Abstract
Purpose
To investigate the effects of ablation time and distance between the radiofrequency ablation (RFA) electrode tip and a large vessel on the ablation zone in beagle livers.
Methods
Sixty-one percutaneous RFA coagulation zones were created near large vessels in 10 beagle livers in vivo. The ablated lesions were divided into four groups based on ablation time and distance between the electrode tip and a large vessel (group A, 3 min 0.5 cm; group B, 3 min 0 cm; group C, 5 min 0.5 cm; group D, 5 min 0 cm). The ablated area, long-axis diameters, short-axis diameters, and vessel wall injury were examined.
Results
With a fixed ablation time, the ablation zone created with the electrode tip at 0.5 cm from the large vessel was significantly larger than at 0 cm (p < .05). At a fixed distance between the electrode tip and vessel, the ablation zone created for 5 min was significantly larger than for 3 min (p < .05). The frequency of vessel wall injury in the 0 cm groups was significantly higher than that in the 0.5 cm groups (37.5% vs. 6.9%; p = .003, odds ratio, 7.43). The ratio of width to depth (Dw/Dz) was larger in the 0.5 cm groups than in the 0 cm groups (p < .001).
Conclusion
The ablation zone increased with longer ablation times and greater distances between the RFA tip and large vessels for perivascular lesions. The distance between the needle tip and blood vessels is an important factor that affects the overall ablation outcome.
Introduction
Radiofrequency ablation (RFA) is a safe and effective treatment option for malignant liver tumors [Citation1–3]. It induces tissue heating, which leads to coagulation necrosis and cell death [Citation4]. The main aim of RFA is to destroy the entire tumor in a minimally invasive manner without damaging adjacent vital structures. However, post-ablation recurrence is common (22–50%) [Citation5,Citation6] and adversely affects the survival of patients.
Tumor size [Citation7], multiple tumor nodules [Citation7] and large adjacent vessels [Citation8,Citation9] are important factors that affect recurrence. Specifically, some studies [Citation10,Citation11] have shown that it is difficult to achieve complete ablation for tumors that are near major vessels because of the heat sink effect. This is when blood flow dispels thermal energy away from the targeted tissue through vessels with a diameter ≥3 mm, leading to reduced coagulation volume and incomplete ablation [Citation11].
To address this limitation, two factors can be considered: reduced blood flow and increased energy deposition. Some clinical groups [Citation12,Citation13] have blocked or reduced blood flow in the liver using methods such as the Pringle maneuver (temporary hepatic inflow occlusion), which effectively increases the ablation area compared to ablation with sustained hepatic blood flow. However, the Pringle maneuver may exacerbate the systemic inflammatory response, thereby causing multi-organ injuries [Citation14]. Additionally, it is difficult to interrupt the blood flow of a large vessel during percutaneous RFA.
Current ablation devices can change the size and shape of the ablation zone by adjusting the power and timing of the generator. The relationship between these variables is complex and non-linear. Currently, ablation sizes provided by various manufacturers for certain ablation times are derived using ex vivo liver tissue. Studies have reported smaller ablation sizes than those reported in the manufacturer’s dataset [Citation15,Citation16]. Furthermore, for perivascular lesions, the distance between the needle tip and blood vessels may affect the overall ablation outcome as well as vascular integrity. There is no general consensus regarding the most appropriate distance [Citation17]. Therefore, the primary aim of the present study was to determine the effect of ablation time and the distance between the RFA electrode tip and a large vessel on the ablation area using in vivo experiments. The secondary aim was to assess the probability of thermal injury to the vessel wall in terms of the distance from the electrode tip and ablation time.
Materials and methods
Experimental animals
Ten beagles that were in good health (Fang Yuanyuan Breeding Base, Beijing, China; animal quarantine certificate no. 110334201100037963) were enrolled in this study; there were nine males and one female with a weight range of 12–16.5 kg. This study was approved by the Institutional Animal Care and Use Committee.
