Abstract
Background
To evaluate the feasibility, efficiency, and safety of microwave ablation (MWA) for papillary thyroid carcinoma (PTC) close to the thyroid capsule.
Methods
The data of 106 cases who underwent thermal ablation from June 2014 to September 2020 were retrospectively analyzed. The mean follow-up time was 25 ± 11 months (range, 9–48 months). The strategy of fluid isolation was successfully applied in all cases, and all PTC nodules underwent extended ablation. The technical feasibility, technical success rate, and safety were analyzed. Changes in tumor size at different time points after MWA were evaluated.
Results
According to the contrast-enhanced ultrasound results after ablation, MWA has been successfully applied in all enrolled cases. The capsular ablation has also been achieved for all cases. Nodules in 71 cases (70.0%) completely disappeared in the follow-up period. No local recurrence was detected. The incidence of lymph node metastasis and new tumors was 1.9% (2/106) respectively. Light voice changes were the only complication, with a rate of 5.7% (6/106), which were relieved within 6 months after MWA. The size of the ablation zone increased firstly in 6 months after MWA compared with the pretreatment tumor size (p < 0.05). At 12, 18, 24, 30, 36 and 42 months after MWA, the ablation zone shrank and the sizes were smaller than the tumor size before MWA (p < 0.05 for all).
Conclusions
MWA is an effective, safe, and feasible method in treating PTC close to the thyroid capsule.
Background
With the improvement of early diagnostic techniques, the incidence of papillary thyroid carcinoma (PTC) has increased significantly in recent years, while disease-related mortality has not changed significantly [Citation1,Citation2]. This has led to the discussion of whether less aggressive treatment should be advocated for PTC [Citation3,Citation4]. The rate of lymph node metastasis in PTC was reported to be 40–90% [Citation5]. Previous studies showed that capsule invasion was an independent risk factor for lymph node metastasis (LNM) in PTC [Citation1,Citation2]. In addition, some studies have suggested that contact of more than 25% of the nodule with the adjacent capsule was independently predictive of LNM [Citation6,Citation7]. The thyroid capsule consists of two layers that are closely adjacent to and envelop the thyroid gland. The connective tissue of the inner layer penetrates through the parenchyma of the gland by forming several fiber bundles, which consist of blood and lymphatic vessels. This provides an anatomical basis for the higher incidence of lymph node metastasis associated with PTC nodules located close to the thyroid capsule. Several studies have examined the correlation between the distance from the capsule and LNM [Citation8,Citation9]. One study enrolled 174 patients, and the results showed that a distance ≥1.9 mm from the capsule was a strong indicator for the absence of LNM (p < 0.05) [Citation8]. Another study indicated that shorter distances between the nodule and the thyroid capsule resulted in a greater risk for LNM (p < 0.0001) [Citation9]. However, another study enrolled 1622 patients with PTC nodules and the results revealed that the distance of the tumor from the capsule was not associated with LNM in PTC (p > 0.05) [Citation10]. Therefore, the issue of LNM remains controversial when treating PTC close the thyroid capsule. Furthermore, a minimally invasive technique is needed to prevent overtreatment for PTC close to the thyroid capsule.
Ultrasound-guided minimally invasive treatments (MITs) for PTC include ethanol ablation, laser ablation, radiofrequency ablation, and microwave ablation [Citation11–15]. Ultrasound (US)-guided microwave ablation (MWA) has been reported to be a reliable minimally invasive technique for effectively and safely treating PTC [Citation16–23]. There has been no study investigating the specific application of MWA for PTC close to the thyroid capsule (≤2 mm). The aim of the present study was to assess the feasibility, efficacy, and safety of US-guided MWA for treating PTC close to the thyroid capsule.
Materials and methods
Patients
This retrospective study was approved by the Institutional Review Board of the China-Japan Friendship Hospital. Written informed consent was obtained from each patient before the ablation procedure. All patients consented to the anonymous publication of their examination results and radiological images.
