Abstract
Purpose
Concurrent chemoradiotherapy (CCRT) is recommended as the standard treatment for locally advanced cervical cancer (LACC). However, the synergistic effect of hyperthermia (HT) with CCRT remains unclear. Therefore, we performed a meta-analysis to evaluate the effect of HT with CCRT on LACC patients.
Methods and materials
A systematic literature search was conducted on the MEDLINE, PubMed, Embase, Cochrane library and SCOPUS databases for articles that compared CCRT with HT and CCRT alone as treatments for LACC. Hazard ratios (HRs) and risk ratios (RRs) were used to compare five-year overall survival (OS), local relapse-free survival (LRFS) and incidence of acute and chronic toxicity between the two treatments.
Results
Two articles out of 2860 were finally selected for analysis. A total of 536 patients were evaluated (CCRT with HT group: 268, CCRT group: 268). FIGO stages I–II and III–IV were found in 295 (55.0%) and 241 patients (45.0%), respectively. The CCRT with HT group had significantly better five-year OS than the CCRT group (HR 0.67, 95% confidence interval [CI] 0.47–0.96, p = 0.03). LRFS of patients was superior in the CCRT with HT group than in the CCRT group, but without significance (HR 0.74, 95% CI 0.49–1.12; p = 0.16). Moreover, there was no difference between the two groups regarding acute and chronic toxicity.
Conclusion
This systematic review and meta-analysis showed that CCRT with HT significantly improved OS in LACC patients without increasing acute and chronic toxicity. Therefore, tri-modality treatment could be a feasible approach for patients with LACC.
Introduction
Cervical cancer is the fourth most common cancer in women globally, and it accounted for approximately 570,000 newly diagnosed cases and 311,000 deaths in 2018 [Citation1]. In South Korea, the incidence of cervical cancer is decreasing owing to the nationwide human papillomavirus vaccination and cervical cancer screening programs [Citation2]; however, it remained the ninth most common cancer among women in 2019 [Citation3]. Radical surgery is an effective treatment modality for patients with early-stage cervical cancer. However, cisplatin-based concurrent chemoradiotherapy (CCRT) is recommended for locally advanced cervical cancer (LACC) patients as a few randomized trials and meta-analyses have proven the efficacy of CCRT in LACC [Citation4–9].
Hyperthermia (HT), which involves exposing body tissues to artificially elevated temperatures of ≥40 °C, has been known to play a role in efficiently killing cancer cells [Citation10,Citation11]. Preclinical studies have also reported that HT could act as an effective sensitizer for radiotherapy (RT) and chemotherapy (CT) through certain mechanisms such as increasing perfusion and oxygenation, inhibiting the DNA repair process, and enhancing drug uptake [Citation12]. Based on these studies, HT has been used clinically for treating various cancers including breast cancer, cervical cancer, head and neck cancer, bladder cancer, melanoma and high-risk sarcoma [Citation13–17]. Specifically, the synergic effect of HT with RT in cervical cancer has been proven in prospective randomized trials. The Dutch Deep Hyperthermia Group has reported that HT with RT improved the three-year local control rate from 41% to 61% and overall survival (OS) from 27% to 51% [Citation18]. Additionally, the 12-year follow-up data confirmed that this effect of HT was maintained for a long period [Citation19].
Although CCRT is recommended for LACC as mentioned above, the effect of CCRT on survival was seen to decrease relative to increasing tumor stage [Citation20,Citation21]. In other words, the efficacy of CCRT seemed less impactful with increasing tumor stage. There have been some attempts to improve the clinical outcome by using HT with RT or CT synergistically. In two network meta-analyses (NMA), CCRT with HT was shown to be a promising treatment approach [Citation22,Citation23]. However, there is inconsistency as to whether CCRT with HT is better than CCRT alone for survival. NMA is a useful tool for summarizing the relative treatment effects, but the treatment rankings and effect estimates are often uncertain and imprecise [Citation24]. Therefore, we performed a meta-analysis by collecting and directly comparing studies to identify the synergistic effect of HT on CCRT for LACC.