The beagles were fasted for 12 h before RFA. They were anesthetized with an intramuscular injection of 20 mg/kg ketamine hydrochloride (Ketalar; Southwest Pharmaceutical, Chongqing, China), and supplemental anesthesia was used during the treatment. After adequate anesthesia was achieved, the beagles were placed on the operating table in the lateral position. The hair on the belly and the posterior limbs were shaved, and the abdomen was disinfected. An electrode plate was attached to the prepared skin on the posterior limbs to form a complete electrode circuit.
Ultrasound and color Doppler ultrasonography were used to detect the diameter and location of the vessel, guide electrode placement and monitor the ablation procedure. Before treatment, the vessel wall thickness, vessel diameter and distance from the electrode tip to the vessel were recorded using ultrasound. The electrode was placed parallel to the long axis of the blood vessel (<30°), and the tip of the electrode was pushed into the target tissue to a depth of at least 2 cm to avoid exposing the bare end to the air. The distance between the electrode insertion points for multiple treatments on the same animal was at least 3 cm, to avoid merging of the respective ablation zones.
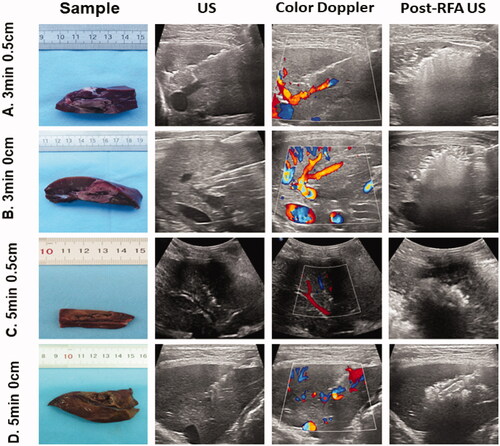
Lesions were grouped as follows: group A, the distance from the tip of the RFA electrode to a large vessel was approximately 0.5 cm and the ablation time set at 3 min (3 min 0.5 cm); group B, the electrode tip was near the large vessel and the ablation time was set at 3 min (3 min 0 cm); In groups C and D, the ablation time was set at 5 min, in group C, the distance was 0.5 cm (5 min 0.5 cm), and in group D, the electrode tip was near the large vessel (5 min 0 cm; ). Positioning the tip of the RFA electrode ‘near the large vessel (the 0 cm groups)’ indicates a distance of no more than 2 mm.
Figure 1. Evaluation of the distance between the radiofrequency ablation (RFA) electrode tip and large vessel, ablation time and specimens/images of different groups. Tissue specimens obtained after euthanizing beagles at 24 h after RFA (first column), conventional ultrasound before RFA (second column), color Doppler ultrasound before RFA (third column) and post-RFA ultrasound (fourth column) are shown. The first row represents a distance of 0.5 cm from the tip of the RFA electrode to a large vessel with the ablation time set at 3 min (3 min 0.5 cm). The second row shows the electrode tip near the large vessel with the ablation time set at 3 min (3 min 0 cm). The third row represents a distance of 0.5 cm from the tip of the RFA electrode to a large vessel with the ablation time set at 5 min (5 min 0.5 cm). The fourth row represents that the electrode tip was near the large vessel with the ablation time set at 5 min (5 min 0 cm).
RFA procedure
RFA was performed using a 90 W, 400 kHz monopolar radiofrequency generator (LDRF-120 S; Mianyang Lide, Sichuan, China). A single internal cooling electrode with a 2 cm exposed tip was used. Dynamic imaging was performed using ultrasound (EPIQ7; Philips, Bothell, WA, USA) with a L12-5 or C5-2 transducer. RFA systems used during this study effectively prevented tissue charring around the exposed tip of the electrode because of their internal cooling and impedance control system. During ablation, we used the set ablation time mode. The ablation device used 90 W of power in the default state. When impedance of the lesion tissue exceeded 300 Ω, the device automatically reduced the output power (5 W) to maintain the basic energy supply and allow heat to be transferred farther away from the electrode tip. When impedance was less than 300 Ω, after a few seconds, the equipment automatically continued to work at the rated power of 90 W. When the set ablation time was reached, the generator stopped automatically. The ablation pattern of the needle path was used for all lesions. Contrast-enhanced ultrasound was performed after RFA, and the ablation size was determined.
