MR-guided focused ultrasound surgery of uterine fibroids: a pictorial guide to relevant findings in screening pelvic MRI. Eur Radiol. 2008;18(12):2997–3006.
- [Consensus for diagnosis and treatment of uterine myoma]. Zhonghua Fu Chan Ke Za Zhi. 2017;52:793–800.
- Duc NM, Keserci B. Review of influential clinical factors in reducing the risk of unsuccessful MRI-guided HIFU treatment outcome of uterine fibroids. Diagn Interv Radiol. 2018;24(5):283–291.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25(5):1317–1328.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19(4):724–732.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30(5):771–777.
- Verpalen IM, Anneveldt KJ, Nijholt IM, et al. Magnetic resonance-high intensity focused ultrasound (MR-HIFU) therapy of symptomatic uterine fibroids with unrestrictive treatment protocols: a systematic review and meta-analysis. Eur J Radiol. 2019;120:108700.
- Spies JB. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(6):1427–1428. author reply 1428–1429
- Chen WZ, Tang LD, Yang WW, et al. [Study on the efficacy and safety of ultrasound ablation in treatment of uterine fibroids]. Zhonghua Fu Chan Ke Za Zhi. 2010;45:909–912.
- Gorny KR, Borah BJ, Brown DL, et al. Incidence of additional treatments in women treated with MR-guided focused US for symptomatic uterine fibroids: review of 138 patients with an average follow-up of 2.8 years. J Vasc Interv Radiol. 2014;25(10):1506–1512.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Kotani Y, Tobiume T, Fujishima R, et al. Recurrence of uterine myoma after myomectomy: open myomectomy versus laparoscopic myomectomy. J Obstet Gynaecol Res. 2018;44(2):298–302.
- Radosa MP, Owsianowski Z, Mothes A, et al. Long-term risk of fibroid recurrence after laparoscopic myomectomy. Eur J Obstetr Gynecol Reprod Biol. 2014;180:35–39.
- Sandberg EM, Tummers FHMP, Cohen SL, et al. Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and meta-analysis. Fertil Steril. 2018;109(4):698–707.e691.
- Froeling V, Meckelburg K, Schreiter NF, et al. Outcome of uterine artery embolization versus MR-guided high-intensity focused ultrasound treatment for uterine fibroids: long-term results. Eur J Radiol. 2013;82(12):2265–2269.
- Lénárd ZM, McDannold NJ, Fennessy FM, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery-imaging predictors of success. Radiology. 2008;249(1):187–194.
- Quinn SD, Vedelago J, Gedroyc W, et al. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2014;182:247–251.
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181–1188.
- Liu Y, Zhang WW, He M, et al. Adverse effect analysis of high-intensity focused ultrasound in the treatment of benign uterine diseases. Int J Hyperthermia. 2018;35(1):56–61.
- Suomi V, Komar G, Sainio T, et al. Comprehensive feature selection for classifying the treatment outcome of high-intensity ultrasound therapy in uterine fibroids. Sci Rep. 2019;9(1):10907.
- Mauermann ML, Amrami KK, Kuntz NL, et al. Longitudinal study of intraneural perineurioma-a benign, focal hypertrophic neuropathy of youth. Brain. 2009;132(Pt 8):2265–2276.