Materials and methods
Search strategy, studies identification and selection
A systematic literature search was performed on the MEDLINE, PubMed, Embase, Cochrane library and SCOPUS databases. All studies published between January 2000 and June 2020 on CCRT with HT for cervical cancer were considered for this meta-analysis. The following terms were used for the search: ((cervical cancer) OR (cervical neoplasm)) AND ((radiotherapy) OR (chemoradiation) OR (chemoradiotherapy) OR (chemo-irradiation) OR (chemo-radiotherapy)) AND (hyperthermia). The search was not limited to any language. The inclusion criteria were studies which: (1) involved patients with FIGO II–IVa cervical cancer, (2) compared treatment outcomes of CCRT alone and CCRT with HT, (3) contained no surgical intervention in the form of hysterectomy, (4) were a full article written in English.
Data extraction and quality assessment
After duplicate publications were deleted, two authors (J.W.Y. and J.P.) independently reviewed the papers to identify eligible studies according to the inclusion criteria. First, articles were screened for eligibility based on the title and abstract. Then, the full text of the remaining articles was accessed and read independently by the two authors. The data extracted from the final, eligible studies included the author names, country, publication date, study type, sample size, age of patients and characteristics of treatment. The data collected on treatment outcome were five-year OS, local relapse-free survival (LRFS) and incidence of acute and chronic toxicity. Disagreements on the evaluation of results were resolved by consensus between the two authors. Tierney’s method was used to calculate the estimated survival date if hazard ratio (HR) and 95% confidence interval (CI) for survival were not presented directly in the article [Citation25]. Survival rates from Kaplan–Meier curves were estimated using the Graph Grabber version 2.0.2 (Quintessa Ltd, England), and the resulting data were then entered in a calculation spreadsheet appended to Tierney’s paper.
The Cochrane Collaboration’s tool was adopted for the assessment of randomized controlled trials. Evaluation of the quality of the literature included assessment of method of randomization, allocation concealment, blinding, data integrity of results, results of selective reporting and other sources of bias. Each element was qualified as having high, low or unclear risk of bias.
Statistical analysis
Review Manager Version 5.3.5 was used to perform the meta-analyses. For time-to-event outcomes such as five-year OS and LRFS, HR and its 95% CI were used as effective measures. For binary outcomes of acute and chronic side effects, the number of events and number of patients were used to calculate the risk ratio (RR) for estimating the effect of treatment. Heterogeneity among studies was evaluated using Cochrane’s Q test and I statistics. A fixed-effect model was used in instances with an I2 of < 50% or a p-value of > 0.1; otherwise, a random effects model was used. Publication bias based on funnel plot analyses was not performed since there were less than 10 studies included in the analysis [Citation26].
Results
Literature search and study selection
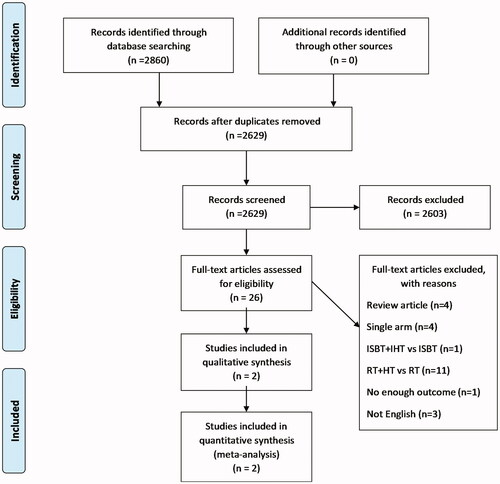
We identified 2860 articles from the aforementioned electronic databases according to the defined search strategy. After removal of duplicate articles, 2629 articles were considered. After screening based on the title and abstract, 26 articles were suitable for full-text evaluation. We excluded review articles, single arm studies, studies comparing RT with HT and RT, studies on interstitial brachytherapy (ISBT) with interstitial hyperthermia (IHT) [Citation27] and studies without enough outcomes [Citation28]. Finally, two articles, with a total of 536 patients were selected for the meta-analysis ().