Tissue samples
At 24 h after ablation, the 10 beagles were euthanized. They were placed in the supine position, and the abdomen was opened with a midline incision in the upper abdomen. Then, the surrounding blood vessels and ligaments were cut to free the liver. Next, the dissected livers and lesions containing RF-induced coagulation were sliced parallel to the electrode tracks under ultrasound guidance. The length (Dl) of the ablation indicated the distance between the proximal and distal edges of the ablation zone following the ablation needle tract, and the width (Dw) was perpendicular to the Dl on the slice. The area was manually drawn and automatically calculated using imaging analysis software (ImageJ v. 1.42; National Institutes of Health, Bethesda, MD, USA) [Citation16] (Supporting Information Figure S1). Another linear dimension, depth (Dz), was the maximum ablation diameter perpendicular to Dl and Dw. To compare the geometries of the ablation zones among the experimental groups, the ratio of Dw to Dz was calculated, measuring the circularity (isometric ratio) of coagulation on a specimen slice [Citation18]. Livers containing RF-induced coagulation were sliced parallel to the electrode tracks. The excised liver samples were fixed with 10% formalin and dehydrated with 75% alcohol. The destruction of the blood vessel wall and thrombus formation were evaluated using hematoxylin and eosin and immunofluorescence staining.
Statistical analyses
Continuous data are shown as the mean ± standard deviation. A one-way analysis of variance (ANOVA) was performed, and all variables were normally distributed according to the Shapiro–Wilk test for normality. Based on the ANOVA results, the Games–Howell post hoc test was applied to compare any two groups. The prevalence of vessel wall injury was compared using the Chi-squared test and Fisher’s exact test. Logistic regression models were fitted to the binary target variables. Statistical analyses were performed using SPSS (version 21.0; SPSS, Chicago, IL, USA). Statistical significance was set at p < .05.
Results
The mean age of the 10 beagles was 2.6 ± 0.18 years (range, 2.3–3.0 years), and the mean weight was 13.75 ± 0.68 kg (range, 12–16.5 kg). A total of 61 ablations (mean, 6.1 ablations per animal; range, 3–8) were performed during this study. The mean distance between the ablation probe active tip and the liver surface was 3.24 ± 0.79 cm (range, 2.2–5.0 cm). The mean Dl, Dw, Dz and area of coagulation were 2.79 ± 0.33 cm (range, 2.07–3.32 cm), 1.64 ± 0.31 cm (range, 2.28–1.10 cm), 2.06 ± 0.21 (range, 1.78–2.50 cm) and 4.03 ± 0.99 cm2 (range, 2.66–6.16 cm2), respectively. The values for the four groups are provided in . The mean vessel diameter was 0.34 ± 0.04 cm (range, 0.30–0.44 cm). The mean vessel wall thickness was 0.72 ± 0.22 mm (range, 0.50–1.40 mm). No statistical differences were observed among the four groups ().
Table 1. Comparison of the ablation zones among different groups.
Comparison of distance and fixed time
A comparison of two distances with a time fixed of 3 min showed that the Dl created by a distance of 0.5 cm was significantly larger than that created by a distance of 0 cm (Dl: 2.85 ± 0.34 cm vs. 2.47 ± 0.19 cm; p = .001). The Dw and Dz created with a distance of 0.5 cm were significantly larger than those created with a distance of 0 cm (Dw: 1.66 ± 0.20 cm vs. 1.33 ± 0.14 cm, p < .001; Dz: 1.96 ± 0.08 cm vs. 1.89 ± 0.73 cm, p = .019). The ablation area created with a distance of 0.5 cm was significantly larger than that created with a distance of 0 cm (4.14 ± 0.71 cm2 vs. 2.93 ± 0.14 cm2; p < .001; ).