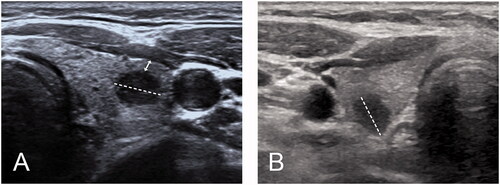
The distance was defined as the shortest distance from the boundary of the PTC nodule to the adjacent thyroid capsule, which was measured on transverse and longitudinal views (double-headed arrow ()). The distance between the PTC nodule and the thyroid capsule was recorded as adjacent when it was greater than 0 mm but no more than 2 mm on ultrasonography. The distance was recorded as 0 mm when the nodule abutted the thyroid capsule without invading surrounding tissues, and the echo of the thyroid capsule was still continuous and complete on ultrasonography. The distance was recorded as a capsular invasion (CI) when the echogenic line of the thyroid capsule was lost due to the PTC nodule [Citation24].
Figure 1. (A) Measurement of distance between PTC tumor to thyroid capsule 348 (double headed arrow); (B) PTC tumor abutted to thyroid capsule without CI.
According to the adjacent structure, the thyroid capsule was divided into anterior, medial, lateral, and posterior capsules on the transverse ultrasonographic views. The anterior capsule refers to the capsule close to the anterior cervical muscle, the medial capsule close to the trachea, the lateral capsule close to the carotid sheath, and the posterior capsule close to the retropharyngeal space.
Patients with PTC who underwent MWA therapy from June 2014 to September 2020 were enrolled. The inclusion criteria of this study were as follows: (1) pathological diagnosis of PTC based on fine-needle aspiration (FNA) results; (2) unifocal PTC abutted or adjacent to the thyroid capsule without CI on US examination (≤2 mm); (3) imaging examination showing no lymph node metastasis (LNM) or distant metastasis; and (4) follow-up time of more than 9 months after MWA (). The exclusion criteria of the present study included: (1) younger than 20 years or older than 80 years; (2) multifocal PTC; (3) PTC showed CI or minimal extra-thyroid extension (mETE) on US examination; (4) PTC with LNM or distant metastasis; (5) follow-up time of fewer than 9 months post-ablation ().
Table 1. Inclusion and exclusion criteria for study designs.
Pretreatment assessments
Before ablation, all patients underwent laboratory examinations, neck and chest computed tomography (CT) scans, US scans and FNA biopsies. Laboratory examinations mainly included routine blood examination and coagulation and thyroid function tests. CT scans were taken to diagnose LNM or distant metastasis. For US examination and FNA guidance, the ultrasound scanners GE LOGIQ E9 (GE Healthcare, Pittsburgh, PA, USA) and Aplio 500 (Toshiba, Tokyo, Japan) US units were used. BRAF V600E mutation tests and cytopathological analyses were conducted. Vocal cord function was also evaluated by US before ablation [Citation19]. Three orthogonal diameters of all PTC nodules, size, location, and distance to thyroid capsule, were recorded by US examination. Tumor volumes were calculated using the following equations: V = πabc/6 (V as volume, a as the largest diameter, and b and c as other two perpendicular diameters) and volume reduction ratio (VRR) = (initial volume − final volume)/initial volume [Citation20].
The medical records of patients were reviewed by three doctors, including the US examination results and CT images.
MWA procedure
MWA was performed by radiologists with more than 3 years of experience in MWA for thyroid nodules. Intravenous access was obtained through the antecubital vein before MWA. A contrast-enhanced US (CEUS) examination was conducted to assess the enhancement mode. 1% lidocaine was applied for local anesthesia. An 18-gauge needle was inserted between the nodule and surrounding critical structures for hydrodissection. After the hypoechoic band expanded to at least 5 mm, a 10-cm internally cooled MWA antenna (17 G) with a 0.3 cm tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China; or KY-2000, Kangyou Medical, Nanjing, China) was inserted into the PTC nodule under US guidance. The output power was 30 W. The strategy of multipoint ablation was applied. During ablation, normal saline was continuously injected as isolating fluid to maintain the thickness of the isolation band and prevent heat injury. The ablation was terminated after the hyperechoic ablation zone covered the whole tumor and extended at least 2 mm from the original tumor margin, including the adjacent thyroid capsule. CEUS was performed after WMA to confirm complete ablation, which indicated that the ablation zone completely covered the tumor and extended at least 2 mm beyond the margin of the tumor. Additional ablation was immediately performed if there was nodular enhancement inside the ablation zone or if the extended ablation zone was less than 2 mm. After the procedure, vocal cord function was reassessed to detect any heat injury to the recurrent laryngeal nerve (RLN) [Citation19]. Patients remained under observation for 2 h for potential complications.
