Abstract
Purpose
To retrospectively compare the efficacy and safety of surgical resection (SR) and thermal ablation for the treatment of adrenal metastases.
Methods
From January 2008 to December 2018, 133 patients with adrenal metastases who underwent SR (n = 76) or thermal ablation (n = 57) were enrolled. The mean tumor size was 58.00 ± 10.65 mm (22-80 mm) in the SR group and 58.03 ± 12.76 mm (34–89 mm) in the thermal ablation group. Local progression-free survival (LPFS) and safety were compared between the two groups using the Kaplan–Meier method and log-rank tests. Cox proportional hazard regression models were used to evaluate the prognostic factors of LPFS. Complications, hospitalization days, and blood loss were also assessed.
Results
The median follow-up was 29.0 months (range, 20.4–37.6 months). No treatment-related mortality was observed. The 1-, 3- and 5-year LPFS rates were 74.0%, 62.8%, and 31.4% in the SR group and 72.8%, 68.7%, and 51.5% in the ablation group, with the median LPFS of 41.5 months (95% CI: 9.3–23.4 months) vs. 47.9 months (95% CI 20.6–75.8 months), respectively (p = 0.784). Tumor size ≥3 cm was the only significant risk factor for LPFS (p = 0.031). The ablation group was superior to the SR group with a lower major complication rate (4.1% vs. 14.5%, p = 0.03), less blood loss (1 ml vs. 100 ml, p < 0.001), and a shorter hospital stay (2 d vs. 6 d, p < 0.001).
Conclusion
Thermal ablation provided a similar LPFS and less comorbidities than SR, indicating that it is an effective and safe treatment for adrenal metastases.
Introduction
The adrenal gland is a common site of metastases, with an incidence rate of 13–27% in patients with malignancy by postmortem review [Citation1,Citation2]. Adrenal metastases are the most common malignant tumors of the adrenal gland [Citation3]. Moreover, lung cancer was reported as the most common site of primary malignancy, followed by melanoma, breast, lung, gastrointestinal, and renal tumors [Citation4]. Most adrenal metastases are typically asymptomatic and are usually discovered by imaging follow-up; only a few patients present with back pain [Citation5]. Adrenal metastases are generally detected within a relatively short period (median period, 7 months) after the diagnosis of the primary tumor, with less than 2% of the patients developing adrenal metastases for more than 5 years [Citation6]. Chemotherapy or palliative supportive care is usually a therapeutic option for adrenal metastases specific to the primary tumor type. Some studies have reported that adrenalectomy for selected patients with isolated metastatic disease of the single adrenal gland would improve survival time, although this remains controversial [Citation7–Citation9]. Stereotactic body radiation therapy (SBRT) is also regarded as an alternative treatment for inoperable adrenal metastases with a local control rate of 77–92.3% [Citation10,Citation11].
Minimally invasive treatments such as arterial embolization and chemical ablation with ethanol or acetic acid have been reported for the treatment of adrenal tumors; however, the application appears to be limited because of the difficulty of embolization of three supplying arteries and an insufficient chemical ablation zone for larger tumors [Citation12–15].
Nowadays, thermal ablations have served as candidates for patients with adrenal neoplasms who are ineligible for surgery, with the advantage of minimal invasiveness and effective short-term local control in the treatment of primary and metastatic adrenal neoplasms [Citation15–21].
To date, there have been limited comparative studies of surgical resection (SR) and thermal ablation of adrenal metastases. Therefore, the purpose of this study was to retrospectively compare the outcomes and safety of SR and thermal ablation as treatments for adrenal metastases.