The detailed characteristics and the methodological quality of these included studies are summarized in and . Both included studies were randomized prospective studies. The studies were published during 2016–2020 and were from Asian countries (Japan and China). Of the total 536 patients, 268 were in the CCRT group and 268 were in the CCRT with HT group. There were 295 (55.0%) and 241 patients (45.0%) with FIGO stages I–II and III–IV, respectively. Most of the patients were pathologically diagnosed as having squamous cell carcinoma (525/536, 97.9%). All patients received cisplatin-based CCRT, and RT consisted of external beam radiotherapy 50.4–52.2 Gy and brachytherapy 20–30 Gy. Most of the patients in the CCRT with HT group were treated with HT that was maintained at ≥40 °C for 60 min.
Figure 2. Methodological quality summary; review of authors’ judgment about each methodological quality item for each included study.
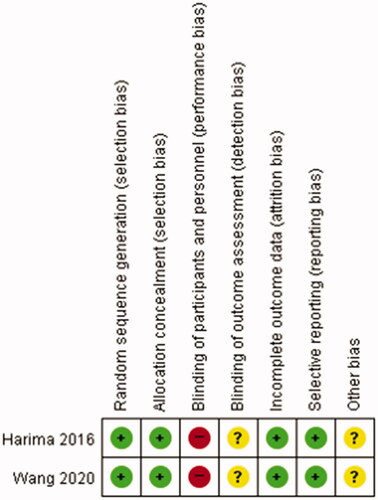
Table 1. Summary of included studies.
OS and LRFS
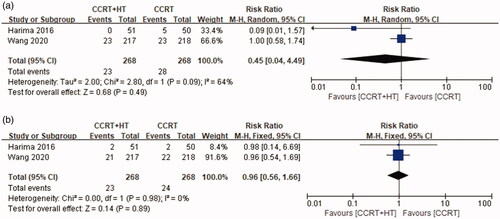
There was no significant heterogeneity in the OS results between the two studies (p = 0.67, I2 = 0%); therefore, a fixed-effects model was used for further analysis. As shown in , patients in the CCRT with HT group had significantly better five-year OS compared with that of patients in the CCRT group (HR 0.67, 95% CI 0.47–0.96, p = 0.03).
Figure 3. Forest plot of (a) overall survival (OS) and (b) local relapse-free survival (LRFS) between chemoradiotherapy with hyperthermia (CCRT + HT) and chemoradiotherapy (CCRT).
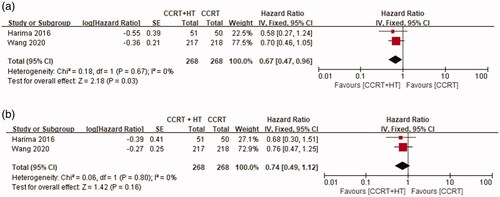
For evaluating LRFS, a fixed-effects model was used for further analysis because there was no significant heterogeneity in the results between the two studies (p = 080, I2 = 0%). As shown in , the differences were not statistically significant between the CCRT group and the CCRT with HT group (HR 0.74, 95% CI 0.49–1.12; p = 0.16).
Treatment toxicity
Data on toxicity were extracted from a total of 536 patients in both studies. For evaluating acute toxicity, random models were applied for nausea and diarrhea owing to heterogeneity among the two studies (I2 = 76%, p = 0.04 and I2 = 64%, p = 0.10, respectively). Meanwhile, fixed models were applied for vomiting and genitourinary toxicity due to the absence of heterogeneity among the studies (I2 = 0%, p = 0.70 and I2 = 0%, p = 0.55). There was no significant difference between the CCRT with HT group and CCRT group ().