Post-ablation assessment and follow-up visits
Patients attended follow-up every 3 months within the first year after MWA and then every 6 months thereafter. Clinical evaluations, ultrasound and laboratory tests were performed at each follow-up. The sizes of the ablation zones were evaluated, and the tumor volumes and percentage volume reductions were calculated and recorded. Patients underwent thorax and chest CT every year to screen for metastasis. CEUS and FNA were performed for suspected adverse events including local recurrences, new lesions, and metastatic lymph nodes. Technical feasibility was defined as the successful targeting of the tumor and performing of the ablation as planned. Technical success was defined as complete ablation at the end of every procedure.
Statistical methods
Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, USA). The data are presented as the mean ± standard deviation (SD), and the median and 25–75% interquartile range (IQR) were used if the data did not fit a normal distribution. Statistical tests were two-sided, and P values less than 0.05 were considered to indicate statistical significance.
The chi-square test and independent t-test were used to assess the heterogeneity of demographic characteristics between enrolled and excluded cases. To compare the changes in tumor size (maximum diameter and volume) before and after MWA, the Wilcoxon signed-rank test was used. The changes in thyroid function from before and after MWA were compared by paired t-test. The complication rates in the T1a and T1b subgroups were compared by Fisher’s exact test. Technical feasibility and technical success were evaluated. During follow-up, tumor disappearance, local recurrence, new tumors, LNM, and complication rates were also evaluated.
Results
Demographic and clinical characteristics of patients with PTC close to the thyroid capsule
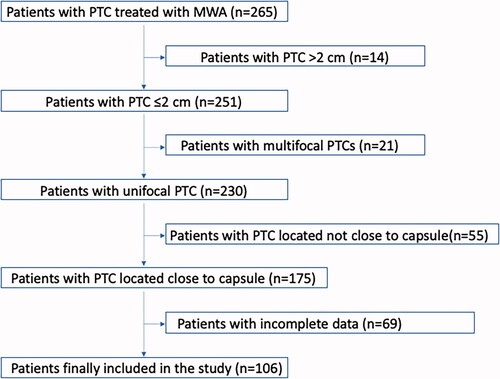
A total of 265 consecutively recruited patients with PTC underwent MWA therapy at our department from June 2014 to September 2020. According to the inclusion criteria and exclusion criteria, 159 patients were excluded, and 106 patients with PTC close to the thyroid capsule were enrolled in the present study (). Compared to the enrolled patients, there were no significant differences in the demographic characteristics of excluded patients (). The demographic characteristics of the enrolled patients are summarized in and . Of the 106 patients, 19 (17.9%) were men and 87 (82.1%) were women. The mean age was 44.39 ± 11.13 years (range, 26–74 years). 76 nodules abutted the thyroid capsule, and 30 nodules were adjacent to the thyroid capsule. Eighty-nine nodules belonged to the T1a subgroup, and 17 nodules belonged to the T1b subgroup. 14 (13.2%) patients had Hashimoto’s thyroiditis. 88 (83.0%) patients tested positive for the BRAF V600E mutation, 12 (11.3%) patients were negative, and 6 (5.7%) patients were not tested. The mean follow-up time was 24.95 ± 10.55 months (range, 9–48 months) after MWA, and 102, 88, 58, 37, 27, 14, and 6 patients attended follow-up for more than 12, 18, 24, 30, 36, 42 and 48 months, respectively. The mean volume for hydrodissection was 43.21 ± 22.62 ml (range, 50–80 ml).
Table 2. Demographic characteristics of enrolled and excluded cases in this study.
Table 3. Demographic characteristics of patients with PTC close to capsule included in this study (n = 106).