Figure 2. Comparison of protocols with a fixed time. The graph depicts the mean (± standard deviation) distance and area of the two groups. (A) With the time fixed at 3 min, the ablation length, width, depth and area created using a distance of 0.5 cm were significantly larger than those created using a distance of 0 cm (length: p = .001; width: p < .001; depth: p = .019; area: p < .001). (B) With the time fixed at 5 min, there was no significant difference when the distances were 0 cm and 0.5 cm (length: p = .140; depth: p = .611). The ablation width and area when the distance was 0 cm were significantly smaller than when the distance was 0.5 cm (width: p = .007; area: p = .001). ***p < .001. **p < .01. *p < .05.
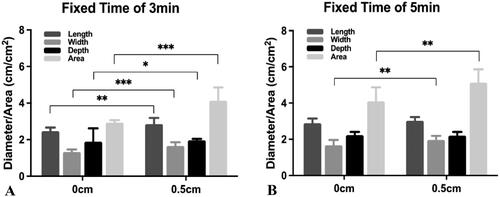
A comparison of two distances with a time fixed of 5 min showed no statistically significant differences in the Dl and Dz (Dl: 2.89 ± 0.26 vs. 3.02 ± 0.21 cm, p = .140; Dz: 2.23 ± 0.18 cm vs. 2.20 ± 0.21 cm, p = .611). The Dw and area with a distance of 0 cm were significantly lower than those with a distance of 0.5 cm (Dw: 1.67 ± 0.29 cm vs. 1.96 ± 0.23 cm, p = .007; area: 4.10 ± 0.77 cm2 vs. 5.13 ± 0.74 cm2, p = .001; ).
Comparison of protocols with a fixed distance
With a fixed distance of 0 cm, a statistically significant difference was observed between ablation times of 3 min and 5 min (Dl: 2.47 ± 0.19 cm vs. 2.89 ± 0.26 cm, p < .001; Dw: 1.33 ± 0.14 cm vs. 1.67 ± 0.29 cm, p < .001; Dz: 1.89 ± 0.07 cm vs. 2.23 ± 0.18 cm, p < .001; area: 2.93 ± 0.14 cm2 vs. 4.10 ± 0.77 cm2, p < .001; ).
Figure 3. Comparison of protocols with a fixed distance. The graph depicts the mean (± standard deviation) distance and area of the two groups. (A) At a fixed distance of 0 cm, the statistically significant differences were observed when the ablation times were 3 min and 5 min (length: p < .001; width: p < .001; depth: p < .001; area: p < .001). (B) At a fixed distance of 0.5 cm, the ablation length created with an ablation time of 3 min was not statistically different from that created with an ablation time of 5 min (length: p = .112). The ablation width and ablation zone created with an ablation time of 5 min were significantly larger than those created with an ablation time of 3 min (width: p = .001; depth: p = .002; area: p = .001). ***p < .001. **p < .01. *p < .05.
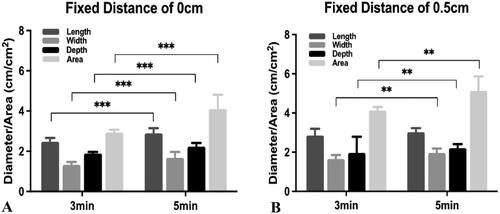
With a fixed distance of 0.5 cm, the length created with an ablation time of 3 min was 2.85 ± 0.34 cm, and the length created with an ablation time of 5 min was 3.02 ± 0.21 cm; however, no statistically significant difference was observed (p = .112). The Dw, Dz and area created with an ablation time of 5 min were significantly greater than those created with an ablation time of 3 min (Dw: 1.96 ± 0.23 cm vs. 1.66 ± 0.20 cm, p = .001; Dz: 2.20 ± 0.21 cm vs. 1.96 ± 0.83 cm, p = .002; area: 5.13 ± 0.74 cm2 vs. 4.14 ± 0.71 cm2, p = .001; ).