Figure 4. Forest plot of acute toxicity: (a) nausea, (b) vomiting, (c) diarrhea and (d) acute genitourinary toxicity.
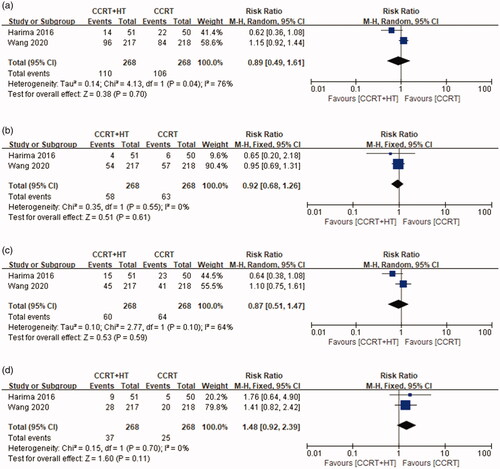
For evaluating chronic toxicity, random models were applied for rectal bleeding owing to heterogeneity among the studies (I2 = 64%, p = 0.09), and fixed models were applied for genitourinary toxicity due to the absence of heterogeneity among the studies (I2 = 0%, p = 0.98). There was no significant difference between the CCRT with HT and CCRT groups ().
Discussion
This study demonstrated that combining HT with CCRT for cervical cancer significantly improves OS (HR 0.67, p = 0.03). Datta et al. [Citation22,Citation23] also showed that CCRT with HT is a promising approach through two NMA. Of the two, the latest study suggested that RT with HT, CCRT with HT and CCRT (triweekly cisplatin) were the three best options for LACC; however, CCRT (triweekly cisplatin) was superior to CCRT with HT in the OS through indirect comparison such as surface under the cumulative ranking curve and rankogram [Citation23]. Even with such improvements in clinical outcomes, it was observed that HT did not cause an increase in acute or chronic side effects.
The benefits of HT for cervical cancer have been reported in several studies. The Dutch Deep Hyperthermia Group trial showed better clinical outcomes with combined RT and HT as compared with RT alone in patients with FIGO IIB or higher [Citation18]. Complete response rate, three-year pelvic control and OS were improved from 57% to 83%, 41% to 61% and 27% to 51%, respectively. Acute grades 3–4 radiation toxicity was not statistically different (2.2% in combined RT and HT, 5.9% in RT alone). According to the long-term follow-up study, the effect of HT was sustained; the combined RT and HT group was statistically superior than the RT alone group in 12-year local control rate (37% vs. 56%; p = 0.01) and OS (20% vs. 37%; p = 0.03) [Citation19]. A Cochrane database meta-analysis also showed similar results, with higher CR (RR 0.56 CI 0.39–0.79) and OS (HR 0.67 CI 0.45–0.99) and lower local recurrence rate (HR 0.48 CI 0.37–0.63) in the combined RT and HT group [Citation31]. It should be noted that 74% of the patients included in the analysis were FIGO stage IIIb.
Since the 1999 National Cancer Institute clinical alert, CCRT is widely used and recommended as a standard treatment in clinical practice guidelines for LACC [Citation32,Citation33]. However, the benefits of CCRT decrease with increasing tumor stage, with estimated absolute survival benefits of 10%, 7%, and 3% for stages Ia–IIa, IIb, and III–IVa at five years, respectively [Citation20]. Therefore, the RADCHOC trial was conducted to investigate whether CCRT or RT with HT is effective for bulky and/or cervical cancer of FIGO stage III or higher [Citation34]. They reported that there were no significant differences in event-free survival, pelvic recurrence-free survival and OS between the two groups. This trial was terminated prematurely due to poor accrual and only 23% of the planned patients were analyzed, which did not provide sufficient results to demonstrate the efficacy of combined HT with RT as compared with that of CT alone in LACC.
