Abstract
Purpose
This study aimed to compare the survival benefits between high-intensity focused ultrasound (HIFU) combined with chemotherapy and chemotherapy alone in patients with unresectable pancreatic ductal adenocarcinoma (PDAC).
Methods
All randomized clinical trials (RCTs) and observational studies were systematically searched through the databases of PubMed, EMBASE, CNKi and CQVIP up to December 2020. Case reports, case series and nonsystematic reviews were excluded. A meta-analysis was conducted to generate combined hazard ratios (HRs) with 95% confidence intervals (CI) for overall survival (OS).
Results
Seven trials, containing a total of 992 patients, were included in this study. The meta-analysis showed that a combination of HIFU and chemotherapy increased overall survival compared with chemotherapy alone, with a pooled HR of 0.40 (95% confidence interval [CI], 0.28–0.58). The combined therapy group had a significant advantage in 1-year survival rate (OR: 0.35, 95% CI: 0.22–0.53, p = 0.00). The trial sequence analysis (TSA) showed that there were enough trials to control for random errors.
Conclusion
Our analysis suggests that HIFU combined with chemotherapy intravenously will prolong survival for unresectable PDAC patients. The TSA showed that the survival benefit of combined therapy was definitive and there was no need to expand the sample size for repetitive exploration.
Keywords:
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death worldwide [Citation1]. It is only curable in a minority of patients with resectable disease. About 80% of PDAC are unresectable at diagnose. The 5-year survival rate at the unresectable stage is only 2% with minimal improvement in prognosis observed for the past decades [Citation2,Citation3]. Despite the development of targeted therapy and immunotherapy [Citation4–7], chemotherapy is still the main treatment for PDAC. According to different clinical trials, the median overall survival time is 8–11 months [Citation8–11].
Because of its special tumor microenvironment and anatomical location, pancreatic cancer has poor sensitivity to treatment and limited local treatment methods. Pancreatic cancer cells are surrounded by a large amount of extracellular matrix, which forms a biological barrier that can cause ischemia, hypoxia [Citation12] and increased osmotic pressure [Citation13–15]. At the same time, the pancreas is located close to the intestinal tract and blood vessels, increasing the difficulty of local treatment such as radiofrequency ablation and irreversible electroporation [Citation16–19].
High-intensity focused ultrasound (HIFU) uses ultrasound to penetrate tissues and focus ultrasonic heat on a tumor in vivo, so as to achieve tumor thermal ablation. HIFU provides a noninvasive and effective treatment for pancreatic cancer patients [Citation20]. So far, there have been numerous studies on HIFU for PDAC. These studies have shown that HIFU plays a very effective role in pain relief and tumor size reduction [Citation20–23]. Whether HIFU can prolong survival is still a matter of opinion.
Despite increasing evidence of the efficiency of HIFU, its status as a standard treatment remains unknown. Enhancing understanding of the role of HIFU in PDAC survival will improve clinical decision-making with respect to trial design and cancer treatment.
Methods
We followed the PRISMA Statement guidelines to design, analyze and report our meta-analytic findings [Citation24,Citation25].
Literature search
We reviewed PubMed, EMBASE, Web of science, Cohrane, CNKi and CQVIP databases for articles published before 10 December 2020. We identified studies by using Medical Subject Headings (MeSH) including pancreatic cancer, HIFU and chemotherapy. These terms were also combined with keywords and manual searches of references in all selected studies.
Selection criteria
The inclusion criteria were as follows: (i) survival comparisons were made between HIFU plus chemotherapy and chemotherapy alone in patients with pancreatic cancer; (ii) outcomes were related to overall survival; (iii) HR or survival curves were provided; (iv) articles were published in English or Chinese. In addition, articles were excluded if (i) no HRs or survival data were extracted; (ii) they were review articles; (iii) it was a single-arm study.