Gross observations and the shape of the ablation zone
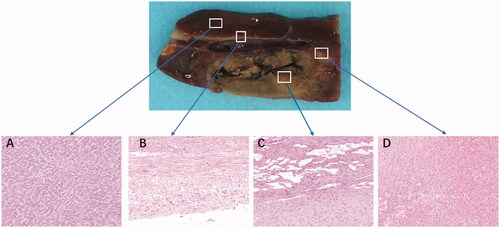
After dissection, the gross specimens revealed that the center needle track was gray, the ablation necrosis zone was brown and yellow, and the pink outer area was the RFA necrosis area–normal liver tissue transition area. The normal liver tissue around the RFA zone was red and dark red ().
Figure 4. Comparison of the ablation zones in the specimens and hematoxylin and eosin tissue taken from different areas using a light microscope (×200). (A) Normal liver. (B) Vascular wall. (C) Central ablation zone. (D) Peripheral ablation zone. All specimens were obtained 24 h after ablation.
In the 0.5 cm groups, the shape of the RFA ablation was similar to being ellipsoidal and the ratio of Dw to Dz was 0.87 ± 0.07 (3 min and 0.5 cm: 0.85 ± 0.09; 5 min and 0.5 cm: 0.89 ± 0.03). In the 0 cm groups, the shape was irregular, there was a depression in the ablation lesion near the blood vessel (Supporting Information Figure S2), and the ratio of Dw to Dz was 0.73 ± 0.07 (3 min and 0 cm: 0.71 ± 0.06; 5 min and 0 cm: 0.75 ± 0.09). In summary, the ratio of Dw to Dz was larger in the 0.5 cm groups than in the 0 cm groups (p < .001).
Pathological evaluation of the vessels above the ablation zone
H&E and TUNEL staining of the ablation samples showed vascular wall destruction in 5 of 16 (31.3%) lesions in group B (3 min 0 cm), 7 of 16 lesions (43.8%) in group D and 2 of 13 (15.4%) lesions in group C (5 min 0.5 cm). However, the vascular wall was not obviously obliterated in group A (3 min 0.5 cm). There were obvious differences among the four groups (p = .010), and the frequency of vascular wall injury in group D (5 min 0 cm) was significantly higher than that in group A (3 min 0.5 cm) (p = .007). There was one thrombus in group B (3 min 0 cm) and one thrombus in group D (5 min 0 cm; and ).
Figure 5. Pathological staining and TUNEL immunofluorescence staining results of beagle liver vessels. Results of pathological staining with hematoxylin and eosin (HE), staining with DAPI (blue), co-stained with TUNEL (green representing apoptosis) and merging (blue and green) (from the first column to the fourth column) (×50). The vascular wall cells are still well-formed in (A–D). The vascular wall is damaged in (E–H). The thrombus is observed in (I–L).
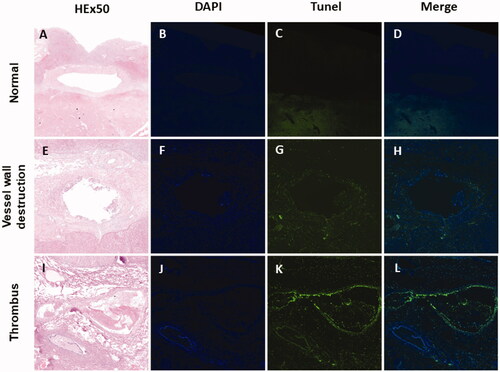
Table 2. Pathological evaluation of the vessels.