HT is known to have a synergic effect with RT and CT. RT causes damage to the DNA of the cancer cells leading to cellular death; in particular, it causes DNA double strand breaks that are extremely lethal. There are repair systems, such as homologous recombination and non-homologous end joining, for these breaks, but heat interferes with these systems [Citation35]. Combining HT with RT increases unrepaired DNA breaks by inhibiting the repair system, that causes an increase in cell cycle arrest and apoptosis through an increase in transcriptional activation of p53 [Citation36]. HT also enhances the cytotoxic effects of cisplatin by increasing absorption that leads to increased intracellular accumulation [Citation37].
Clinical studies have proven the synergic effects of HT. Zolciak-Siwinska et al. [Citation27] conducted a prospective trial to investigate whether the addition of HT affected local control (LC) or disease-free survival (DFS) in patients undergoing CCRT. There was no significant difference in the three-year LC and DFS between HT group and no HT group (60% vs. 67%, p = 0.99; 88% vs. 84%, p = 0.18, respectively). However, it was difficult to infer the synergic effect of HT in CCRT since this study investigated the combined effect of HT and ISBT. Flameling et al. [Citation38] conducted a prospective randomized trial to investigate the role of locoregional HT in CCRT. The study was closed prematurely due to slow recruitment. CCRT with HT showed high CR rate and five-year DFS, but it was not statistically significant (86% vs. 76%, p = 0.54; 61.0% vs. 58.7%, p = 0.65). Minnaar et al. [Citation28] also conducted a randomized controlled study to determine whether HT improves treatment outcomes for patients with CCRT. They used modulated electro-hyperthermia (mEHT), which is a capacitive technology to enhance the efficiency of HT by amplitude modulation, and it is known that the temperature reached during mEHT is lower than that of conventional HT [Citation39]. Although the evaluation of the efficacy of HT was limited because the temperature was not measured, it was reported that the CCRT with HT group had a significantly higher six month-local disease-free survival (38.6% vs. 19.8%, p = 0.003). In addition, HT did not increase CCRT-related toxicity except for mild neurological toxicity [Citation40].
Harima et al. [Citation29] prospectively investigated clinical outcomes of 101 patients without para-aortic lymph node metastasis. They reported that the five-year LRFS, disease-free survival, OS and CR rate in CCRT with HT were superior to those of CCRT alone; however, there was no statistically significant difference observed (80.1% vs. 71.0%, p = 0.25; 70.8% vs. 60.6%, p = 0.18; 77.8% vs. 61.8%, p = 0.14; 88% vs. 77.6%, p = 0.19, respectively). A limitation of this trial was that they recruited a small number of patients by assuming the difference in CR rate between the two groups as 30% while calculating the sample size. The authors regarded this limitation as the main reason for absence of any statistically significant differences.
Wang et al. [Citation30] recently published the results of a randomized controlled trial of 435 patients. They reported similar results that CCRT with HT was superior to CCRT alone in locoregional control; however, the superiority was not statistically significant (86.2% vs. 83.0%. p = 0.21). There are several possible reasons to explain these unexpected results. First, it is possible that the performance of the HT system used in this study was insufficient. Wang et al. used an unconventional HT system consisting of two pairs of electrodes with different frequencies (30.32 and 40.68 MHz) and a power of 1500 W. Second, the dose of the chemotherapy was relatively low compared to other trials using a thrice-weekly regimen consisting of cisplatin and 5-fluorouracil [Citation41,Citation42]. Furthermore, the degree of synergic effect of HT according to the anticancer drugs used should be considered. The thermal enhancement ratio was low when 5-fluorouracil was included in a multidrug regimen [Citation43]. The five-year OS was significantly superior in the CCRT with HT than in the CCRT alone (81.9% vs. 72.3%, p = 0.04) as per protocol (PP), but not in the intention to treat (ITT) analysis (81.1% vs. 72.9%, p = 0.05). These results may be attributed to selection bias due to the exclusion of approximately 14% of patients from the PP analysis. Another possible cause is that more patients in the CCRT group died from distant metastases. There were about 5% more lymph node positive patients in the CCRT group than in the CCRT with HT group. Lymph node status is an important prognostic factor and is associated with distant recurrence [Citation44–46].