Study selection, data extraction and endpoints
Initial screening of potentially eligible records was performed by two investigators (Jing Guo, Yunbing Wang). Subsequent full-text record screening was performed independently by two investigators (Jing Guo, Jinyun Chen). Disagreements were resolved by consensus. Baseline characteristics and outcomes were extracted from the selected articles. We chose overall survival (OS) as our endpoint for meta-analysis.
Quality assessment
The quality of each study was independently rated by two investigators (Jing Guo, Wenzhi Chen), using the Newcastle-Ottawa Scale and Altman framework.
Statistical analysis
HR was used as a prognostic measurement. HRs and 95% confidence intervals (CI) were extracted from research studies. For studies without HRs and CIs, we used the extracted data from survival curves by Engauge Digitizer and calculated corresponding HRs and CIs using a method proposed by Parmar et al. (http://markummitchell.github.io/engauge-digitizer/). To improve accuracy, we excluded HRs derived from studies if the number of events in one group was less than 5. Heterogeneity was expressed by the I2 index (25% corresponds to low heterogeneity, 50% medium, 75% high). Random effects models were initially used to estimate pooled HRs. If there was no heterogeneity between the results (p > 0.05), the fixed-effect model was used. Then, subgroup analyses were performed on the basis of administration route (by venous, by a venous plus artery or by oral administration), treatment mode (once or repeated) and randomization. Publication bias was assessed by inspecting the symmetry of the funnel plot and tested with Egger’s test. STATA 15.0 was also used to test for publication bias. All other analyses were conducted using Review Manager 5.2.
Trial sequential analysis performance
Trial sequential analysis (TSA) is used to estimate the sample size of systematic review or meta-analysis, overcoming the shortcomings of classical systematic review or meta-analysis. First of all, when the number of cases included in the meta-analysis did not reach enough sample size, the application of TSA could minimize the false-positive results caused by random error. Secondly, meta-analysis is a retrospective study and the Required Information Size (RIS) obtained by TSA refers to the number of cases required for meta-analysis to obtain statistically significant differences. It is generally believed that the number of cases required for meta-analysis should not be less than the sample size required for a single well-designed randomized controlled trial with sufficient statistical certainty. Thirdly, meta-analysis aims to find evidence of the efficacy of medical interventions as early as possible. By estimating RIS, TSA provides a termination standard for clinical trials, that is, when the cumulative number of cases in meta-analysis reaches the expected information, similar clinical trials can be terminated, so as to avoid the waste of scientific research and medical resources.
TSA to assess for random errors and to calculate the required sample size by using an α of 0.05 (two-sided) and a β of 0.20 (80% power). The control group proportions were calculated from the chemotherapy group of the trials [Citation26–28].
According to our clinical experience, the truly effective treatments for reducing the risk of mortality had previously yielded a relative risk reduction of 35%, thus, an α-spending monitoring boundary was used to detect an intervention effect with a relative risk reduction set at a level of 35%. We used two-tailed tests because it was unclear whether a combination of HIFU and chemotherapy performed better than chemotherapy in terms of one-year OS. We applied a random-effects model according to the calculated I2 in the meta-analysis. TSA was performed by using TSA V.0.9.5.10 beta (http://www.ctu.dk/tsa/).
Results
Identification of relevant studies
Our initial search yielded 179 records. After reviewing the titles and abstracts, the full texts of 11 studies were examined. Four studies were further excluded for the following reasons: no comparisons between two groups or no survival data, such as HRs and survival curves. As a result, seven studies [Citation29–35] were included in this meta-analysis ().