Materials and methods
Patient population
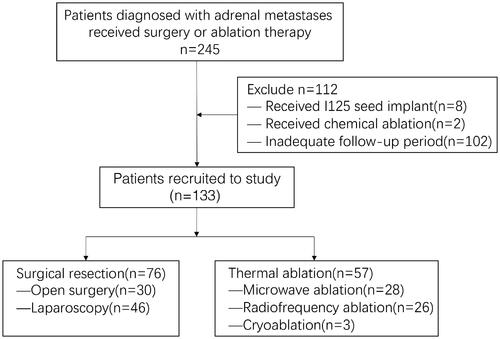
This retrospective study was approved by the Institutional Review Board of the Sun Yat-sen University Cancer Center (2021-FXY-416) and the requirement for informed consent is waived. Between January 2008 and December 2018, patients diagnosed with adrenal metastases who received corresponding treatment at our institute were considered as potential candidates for this study. The treatment strategy was determined by a multidisciplinary team (MDT), including medical and radiation oncologists, urologists, and interventional radiologists. If adrenal lesions are curable, surgical resection is recommended as the preferred treatment. Thermal ablation is recommended as an alternative therapy for patients with surgical contraindications or refusal of resection. Patients who met the following criteria were enrolled in this study [Citation1]: received treatment with surgical resection or thermal ablation, and [Citation2] follow-up with radiological examination for at least 6 months after the treatment. Two-hundred-and-forty-five consecutive patients received treatment for adrenal metastases. According to the inclusion criteria, 112 patients were excluded because of a combination of I125 seed implant treatment, chemical ablation, or an inadequate follow-up period. The flowchart of the patients is shown in .
Finally, 133 patients (108 men and 25 women; median age, 58 years; range, 22–89 years) with 140 adrenal metastases who underwent SR (n = 76), including open resection (n = 30) and laparoscopic resection (n = 46), or thermal ablation (n = 57) were enrolled in this study. The mean diameter of the tumors was 34 ± 18.86 mm (range, 6–101 mm). In the thermal ablation group, adrenal metastases were histologically diagnosed in two patients by percutaneous biopsy, whereas the remaining 55 patients were diagnosed based on radiologic findings by two radiologists with consensus. In the SR group, all patients were diagnosed with pathology after treatment. The adrenal metastases included hepatocellular carcinoma (n = 44, 33.1%), non-small cell lung cancer (n = 50, 37.6%), renal cell carcinoma (n = 8, 6%), esophageal carcinoma (n = 5, 3.8%), colorectal cancer (n = 7, 5.3%), gastric cancer (n = 3, 2.3%), breast cancer (n = 2, 1.5%), thyroid carcinoma (n = 2, 1.5%), bone and soft tissue sarcoma (n = 4, 3%), cervical cancer (n = 3, 2.3%), melanoma (n = 2, 1.5%), nasopharyngeal carcinoma (n = 1, 0.7%), moderately differentiated squamous cell carcinoma (n = 1, 0.7%), and anaplastic meningioma (n = 1, 0.7%). Two patients in the ablation group and three patients in the SR group experienced back pain. The patient’s baseline characteristics are demonstrated in . The primary tumors were well controlled by resection, chemotherapy, radiotherapy, targeted therapy, or interventional therapy.
Table 1. Baseline characteristics of ablation and surgery group.
Surgical resection
SR of the adrenal metastases was performed by experienced urologists under general anesthesia. Open surgical adrenalectomy or laparoscopic adrenalectomy was performed on the basis of tumor location and size, preference and experience of the surgeon, and previous abdominal surgery. The fatty tissue around the tumor was dissected prudently to clearly identify the tumor margin, and then the whole adrenal gland was sufficiently exposed. Subsequently, the neoplasm was resected along with an adequate safety margin of the normal adrenal tissue. Finally, the adrenal gland lesion was confirmed grossly in detail to ensure adequate excision. After the surgery, patients were observed for at least 2 h in the post-anesthesia care unit.