There were some limitations to this study. First, although this study confirmed that HT improved the clinical outcomes through synergic effects with CCRT in all patients, it did not evaluate whether these differences were significant for each FIGO stage. However, since stages I–II and III–IV in this study were relatively evenly distributed at 55% and 45%, respectively, the effect of stage on the results is thought to be small. Second, there is a limit to applying research results globally due to small number of included studies. In particular, Chinese articles that have conducted active research in this area were not included. However, both studies were highly qualified, prospective, randomized controlled trials and the treatment modalities used in both studies were similar in terms of regional HT and cisplatin-based CCRT. Third, the staging of patients included in this analysis was based on the FIGO 2009. Therefore, it seems necessary to perform this analysis according to the FIGO 2018, i.e., a revised version that reflects lymph node status.
Conclusion
Although the effect of HT on the survival rate based on the stage of cervical cancer could not be analyzed, CCRT in combination with HT significantly improved OS in LACC without increasing acute or chronic toxicity. Therefore, it is believed that this tri-modality approach could be a good option for treating LACC patients. Further large randomized studies are needed to confirm whether the synergic effect of HT and CCRT is significant for each stage, and these studies should be based on the recently revised FIGO 2018.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203.
- Lim MC, Won YJ, Ko MJ, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999-2015. J Gynecol Oncol. 2019;30(1):e38.
- Jung KW, Won YJ, Kong HJ, et al. Prediction of cancer incidence and mortality in korea, 2019. Cancer Res Treat. 2019;51(2):431–437.
- Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–1161.
- Peters WA, Liu PY, Barrett RJ, Stock RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18(8):1606–1613.
- Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a gynecologic oncology group and southwest oncology group study. J Clin Oncol. 1999;17(5):1339–1348.
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–1153.
- Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340(15):1137–1143.
- Lukka H, Hirte H, Fyles A, et al. Concurrent cisplatin-based chemotherapy plus radiotherapy for cervical cancer-a meta-analysis. Clin Oncol. 2002;14(3):203–212.
- Sakaguchi Y, Stephens LC, Makino M, et al. Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res. 1995;55(22):5459–5464.
- Vertrees RA, Das GC, Coscio AM, et al. A mechanism of hyperthermia-induced apoptosis in ras-transformed lung cells. Mol Carcinog. 2005;44(2):111–121.
- Oei AL, Kok HP, Oei SB, et al. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv Drug Deliv Rev. 2020;163–164:84–97.
- Zhao C, Chen J, Yu B, et al. Improvement in quality of life in patients with nasopharyngeal carcinoma treated with non-invasive extracorporeal radiofrequency in combination with chemoradiotherapy. Int J Radiat Biol. 2014;90(10):853–858.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and Meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- van der Zee J, González González D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch deep hyperthermia group. Lancet. 2000;355(9210):1119–1125.
- Overgaard J, Bentzen SM, Overgaard J, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet. 1995;345(8949):540–543.
- Issels RD, Lindner LH, Verweij J, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492.
- van der Zee J, González GD. The Dutch deep hyperthermia trial: results in cervical cancer. Int J Hyperthermia. 2002;18(1):1–12.
- Franckena M, Stalpers LJ, Koper PC, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch deep hyperthermia trial. Int J Radiat Oncol Biol Phys. 2008;70(4):1176–1182.
- Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26(35):5802–5812.
- Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2005;3:CD002225.
- Datta NR, Rogers S, Klingbiel D, et al. Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: a systematic review with conventional and network meta-analyses. Int J Hyperthermia. 2016;32(7):809–821.
- Datta NR, Stutz E, Gomez S, et al. Efficacy and safety evaluation of the various therapeutic options in locally advanced cervix cancer: a systematic review and network meta-analysis of randomized clinical trials. Int J Radiat Oncol Biol Phys. 2019;103(2):411–437.