The detailed relationship between PTC nodules and the thyroid capsule
Thirty-five PTC nodules were adjacent to the capsule, of which 33 nodules were T1a class and 2 were T1b class. Seventy-one PTC nodules abutted the thyroid capsule, of which 36 nodules were T1a class and 15 were T1b class. 45 nodules abutted to thyroid capsule on one side, 22 nodules abutted two sides, and 4 nodules abutted three sides simultaneously. For those that abutted only one side of the thyroid capsule, 25 nodules abutted the anterior capsule, 6 abutted the lateral capsule, 6 abutted the posterior capsule and 8 abutted the medial capsule. For those that abutted two sides of the thyroid capsule, 11 nodules abutted the anterior and medial capsule simultaneously, 6 abutted the anterior and lateral capsule, 3 abutted the lateral and posterior capsule, and 2 abutted the posterior and medial capsule. For those that abutted three sides of the thyroid capsule, 2 nodules abutted the anterior, lateral and medial capsules simultaneously, 1 nodule abutted the lateral, posterior and medial capsules and 1 nodule abutted the anterior, lateral and posterior capsules.
Technical feasibility and technical success of MWA for treating PTC close to the thyroid capsule
All ablations were performed according to the protocol (). The median ablation time was 89 s (26-382 s). The median ablation time was 87.90 ± 43.15 s (26-223 s) for T1a PTC and 181.47 ± 91.30 s (64-382 s) for T1b PTC. According to the CEUS results after ablation, complete ablation was achieved in all patients. The rate of technical success was 100%.
Changes in thyroid function and size of PTC tumors close to the thyroid capsule after MWA
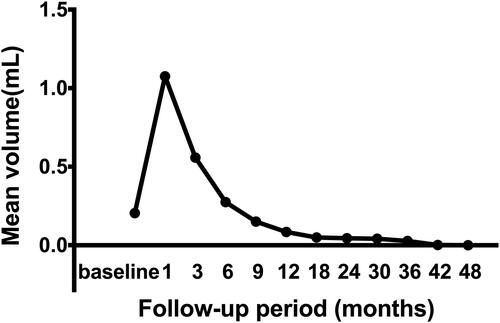
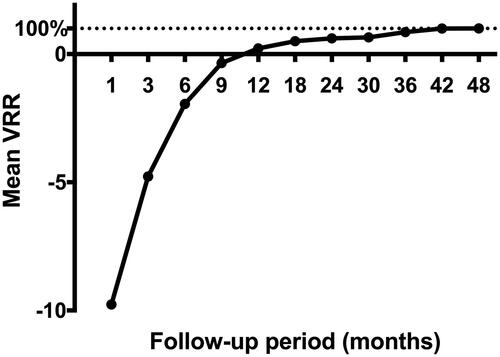
There was no significant difference in the measured values of T3, fT4, and TSH between pre-MWA and after MWA (). The nodules disappeared completely in 71 patients (71/106, 70.0%), in which 51 nodules disappeared at the 12th month after ablation, 11 at the 18th month, 4 at the 24th and 36th months and 1 at the 48th month. In PTC patients, the mean maximum tumor diameter was 0.77 ± 0.35 cm (range, 0.3–2 cm) before MWA, and the mean volume of target lesions was 0.20 ± 0.30 ml (range, 0.01–1.77 ml) before ablation. Significant differences were shown in the maximum diameters and mean tumor volume between the tumor pre-ablation and the ablation zone at each follow-up time point (p < 0.05), except for those between baseline and 9 months after ablation (). At the 1-, 3- and 6-month follow-ups, the sizes of the ablation zones were larger than those of the original tumor before MWA because of the expanding ablation procedure (p < 0.01). However, the sizes of the ablation zone at 12, 18, 24, 30, 36 and 42 months after MWA were smaller than those of the original tumor before MWA (p < 0.05). The mean tumor volume decreased from 0.20 ± 0.30 ml (range, 0.01–1.77 ml) before ablation to 0.04 ± 0.16 (range, 0.01–0.26 ml) at the 30-month follow-up (; ). The VRR value was negative at the 6-month follow-up but positive by 12 months after ablation (). There were significant differences in the VRR between every 2 follow-up time points before 24 months (p < 0.01). The mean VRR was 0.22 ± 2.45 (range: −19.3 to −1) at 12 months after ablation. There were no differences in mean VRR between the T1a and T1b subgroups. There were also no differences in mean VRR between the abutted and adjacent subgroups.