Thermal ablation procedure
Thermal ablation was performed under computer tomography (CT) (Siemens, Munich, Germany) guidance by an interventional radiologist (Weijun Fan) with more than 20 years of experience in interventional radiology. The choice criteria for the ablation method were determined mainly by the size of the lesion. Cryoablation was performed in relatively large tumors (size >5 cm), and RFA was only performed in small tumors (size < 3cm). Patients were in the prone or ipsilateral decubitus position. Conscious analgesic sedation (intravenous administration of remifentanil, 0.1 mg/kg/min) and local anesthesia (subcutaneous 1% lidocaine) were performed. Vital signs were continuously and noninvasively monitored. Microwave ablation was performed using an MTC-3C microwave system (Vision Microwave Electronic Institute, Nanjing, China). Radiofrequency ablation was performed using a Cool-tip RF Ablation System (Valleylab, Boulder, CO, USA), RITA Medical Systems (StarBurst XL-AngioDynamics, Queensbury, NY, USA), or VIVA RF System (STARmed, Goyang-si, Gyeonggi-do, Korea). Cryoablation was performed using a Cryo-Hit system (CryoHit, Galil Medical Inc., Yokneam, Israel). For lesions larger than 3 cm, the ablation was overlapped ablation and the treatment duration was prolonged. Following the treatment, a CT scan was performed to evaluate ablation efficacy and to examine complications. Blood cell count, biochemical tests, and chest radiography were performed on the first day after the ablation. All patients were discharged within 3 d if no complications occurred ().
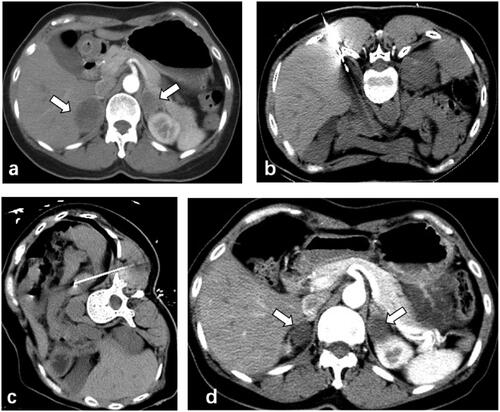
Figure 2. 56-year-old male patient with lung adenocarcinoma presented bilateral adrenal metastases after resection of the primary tumor (a) CT-guided microwave ablation was performed on the adrenal metastases in lateral position (b) and prone position (c) respectively. The adrenal metastases showed complete necrosis 6 months after ablation (d).
Treatment assessment and follow-up
For thermal ablation, technique success was defined as completion of adrenal ablation according to the planned treatment protocol, and the tumor was covered completely by the ablation zone in the images. Abdominal contrast-enhanced CT scans were performed 1 month after treatment and at 3, 6, and 12 months for the first year, and then every 6–12 months thereafter. Technique efficacy was defined as the absence of tumor enhancement on the contrast-enhanced CT or magnetic resonance imaging (MRI) estimated by two senior radiologists. Local tumor progression (LTP) was defined as the appearance of a 20% increase in the diameter of the intra-adrenal lesions, taking the baseline diameter as a reference [Citation22]. Local progression-free survival (LPFS) was calculated from the time of initial adrenalectomy or ablation to the time of adrenal metastasis recurrence or the last follow-up. The overall survival time was not calculated in this study because the biology of different malignancies would largely affect the outcomes. Residual tumors were not considered as local progression until positive failure from radiography.
Procedural complications were evaluated based on the Clavien–Dindo classification. Major complications were defined as events that led to substantial comorbidity and disability, resulting in lengthened hospitalization or hospital admission. All other complications were considered minor complications [Citation23,Citation24]. The hypertensive crisis was defined as acute systolic blood pressure >180 mmHg or diastolic pressure >110 mmHg during the procedure [Citation25]. Hospitalization stays were also estimated.
Statistical analysis
Continuous variables are presented as the mean ± SD. Categorical variables were presented as counts and percentages. Variables in the two groups were compared using the Mann–Whitney U test for continuous variables and Pearson’s x2 test for categorical variables. LPFS curves were calculated using the Kaplan–Meier method. Differences in the curves between the two groups were statistically compared using the log-rank test. Univariate and multivariate Cox proportional hazard regression models were used to evaluate the prognostic factors for LPFS. All statistical analyses were performed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, and p-values < 0.05, were considered statistically significant.
Table 2. Univariate and multivariate analysis of the relative risk of local progression-free survival.
Results
Ablation and resection sessions
In the ablation group, 57 patients with 60 adrenal metastases (three patients received treatment for two lesions) were treated in 73 sessions (29 sessions of radiofrequency ablation, 36 sessions of microwave ablation, and eight sessions of cryoablation). One patient underwent ablation of bilateral adrenal lesions (). Technical success was achieved in all patients. Forty patients (70.2%, 40/57) achieved complete response after the ablation treatment, including 32 patients in one session, six patients in two sessions, one patient in three sessions, and one patient in four sessions. The remaining 17 patients (28.8%, 17/57) achieved a partial response, including 12 patients with one session and five patients with two sessions. In the SR group, 80 adrenal metastases were removed in 76 patients (four patients underwent resection for two lesions).