- Trinquart L, Attiche N, Bafeta A, et al. Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials. Ann Intern Med. 2016;164(10):666–673.
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
- Ioannidis JP, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ. 2007;176(8):1091–1096.
- Zolciak-Siwinska A, Piotrkowicz N, Jonska-Gmyrek J, et al. HDR brachytherapy combined with interstitial hyperthermia in locally advanced cervical cancer patients initially treated with concomitant radiochemotherapy – a phase III study. Radiother Oncol. 2013;109(2):194–199.
- Minnaar CA, Kotzen JA, Ayeni OA, et al. The effect of modulated electro-hyperthermia on local disease control in HIV-positive and -negative cervical cancer women in South Africa: early results from a phase III randomised controlled trial. PLoS One. 2019;14(6):e0217894.
- Harima Y, Ohguri T, Imada H, et al. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hyperthermia. 2016;32(7):801–808.
- Wang Y, Hong W, Che S, et al. Outcomes for hyperthermia combined with concurrent radiochemotherapy for patients with cervical cancer. Int J Radiat Oncol Biol Phys. 2020;107(3):499–511.
- Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev. 2010; 3:Cd006377.
- Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(1):64–84.
- Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv72–iv83.
- Lutgens LC, Koper PC, Jobsen JJ, et al. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: results of the randomized RADCHOC trial. Radiother Oncol. 2016;120(3):378–382.
- Ihara M, Takeshita S, Okaichi K, et al. Heat exposure enhances radiosensitivity by depressing DNA-PK kinase activity during double strand break repair. Int J Hyperthermia. 2014;30(2):102–109.
- Luo Z, Zheng K, Fan Q, et al. Hyperthermia exposure induces apoptosis and inhibits proliferation in HCT116 cells by upregulating miR-34a and causing transcriptional activation of p53. Exp Ther Med. 2017;14(6):5379–5386.
- Haveman J, Bergs JW, Franken NA, et al. Effect of hyperthermia on uptake and cytotoxicity of cisplatin in cultured murine mammary carcinoma cells. Oncol Rep. 2005;14(2):561–567.
- Flameling B, Nordberg T, Ott O, et al. An international multicenter phase III study of chemoradiotherapy versus chemoradiotherapy plus hyperthermia for locally advanced cervical cancer. J Clin Oncol. 2016;34(15_suppl):e17023.
- Lee SY, Kim JH, Han YH, et al. The effect of modulated electro-hyperthermia on temperature and blood flow in human cervical carcinoma. Int J Hyperthermia. 2018;34(7):953–960.
- Minnaar CA, Kotzen JA, Naidoo T, et al. Analysis of the effects of mEHT on the treatment-related toxicity and quality of life of HIV-positive cervical cancer patients. Int J Hyperthermia. 2020;37(1):263–272.
- Likhacheva A, Jhingran A, Bodurka DC, et al. Prospective study of symptom assessment among patients with cervical cancer during concurrent chemoradiotherapy with weekly cisplatin or every-3-week cisplatin and 5-fluorouracil. Int J Gynecol Cancer. 2013;23(8):1520–1527.
- Kim YS, Shin SS, Nam JH, Kim YT, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008;108(1):195–200.
- Matsushita S, Reynolds R, Urano M. Synergism between alkylating agent and cis-platin with moderate local hyperthermia: the effect of multidrug chemotherapy in an animal system. Int J Hyperthermia. 1993;9(2):285–296.
- Kidd EA, Siegel BA, Dehdashti F, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010;28(12):2108–2113.
- Harkenrider MM, Altoos B, Small W. Jr. Prognostic significance of residual lymph node status after definitive chemoradiotherapy in patients with node-positive cervical cancer. Gynecol Oncol. 2018;148(3):437–438.
- Kang S, Park JY, Lim MC, et al. Pelvic lymph node status assessed by 18F-fluorodeoxyglucose positron emission tomography predicts low-risk group for distant recurrence in locally advanced cervical cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2011;79(3):788–793.