The frequency of vessel wall injury in the 5 min group was higher than that in the 3 min group; however, there was no statistical difference between these two groups (15.6% vs. 31.0%; p = .153). However, the frequency in the 0 cm group (12/32) was significantly higher than that in the 0.5 cm group (2/29) (37.5% vs. 6.9%; p = .003; ). Logistic regression showed that the distance between the RFA electrode tip and large vessel was an important factor affecting vascular wall destruction (p = .014). The probability of vascular wall injury in the adjacent blood vessel group was significantly higher than that in the 0.5 cm group (odds ratio, 7.43; 95% confidence interval, 1.49–36.93).
Discussion
In this study, we explored an extreme needle placement strategy for perivascular RFA targets, providing for a minimum distance between the electrode tip and blood vessel wall (0 cm groups). Under these operating conditions, despite the continuous heat removal created by blood circulation, vessel wall injury and thrombosis were systematically observed. Therefore, great caution should be used in clinical scenarios when ablating tumors in close proximity to vessels that need to be protected from thermal damage.
Currently, the treatment of adjacent vascular lesions is a difficult clinical problem. Surgical resection is the most effective treatment for liver cancer. However, some lesions are located close to a main blood vessel, and surgical treatment sacrifices significant liver volume and may be intolerable for the patient. RFA is a minimally invasive treatment that can be used for perivascular tumors. However, the outcomes of RFA for perivascular tumors have been inconclusive. Other studies have reported lower progression-free survival rates because of the heat sink effect [Citation19,Citation20]. Moreover, some researchers have shown that the long-term therapeutic outcomes of RFA for perivascular liver cancer are similar to those for non-perivascular lesions [Citation2,Citation21].
This in vivo study shows that ad hoc RFA protocols (in terms of electrode placement strategy, treatment power and time settings) are feasible for obtaining consistent ablation volumes while ensuring the integrity of nearby vessels. Continuous thermal ablation of tumors close to large vessels could compensate for the limitations of the heat sink effect inside large vessels. Therefore, during this study, we created 3 min groups and 5 min groups to compare the effects of different ablation durations. Our results revealed that when using a fixed distance, the zone with an ablation time of 5 min was significantly larger than that of 3 min. This indicates that appropriate extension of the ablation time may increase the ablation area. These results are similar to those of previous studies [Citation22,Citation23]. Because the blood vessel flow removes heat, the blood vessel wall may be protected. No distance between a large vessel and the RFA electrode has been defined as safe. Huang et al. [Citation17] showed that RFA is relatively safe and not a threat to the patient’s safety when the distance between the needle tip and blood vessels is more than 2 mm. Although vascular integrity may be affected, no thrombosis or stenosis was observed. Another study [Citation24] showed that when the distance between the tip of the RFA electrode and the portal vein was <5 mm, thermal injury to the liver tissue and corresponding vessel occurred. Therefore, we set different distances between the needle tip and blood vessel wall to compare the effects on ablation. Our results revealed that using a fixed time, the ablation zone created with a distance of 0.5 cm between the electrode tip and large vessel was significantly larger than that created with a distance of 0 cm. The probability of vascular wall injury in the adjacent blood vessel group (0 cm group) was significantly higher than that in the 0.5 cm group.
During this study, the mean value of circularity (Dw/Dz) of coagulation on a specimen slice was <1 (0 cm group: 0.87 ± 0.07; 0.5 cm group: 0.73 ± 0.07; p < .001). These data and shape indicate the inability of the ablation zone to cross the adjacent vessel, the lack of symmetry of the ablation zone with regard to the RF electrode and the non-negligible influence of blood perfusion on the thermal treatment outcome. Hence, if the intended ablation target was positioned across the vessel, then it would be impossible perform complete ablation with a single deposition of energy; an overlapping ablation technique would be required to achieve lager ablation zones with multiple depositions of energy all around the vessel.