Figure 3. A 33-year-old woman with papillary thyroid cancer close to the capsule was treated with microwave ablation (MWA). (A) pre-MWA, ultrasound (US) showed a hypoechoic target tumor (arrows); (B) before MWA, contrast-enhanced US (CEUS) showed a hypo-enhancement pattern in the artery phase (arrows); (C) the hydrodissection technique (triangles) was used to protect the trachea surrounding the tumor (arrows); (D) US showed a hyperechoic pattern in the tumor (arrows) during ablation; (E) post-MWA, CEUS showed no enhancement (arrows) in the tumor area; and (F) on 1 month post-MWA, US showed a hypoechoic ablation zone (arrows).
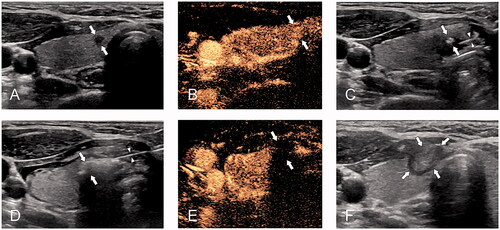
Table 4. Indicators of thyroid function before MWA and after MWA.
Table 5. Tumor size (maximum diameter and volume) before MWA and at each follow-up time-point after MWA.
Recurrence and metastasis after ablation
New lesions or LNM were detected in three patients (3/106, 2.83%). A new PTC nodule in the contralateral thyroid lobe along with metastatic lymph nodes occurred in one patient 12 months after ablation. A new PTC nodule was monitored in the contralateral thyroid lobe in the second patient 24 months after ablation. One ipsilateral metastatic lymph node was detected in the third patient at 12 months after ablation. Further ablations were employed to successfully inactivate all the new PTC nodules and metastatic lymph nodes. In the above patients, the original nodules were all T1a positive for the BRAF V600E mutation. No malignant thyroid nodules or LNM were detected in patients with T1b PTCs. The incidence of adverse events in the T1a subgroup (3/89, 3.4%) was higher than that in the T1b subgroup (0/17, 0%), but there was no significant difference (p > 0.05).
For the three patients who experienced adverse events, the original nodule and thyroid capsule was abutted in two patients and the distance between the nodule and the thyroid capsule was 0.7 mm in the third patient. After further ablation, no local recurrence, new recurrence, or distant metastases were detected during follow-up.
Complications of MWA in treating PTC close to the thyroid capsule
The treatment was well-tolerated by all patients, and no major complications were observed. Light voice changes were encountered as the primary complication in four patients (4/89, 4.49%) with T1a PTCs and two patients (2/17, 11.76%) with T1b PTCs. No significant difference was found in the incidence of light voice changes between the T1a and T1b subgroups (p > 0.05). Moreover, for the six nodules, 3 (3/6, 50%) nodules abutted the medial capsule, 1 (1/6, 16.7%) abutted the lateral capsule, and 2 (2/6, 33.3%) were adjacent to the posterior capsule. There was no significant difference in the incidence of light voice change between the abutted and adjacent subgroups (p > 0.05). The voice symptoms were relieved at 3 months in four patients, 4 months in two patients, and 6 months in one patient without any specific therapy. No hematomas or skin burns occurred.
Discussion
Surgery is considered to be the standard treatment for PTC [Citation25]. There is substantial controversy surrounding the optimum clinical treatment for PTC close to the thyroid capsule. Recent studies have shown that the application of thermal ablation for the treatment of T1aN0M0 and T1bN0M0 PTC achieves promising results [Citation19,Citation23,Citation26]. According to the 2021 European guidelines, thermal ablation could be considered in patients with low-risk PTMC and radioiodine-refractory metastases not amenable to surgery [Citation27,Citation28]. However, according to the 2019 edition of the ‘Expert Consensus Workshop Report: Guidelines for Thermal Ablation of Thyroid Tumours’, the distance between nodules and the posterior medial capsule should be >2 mm to benefit from ablation [Citation16]. At present, there is no relevant research on the thermal ablation of T1N0M0 nodules close to the thyroid capsule. This retrospective study was undertaken to assess the feasibility, efficacy, and safety of MWA for treating patients with T1N0M0 nodules abutted or adjacent to the thyroid capsule.