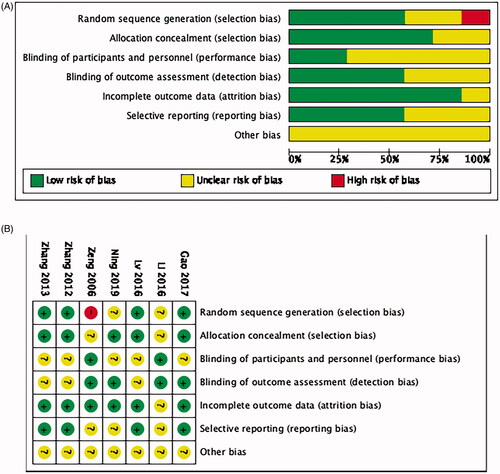
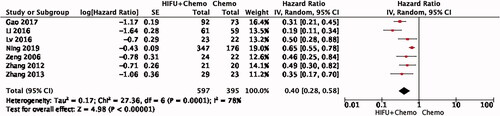
Risk of bias within studies
Quality evaluation of selected literature is summarized in . All of the trials were retrospective studies, not all of the studies used randomization, only four did. Five studies reported using allocation concealment. The allocation concealment methods were inconsistent. Because HIFU is an operational treatment, it cannot be done in a blind way. Even though, two of the study mentioned blinding.
Figure 1. Summarizes the identification of relevant studies [Citation29–35].
![Figure 1. Summarizes the identification of relevant studies [Citation29–35].](/cms/asset/4b280443-3e73-4db3-b0fe-02b9a4a88ed6/ihyt_a_1962550_f0001_b.jpg)
Study characteristics
Seven studies were included in this analysis. All these studies were conducted in China and published from 2006 to 2019. All patients enrolled had unresectable pancreatic cancer, classified as stage III and stage IV. In these studies, HIFU ablation was performed once in five studies and more than once in two. Gemcitabine-based chemotherapy was conducted in six studies, which served as the first-line therapy, while S-1 was chosen as the second-line therapy in one study ().
Table 1. Study characteristics in this analysis.
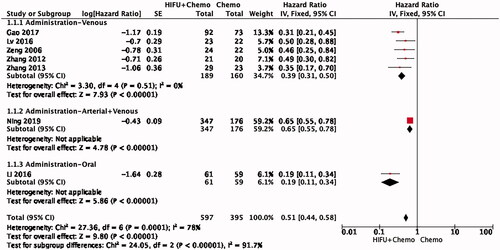
Effect of HIFU on the survival of PDAC patients
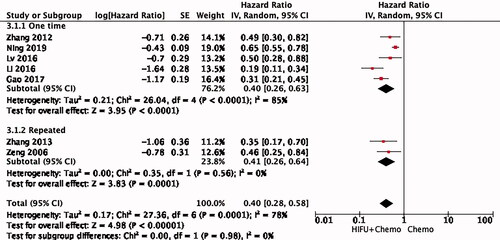
Seven studies were included in the analysis of PDAC OS. The combination of HIFU and chemotherapy had better OS than chemotherapy alone, with a pooled HR of 0.40 (95% CI: 0.28–0.58) using random mode (). Significant heterogeneity was found among these studies (I2 = 78%, p = 0.0001).
Subgroup analysis of HIFU in PDAC
To investigate potential sources of heterogeneity, we conducted a subgroup analysis.
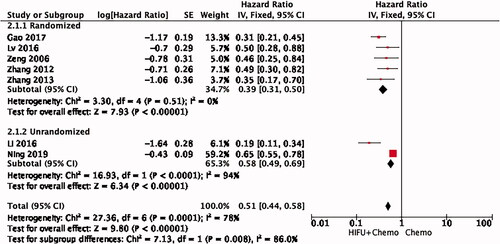
Subgroup analysis of administrative way
Among the seven studies, five were given chemotherapy intravenously, one included both intravenous and arterial chemotherapy and the other one was administered orally. Combination therapy has a significant advantage in prolonging survival in all three subgroups. The venous administration subgroup showed a high degree of consistency, with a pooled HR of 0.39 (95% CI: 0.31–0.50, I2 = 0%) by the fixed effect model ().