Although RFA is a minimally invasive treatment method, it involves the risk of bleeding, vascular wall damage and thrombus formation in lesions adjacent to the blood vessel. However, mild treatment side effects that are not a threat to the patient’s safety in clinical practice are acceptable. During this study, we observed that distance is an important factor affecting ablation outcomes in vivo. The adjacent blood vessel group (0 cm group) may incur thrombus formation; therefore, a safe distance should be considered for perivascular lesions undergoing RFA.
During this study, we probed several issues of clinical concern. First, this pilot study explored ways to reduce the influence of the heat sink effect at the technical level in vivo. Second, the results indicated the clinically important fact that ablation may cause thrombosis when the needle tip is near the blood vessel wall (0 cm). Finally, the results indicated that an appropriate extended ablation time is relatively safety and can increase the ablation zone.
Our study had several limitations. First, the long-term prognosis for vascular wall injury was not investigated because of the lack of short-term follow-up. Second, RFA was performed for healthy beagle livers without tumors. During RFA, heat transfer is different in normal liver tissue and tumor tissue [Citation20]; specifically, tumor tissue seems to be more sensitive to heat damage than normal liver tissue [Citation25]. Third, our study did not include computed tomography or magnetic resonance imaging to assess the extent of tissue ablation. However, contrast-enhanced ultrasound, pathology and immunohistochemistry were used to measure the extent of coagulation necrosis. Fourth, the size of the beagle liver is small, and the ablation length was reported to be close to the distance between the electrode tip and liver surface in all experimental groups. This means that the overall ablation shape and size may have been partly affected by convective phenomena at the hepatic capsule, as indicated by previous studies [Citation18]. Therefore, the reported data may not be representative of the actual coagulative performance using equal RFA settings in a deep location. Fifth, only one RFA system, two ablation time points and two electrode-vessel distances were explored; therefore, the reported findings cannot be retained as fully representative of the outcomes of perivascular RFA in general, nor of the variability trends of the individual quantities subject to investigation. Furthermore, a full three-dimensional characterization of the ablation (i.e., calculation of the ablation volume) was not performed because of the irregular shape of the ablation zone. The commonly used ellipsoid volume formula [Citation18,Citation26] may overestimate the ablation zone; however, new software measurement technologies may facilitate more accurate volume calculations. Finally, blood flow and actual RF energy deposition, which are key to the quantification of heat sinking, were not explicitly measured. Despite these shortcomings, this preliminary study involving an in vivo experiment provides a reliable basis for verifying the effects of ablation time and the distance between the RFA electrode tip and a large vessel on the ablation area.
Conclusion
Our preliminary results showed that the ablation zone increased with the extension of time and increased distance between the RFA electrode tip and the large vessels of perivascular lesions. Additionally, the distance between the needle tip and blood vessels is an important factor that affects the overall ablation outcome, as well as vascular integrity, and should be carefully considered when planning RFA treatments of perivascular lesions.
Author contributions
B.J. and K.Z. contributed equally to this study. Study design: K.Y., B.J., K.Z., S.W. and Y. M.; experimental studies: S.W., Y.M., B.J, B.L., K.Z. and H.W.; pathological analysis: H.W. and H.W.; statistical analysis and manuscript editing: B.J., K.Y., K.Z. and B.L.
Supplemental Material
Download PDF (168 KB)Acknowledgments
We would like to acknowledge equipment-related support from Lide Medical System and Philips Healthcare.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109(9):djx015.
- Jiang B, Luo H, Yan K, et al. Ten-year outcomes of percutaneous radiofrequency ablation for colorectal cancer liver metastases in perivascular vs. non-perivascular locations: a propensity-score matched study. Front Oncol. 2020;10:553556.
- Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70.
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13(2):129–147.
- Shiozawa K, Watanabe M, Wakui N, et al. Risk factors for the local recurrence of hepatocellular carcinoma after single-session percutaneous radiofrequency ablation with a single electrode insertion. Mol Med Rep. 2009;2(1):89–95.