Local progression
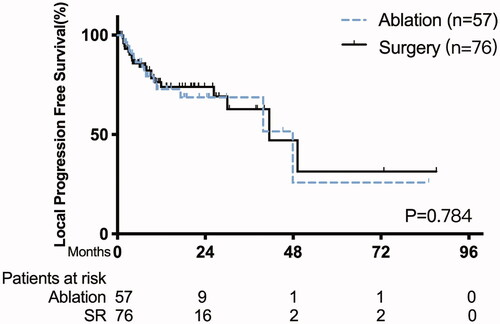
The median follow-up was 29.0 months (range, 20.4 − 37.6 months). During follow-up, local progression occurred in 14 patients in the ablation group and 20 patients in the SR group. The 1-, 3- and 5-year LPFS rates were 74.0%, 62.8%, and 31.4% in the SR group, and 72.8%, 68.7%, and 51.5% in the ablation group, with the median LPFS of 41.5 months (95% CI 9.3–23.4 months) vs. 47.9 months (95% CI 20.6–75.8 months), respectively (p = 0.784) (). The LTP rate in the ablation group was similar to that in the SR group (24.6% vs. 26.3%, p = 0.819) (). Distant metastases occurred in 31 patients after ablation and in 49 patients after surgery. The distant metastasis occurrence rate in the ablation group was similar to that in the SR group (54.4% vs. 64.5%, p = 0.240). For patients with recurrent or progressive disease, various types of treatments, including chemotherapy, targeted drug therapy, and best supportive care, were performed. Univariate analysis revealed that age <65 years (p = 0.097) and tumor size ≥3 cm (p = 0.027) were associated with LPFS. Multivariate analysis showed that tumor size ≥3 cm was an independent prognostic factor for LPFS with a significant difference (p = 0.031) (). There was no significant difference in LPFS between MWA, RFA, and CA (p = 0.725).
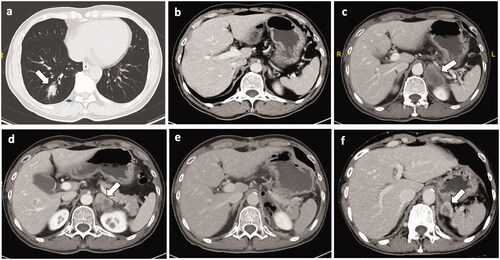
Figure 3. Recurrence after resection of the adrenal metastases. Four months after radical resection of right-lower lung cancer(pT2aN0M0), the 59-year-old male patient was founded with a right adrenal metastasis (size: 40mm × 29mm). (a) The primary lung tumor. (b) No tumor nodule was found in the right adrenal gland before pulmonary tumor resection. (c, d) The right adrenal metastasis. According to suggestion of the MDT, the patient received the right adrenal tumor resection (e). However, right adrenal metastasis (size: 29mm × 22mm) recurred 3 months after adrenal resection (f).
Complications
No treatment-related mortality was observed in the present study. Adverse events in the ablation and SR groups are shown in . According to the Clavien–Dindo classification system, all complications except for one were grade I or II. Only one-grade IIIa pneumothorax developed in the SR group, which needed a drainage tube.
Table 3. Adverse events in ablation group and surgery group.
Hemorrhage was the most common complication in the SR group, and nine patients required blood transfusion. One patient who underwent resection of the left adrenal metastasis experienced palpitations, hypodynamia, and dizziness on the first day after surgery due to adrenal insufficiency, which was relieved after the administration of an intravenous drip of glucocorticoids. Subsequently, the patient received oral glucocorticoid replacement therapy to maintain adrenal gland function. Another patient who underwent cryoablation for right adrenal metastasis suffered from painful shock during the procedure. The patient presented with clammy skin, and his blood pressure decreased to 66/36 mmHg. After rapid rescue measures (dopamine 60 mg, morphine 10 mg, and epinephrine 10 mg), the patient recovered from shock with a blood pressure of 105/65 mmHg and the cryoablation procedure was then performed.