Randomization subgroup analysis
In five of the seven studies, patients were randomized and two did not mention how groups were formed. Therefore, we performed a subgroup analysis based on whether randomization was conducted (). Randomization may be another main source of heterogeneity (HR = 0.39, 95% CI: 0.31–0.50, I2 = 0%).
HIFU ablation schedule subgroup analysis
At the same time, we are very concerned about the effect of different HIFU treatment modes on the survival of PDAC patients. Due to different mechanical principles, some HIFU treatment systems can be used for one-time ablation, while others require repeated treatment. This subgroup analysis shows high heterogeneity in HIFU treatments received (HR = 0.40, 95% CI: 0.26–0.63, I2 = 85%) ().
TSA
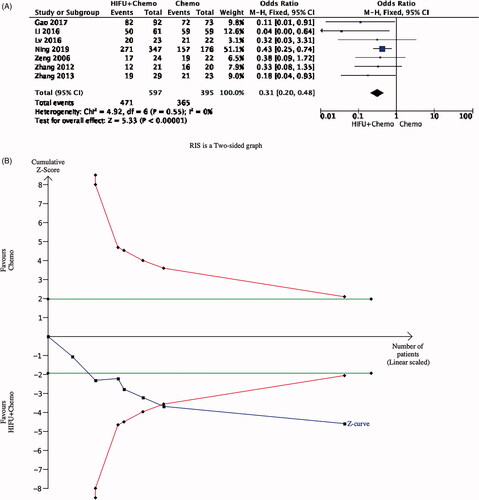
Since most patient survival time has been less than 1 year, one-year survival rates were also analyzed in patients with PDAC. The forest pilot showed that combined therapy had a significant advantage in 1-year survival rate (OR 0.35, 0.22–0.53). Given the small number of existing studies and patients, random errors are possible, which can cause spurious results. For this reason, we conducted TSA. From , we can see that the Z curve crossed the futility boundaries, but it did not reach the sequential monitoring boundary. This indicated that there are enough participants to confirm the survival benefit in HIFU ().
Figure 7. TSA analysis. The blue cumulative Z-curve was constructed by using a random-effects model. The horizontal green lines represent the conventional boundaries for benefit or harm. The red lines represent the trial sequential boundaries for benefit or harm. (A) Forest pilot of 1-year survival rate; (B) TSA of 1-year mortality.
Publication bias
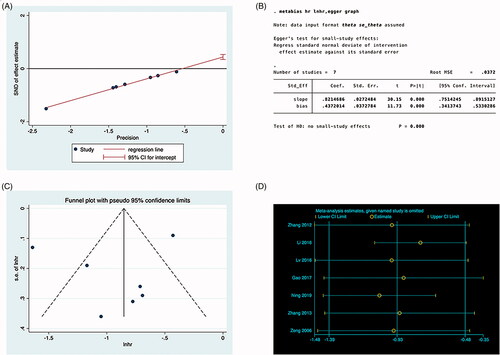
Formal investigation using Egger’s tests indicated publication bias in the meta-analysis. The results showed that there was no publication offset, and Egger’s test showed no statistical significance (p = 0.027) ().
Discussion
As far as we know, this is the first systematic review about the OS benefits of HIFU in PDAC patients by pooling HRs. We found HIFU plus chemotherapy will decrease the risk of death by 60%. To prevent the risk of random error and the multiplicity phenomenon due to repeated significance testing in the meta-analysis, we performed TSA. This further confirmed the deterministic effect of HIFU on prolonged survival.