All 106 enrolled patients were ineligible for or refused surgery. All PTC nodules were radically ablated according to the described protocol, and no local recurrence was encountered in the follow-up period. The technical feasibility and success rate were both 100%. During a mean follow-up period of 24.95 ± 10.56 (range, 9–48) months, 71 nodules (70.0%) completely disappeared. The median VRR was 100% (range, 90.8–100%) at the 42-month follow-up. Meta-analyses indicated that the disappearance rate of PTC nodules varies from 34% to 91% post-ablation [Citation13,Citation14], which is comparable with the present study. Further analysis showed that there were no differences in the mean VRR between the T1a and T1b subgroups. In the present study, the rate of new tumors and LNM was 1.9%, which is comparable with the rate of 0.6% to 2.0% reported in previous studies [Citation14]. There was no local recurrence during follow-up in the present study, and the incidence of local recurrence was lower than 0.5% in previous studies [Citation29]. These results indicated that MWA is a potentially feasible and effective modality for treating T1N0M0 PTC nodules close to the thyroid capsule, especially when the patient is ineligible for or refuses surgery.
In the present study, no life-threatening complications were encountered. The overall complication rate was 5.7%, and the voice change rate was 5.7%. No significant difference was found between the T1a and T1b subgroups (p > 0.05). None of the patients developed permanent hoarseness. Previous studies showed that the overall rate of definitive complications was 7.1% after surgery, in which the persistent hypoparathyroidism rate was 1.7% and the rate of permanent palsy of the laryngeal recurrent nerve (LRN) was 1.0% [Citation30]. None of these complications occurred in the present study. In a meta-analysis of T1a PTC in 12 studies, the overall incidence of complications was 6.0% after ablation, in which the voice hoarseness rate was 4.4% [Citation31], which is comparable to the results of the present study. The complication rates were not significantly different between the abutted and adjacent subgroups (p > 0.05). In addition, when the nodule is close to the medial or posterior capsule, it is necessary to protect nerve function and prevent complications. Therefore, the present results indicate that MWA is a safe treatment for T1N0M0 PTC nodules close to the thyroid capsule.
In the present study, the lack of local recurrence and the low rate of complications after ablation may be attributed to some innovations in the strategy. Generally, when the PTC is close to the thyroid capsule, it is difficult to perform extended ablation to ensure complete ablation and protect surrounding structures. In the present study, the thyroid lobe was successfully separated from the surrounding critical structures through continuous injection of normal saline. This strategy could ensure complete ablation and protect surrounding structures from heat injury by creating a barrier and offers the following advantages: (1) Continuous injection of normal saline can maintain the distance between the thyroid capsule and critical structures during the whole ablation process, which effectively prevents heat damage; (2) Complete ablation is ensured by achieving an expanded ablation at least 2 mm outside of the thyroid capsule.
There were several limitations in this study. First, this was a retrospective nonrandomized study, which may have resulted in selection bias. Second, the diagnosis of PTC was based on cytopathology or histopathology instead of the complete pathology of the tumors. Therefore, the relationship between PTC nodules and thyroid capsules was only evaluated by ultrasound signs. There was no specific pathological diagnosis on capsule invasion or other minimal extra-thyroid extensions in the present study. Third, there were no other tumor markers beyond BRAF tested in the present study. In the end, the number of cases in this study was relatively small, and there were only 17 (17/106, 16.04%) patients in the T1b subgroup. Additionally, these patients in this study had relatively short follow-up times in relation to the indolent progression of PTC. Therefore, further studies are necessary to obtain more definite results.
Conclusions
In conclusion, MWA is a feasible, effective, and safe modality for treating T1N0M0 PTC nodules close to the thyroid capsule, especially for patients who are ineligible for or refuse surgery.
Ethics approval and consent to participate
Our retrospective study was approved by the institutional review board of our hospital. Written informed consent was obtained from each patient before the ablation procedure.