- Yang B, Zou J, Xia J, et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur J Radiol. 2011;79(2):196–200.
- Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10(1):52–58.
- Vietti Violi N, Duran R, Demartines N, et al. Local recurrence rate in patients with colorectal cancer liver metastasis after wedge resection or percutaneous radiofrequency ablation. Int J Hyperthermia. 2018;34(7):1020–1028.
- Toshimori J, Nouso K, Nakamura S, et al. Local recurrence and complications after percutaneous radiofrequency ablation of hepatocellular carcinoma: a retrospective cohort study focused on tumor location. Acta Med Okayama. 2015;69(4):219–226.
- Tamaki K, Shimizu I, Oshio A, et al. Influence of large intrahepatic blood vessels on the gross and histological characteristics of lesions produced by radiofrequency ablation in a pig liver model. Liver Int. 2004;24(6):696–701.
- Lu DS, Raman SS, Vodopich DJ, et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178(1):47–51.
- Poch FGM, Neizert CA, Geyer B, et al. Influence of interapplicator distance on multibipolar radiofrequency ablation during physiological and interrupted liver perfusion in an in vivo porcine model. Sci Rep. 2020;10(1):16210.
- Poch FGM, Neizert CA, Gemeinhardt O, et al. Intermittent pringle maneuver may be beneficial for radiofrequency ablations in situations with tumor-vessel proximity. Innov Surg Sci. 2018;3(4):245–251.
- Ypsilantis P, Lambropoulou M, Anagnostopoulos C, et al. Pringle maneuver exacerbates systemic inflammatory response and multiple-organ injury induced by extended liver radiofrequency ablation. Hum Exp Toxicol. 2011;30(11):1855–1864.
- Vahldiek JL, Erxleben C, Bressem KK, et al. Multipolar RFA of the liver: influence of intrahepatic vessels on ablation zones and appropriateness of CECT in detecting ablation dimensions - results of an in-vivo porcine liver model. Clin Hemorheol Microcirc. 2018;70(4):467–476.
- Jiang AN, Wang S, Yang W, et al. The role of a curved electrode with controllable direction in the radiofrequency ablation of liver tumors behind large vessels. Cardiovasc Intervent Radiol. 2019;42(6):893–904.
- Huang J, Li T, Liu N, et al. Safety and reliability of hepatic radiofrequency ablation near the inferior vena cava: an experimental study. Int J Hyperthermia. 2011;27(2):116–123.
- Lee JM, Han JK, Kim HC, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007;42(3):163–171.
- Lee S, Kang TW, Cha DI, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69(1):70–78.
- Ahmed M, Liu Z, Afzal KS, et al. Radiofrequency ablation: effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;230(3):761–767.
- Kang TW, Lim HK, Lee MW, et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: retrospective comparison of long-term therapeutic outcomes. Radiology. 2014;270(3):888–899.
- Cosman ER Jr, Dolensky JR, Hoffman RA. Factors that affect radiofrequency heat lesion size. Pain Med. 2014;15(12):2020–2036.
- Patterson EJ, Scudamore CH, Owen DA, et al. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227(4):559–565.
- Feng J, Wang S, Jiang K. Influence of the heat irrigating effect of radiofrequency ablation on regional liver tissue in Bama miniature pigs. World J Gastrointest Oncol. 2021;13(2):109–118.
- Maehara Y, Kusumoto T, Kusumoto H, et al. Excised human neoplastic tissues are more sensitive to heat than the adjacent normal tissues. Eur Surg Res. 1988;20(4):254–259.
- Cha DI, Lee MW, Jeong WK, et al. Comparison of ablation performance between dual internally cooled wet tip and conventional dual internally cooled tip radiofrequency electrodes: an experimental study in ex vivo bovine liver. Int J Hyperthermia. 2021;38(1):332–340.