HIFU has been applied in the treatment of pancreatic cancer for nearly 20 years [Citation36,Citation37]. A large number of studies have confirmed that HIFU has a significant effect on tumor shrinkage and pain relief [Citation21,Citation38,Citation39]. As for the survival time, many current studies involved a single-arm cohort. The median OS in PDAC with HIFU is from 8 months to 16.72 months [Citation40–43]. Holger et al. demonstrated that HIFU has an advantage in pancreatic cancers enveloping large mesenteric vessels [Citation21]. Zeng et al. [Citation29] firstly published a case-control study with an overall survival statistic in 2006. In the HIFU plus Gemcitabine cohort, the median survival time was 10.56 months, while it was 6.71 months in the controlled cohort. This result was less than the average result nowadays, taking into consideration the development of more chemotherapy regimens since 2010, such as nab-Paclitaxel and FOLFIRINOX. In 2019, Ning et al. [Citation35] demonstrated the survival benefit of HIFU among PDAC patients treated with Gemcitabine. The benefit was most obvious in PDAC patients treated with HIFU plus RIAC (regional intra-arterial chemotherapy) and systemic chemotherapy. However, the role of HIFU in the treatment of PDAC remains unclear due to a large number of uncontrolled studies. We hoped to provide clinicians with an exact treatment choice through meta-analysis.
There are several limitations to our study. First, most of the articles included in this meta-analysis were retrospectively small sample studies, lacking data from large-scale prospective clinical trials. Secondly, the included patients were all Chinese patients, which may be because HIFU technology was first developed and applied to clinical treatment in China, so a larger amount of patient data has been accumulated. However, further clinical research is needed to explore whether there are survival benefits for non-Chinese patients. Thirdly, in the excluded articles, we found that published abstracts or conference reports presented negative results [Citation44]. Without HR data or a survival curve, we could not obtain the data for analysis. This may produce publication bias to our findings. Finally, the included articles neither distinguished between stages nor distinguished between treatment lines. This still needs to be further explored for making accurate clinical treatment decisions.
This meta-analysis and TSA showed that HIFU combined with the chemotherapy treatment group has long-term survival benefits in patients. Based on these data, HIFU has given us great confidence in the treatment of unresectable pancreatic cancer, which is worthy of further promotion and application in subsequent clinical work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30.
- Hussain SP. Pancreatic cancer: current progress and future challenges. Int J Biol Sci. 2016;12(3):270–272.
- Daniel Ansari BT, Andersson B, Holmquist F, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929–1946.
- Li S, Xu HX, Wu CT, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36.
- Krepline A, Tsai S. Has personalized medicine for pancreatic cancer arrived? Adv Surg. 2019;53:103–115.
- Johansson H, Andersson R, Bauden M, et al. Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol. 2016;22(43):9457–9476.
- Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30(8):1232–1243.
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513–5518.
- Conroy T, Desseigne F, Ychou M, et al. ftGTDoUatP: FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-Paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703.
- Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–605.
- Erkan M, Kurtoglu M, Kleeff J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Rev Gastroenterol Hepatol. 2016;10(3):301–316.
- He G, Jiang Y, Zhang B, et al. The effect of HIF-1α on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014;23(1):174–180.
- Li D, Duell EJ, Yu K, et al. Pathway analysis of genome-wide association study data highlights pancreatic development genes as susceptibility factors for pancreatic cancer. Carcinogenesis. 2012;33(7):1384–1390.
- Giovannetti E, van der Borden CL, Frampton AE, et al. Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol. 2017;44:43–59.
- Gupta R, Amanam I, Chung V. Current and future therapies for advanced pancreatic cancer. J Surg Oncol. 2017;116(1):25–34.
- Reccia I, Kumar J, Habib N, et al. The use of radiofrequency ablation in pancreatic cancer in the midst of the dawn of immuno-oncology. Med Oncol. 2018;35(12):151.
- Ruarus AH, Vroomen L, Geboers B, et al. Percutaneous irreversible electroporation in locally advanced and recurrent pancreatic cancer (PANFIRE-2): a multicenter, prospective, Single-Arm, phase II study. Radiology. 2020;294(1):212–220.
- Tasu JP, Vesselle G, Herpe G, et al. Irreversible electroporation for locally advanced pancreatic cancer. Diagn Interv Imaging. 2016;97(12):1297–1304.