Consent for publication
The patients consented to publish their examination results and radiological images anonymously. Written informed consent was waived.
Author contributions
WJ, ZZL, WY, YMA have reviewed the ultrasound images and did the main measurement. WY, CXJ, PLL, and LY have made substantial contributions to study design and revised the manuscript critically; WJ drafted the article critically and contributed substantially to data collection and data analysis. All authors have provided final approval of the version to be published and have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Acknowledgments
We would like to thank all participants for their support in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Mendelsohn AH, Elashoff DA, Abemayor E, et al. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136(11):1055–1061.
- Saravana-Bawan B, Bajwa A, Paterson J, et al. Active surveillance of low-risk papillary thyroid cancer: a meta-analysis. Surgery. 2020;167(1):46–55.
- Koshkina A, Fazelzad R, Sugitani I, et al. Association of patient age with progression of low-risk papillary thyroid carcinoma under active surveillance: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(6):552–560.
- Lundgren CI, Hall P, Dickman PW, et al. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer-Am Cancer Soc. 2006;106(3):524–531.
- Cai YF, Wang QX, Ni CJ, et al. A scoring system is an effective tool for predicting central lymph node metastasis in papillary thyroid microcarcinoma: a case-control study. World J Surg Oncol. 2016;14(1):45.
- Jin WX, Ye DR, Sun YH, et al. Prediction of central lymph node metastasis in papillary thyroid microcarcinoma according to clinicopathologic factors and thyroid nodule sonographic features: a case-control study. Cancer Manag Res. 2018;10:3237–3243.
- Seong CY, Chai YJ, Lee SM, et al. Significance of distance between tumor and thyroid capsule as an indicator for central lymph node metastasis in clinically node negative papillary thyroid carcinoma patients. PLoS One. 2018;13(7):e200166.
- Wang QC, Cheng W, Wen X, et al. Shorter distance between the nodule and capsule has greater risk of cervical lymph node metastasis in papillary thyroid carcinoma. Asian Pac J Cancer Prev. 2014;15(2):855–860.
- Zhu M, Zheng W, Xiang Y, et al. The relationship between central lymph node metastasis and the distance from tumor to thyroid capsule in papillary thyroid microcarcinoma without capsule invasion. Gland Surg. 2020;9(3):727–736.
- Mauri G, Gennaro N, Lee MK, et al. Laser and radiofrequency ablations for benign and malignant thyroid tumors. Int J Hyperthermia. 2019;36(2):13–20.
- Lim HK, Cho SJ, Baek JH, et al. US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20(12):1653–1661.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720–731.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26(3):420–428.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab. 2020;105(6):dgaa128.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117.
- Li J, Liu Y, Liu J, et al. Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia. 2018;34(5):653–659.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581.
- Cao XJ, Zhao ZL, Wei Y, et al. Microwave ablation for papillary thyroid cancer located in the thyroid isthmus: a preliminary study. Int J Hyperthermia. 2021;38(1):114–119.
- Cao XJ, Wang SR, Che Y, et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: a multicenter retrospective study. Radiology. 2021;300(1):209–216.
- Cao XJ, Yu MA, Zhu YL, et al. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: a multicenter retrospective study. Int J Hyperthermia. 2021;38(1):916–922.
- Mauri G, Orsi F, Carriero S, et al. Image-guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol. 2020;11:575152.
- Chung SR, Baek JH, Choi YJ, et al. Sonographic assessment of the extent of extrathyroidal extension in thyroid cancer. Korean J Radiol. 2020;21(10):1187–1195.
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(12):1856–1883.
- Zhang Y, Zhang MB, Luo YK, et al. Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med. 2019;8(12):5450–5458.
- Mauri G, Hegedus L, Cazzato RL, et al. Minimally invasive treatment procedures have come of age for thyroid malignancy: the 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Cardiovasc Intervent Radiol. 2021;
- Mauri G, Hegedus L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197.
- Wang L, Xu D, Yang Y, et al. Safety and efficacy of ultrasound-guided percutaneous thermal ablation in treating low-risk papillary thyroid microcarcinoma: a pilot and feasibility study. J Can Res Ther. 2019;15(7):1522–1529.
- Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–276.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717.