- Marinova M, Strunk HM, Rauch M, et al. High-intensity focused ultrasound (HIFU) for tumor pain relief in inoperable pancreatic cancer: evaluation with the pain sensation scale (SES). Schmerz. 2017;31(1):31–39.
- Strunk HM, Lutzow C, Henseler J, et al. Mesenteric vessel patency following HIFU therapy in patients with locally invasive pancreatic cancer. Ultraschall Med. 2018;39(6):650–658.
- Marinova M, Huxold HC, Henseler J, et al. Clinical effectiveness and potential survival benefit of US-guided high-intensity focused ultrasound therapy in patients with advanced-stage pancreatic cancer. Ultraschall Med. 2019;40(5):625–637.
- Marinova M, Wilhelm-Buchstab T, Strunk H. Advanced pancreatic cancer: high-Intensity focused ultrasound (HIFU) and other local ablative therapies. Rofo. 2019;191(3):216–227.
- Cheung MW, Vijayakumar R. A guide to conducting a meta-analysis. Neuropsychol Rev. 2016;26(2):121–128.
- David Moher LS, Clarke M, Ghersi D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38(1):276–286.
- Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75.
- Janice M, Pogue M, Yusuf S. FRCPC: cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta-analysis. Contr Clin Trials. 1997;18(6):580–593.
- Zeng Qingdong LL, Xiuguo Z, Bin L, et al. Clinical study of high intensity focused ultrasound combined with genze in the treatment of advanced pancreatic cancer. Chinese J Hepatobil Surg. 2006;12(11):748–750.
- Zhang Tao ZD, Wei L, Guojing W, et al. Clinical effect of high intensity focused ultrasound combined with gemcitabine in the treatment of unresectable pancreatic cancer. Compr Clin Pract China. 2012;28(8):793–796.
- Yu Z, Yongshuo J, Hong Z, et al. Efficacy of intensive focused ultrasound in the treatment of senile pancreatic cancer. Geriatrics Health Care. 2013;19(5):308–311.
- Lv W, Yan T, Wang G, et al. High-intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther Clin Risk Manag. 2016;12:687–691.
- Li X, Wang K, Zheng L, et al. Retrospective analysis of high intensity focused ultrasound combined with S-1 in the treatment of metastatic pancreatic cancer after failure of gemcitabine. Am J Cancer Res. 2016;6(1):84–90.
- Zhiqiang G, Gong Z, Yang W, et al. Application of HIFU combined with gemcitabine in the treatment of advanced pancreatic cancer. J Hepatobil Pancreatic Surg. 2018;30(2):98–101.
- Ning Z, Xie J, Chen Q, et al. HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients. OTT. 2019;12:1021–1029.
- Lynn JG, Zwemer RL, Chick AJ, et al. A new method for the generation and use of focused ultrasound in experimental BIOLOGY. J Gen Physiol. 1942;26(2):179–193.
- Van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66(2):247–258.
- Xiao W, Jingzhong S. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chinese. Chinese. 2002;115(9):1332–1335.
- Strunk HM, Henseler J, Rauch M, et al. Clinical use of High-Intensity focused ultrasound (HIFU) for tumor and pain reduction in advanced pancreatic cancer. Rofo. 2016;188(7):662–670.
- Wang K, Chen Z, Meng Z, et al. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27(2):101–107.
- Wu F, Wang Z-B, Zhu H, et al. Feasibility of US-guided High-Intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236(3):1034–1040.
- Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452.
- Sung HY, Jung SE, Cho SH, et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas. 2011;40(7):1080–1086.
- Shen H, Xiaoye H, Tianzhou C. Concurrent chemotherapy and high intensity focused ultrasound treatment for patients with local advanced pancreatic cancer or metastatic pancreatic cancer: a retrospective study. J Clin Oncol. 2014;32(15):e15242.