Seven patients (9.6%) experienced a hypertension crisis (186–230 mmHg) during the procedure in the ablation group without cardiac arrhythmia or any other symptoms, and only one (1.3%) patient developed hypertension in the SR group (p = 0.031).
Blood loss and hospital stay
The median blood loss was 1 ml (range, 1–20 ml) in the ablation group and 100 ml (range 5–3500 ml) (p < 0.001) in the SR group, including 560 ml (range, 20–3500 ml) in open resection and 138.20 ml (range, 5–1800 ml) in laparoscopic resection, respectively. The median hospital stay was 6 d (range, 3–28 d) and 2 d (range, 1–18 d) in the SR and ablation groups, respectively (p < 0.001).
Discussion
Our results demonstrated that ablation could provide a similar local control rate compared with SR (75.4% vs. 73.7%, p = 0.819), which was similar to the reported local control rate of 77% after adrenalectomy, but inferior to those reported with isolated adrenal metastases of 83% [Citation26,Citation27]. The 1-, 3-, and 5-year LPFS rates were 72.8%, 68.7%, and 51.5% in the ablation group, which are similar to the 1-, 3-, and 5-year LPFS rates of 70–88%, 56–88%, and 55–56% previously reported, respectively [Citation21,Citation28,Citation29]. Given that most patients in the ablation group were not candidates for adrenalectomy, considering these satisfactory results, thermal ablation can serve as an effective local therapy for patients with adrenal metastases. The adrenal gland is also an ideal site for ablation because it is surrounded by adipose tissue in the perinephric space, preventing thermal injury to adjacent structures as a heat insulator, and to some extent to ensure the distribution of heat within the lesion to improve ablation efficacy.
In this study, tumor size ≥3 cm was a determining risk factor for local control, consistent with a previous study indicating that the smaller the tumor size, the greater local control that could be achieved [Citation30]. However, the cutoff size for adrenal metastases ablation remains undetermined. Wood et al. suggested that tumors smaller than 5 cm acquired better local control, and similar findings were reported by Mayo et al. [Citation16,Citation20] Moreover, tumor size ≥3 cm was also a risk factor for overall survival. An association between tumor size and survival after adrenalectomy was first suggested by Strong et al. [Citation27], who also revealed that tumor size smaller than 5 cm was associated with better survival [Citation31].
No deaths related to treatment were observed in this study. Some studies reported an overall mortality rate of 2.7–3.9% [Citation7,Citation8]. Prolonged operative time may increase the risk of comorbidities, while thermal ablation has an obviously short duration; it also has several advantages over surgery with its concomitant risks, increased cost, and extended hospital stay. In our study, there were a fewer adverse events, blood loss, and shorter hospital stay in the ablation group than in the adrenalectomy group; similar findings were reported by Yang et al. and Liu et al. in the treatment of benign adrenal adenoma in a comparative study [Citation32,Citation33]. Moreover, thermal ablation has the advantage of repeatability. In our study, six patients achieved a complete response after repeated ablation.
Figure 4. Kaplan–Meier curve of local progression-free survival between patients with thermal ablation versus surgery.
However, the incidence of hypertensive crisis in the ablation group was higher than that in the resection group (9.6% [7/73] vs. 1.3% [1/76]; p = 0.031), which was considered to be the result of catecholamine release induced by the destruction of normal adrenal gland tissue. Furthermore, ligation of the adrenal vein during surgery before manipulation of the adrenal gland would decrease the risk of hypertensive crisis [Citation34–36]. Notably, there were no severe outcomes of hypertensive crisis in either group, indicating good safety. Although administration of α- and β-blocker medications was recommended by some researchers in the treatment of functional adrenal tumors to prevent hypertensive crisis, for adrenal metastases ablation, we considered it unnecessary because the incidence of the hypertensive crisis of adrenal metastases ablation is relatively low and the blood pressure is well controlled by the whole course management of the anesthetist [Citation34,Citation37].
Adrenal insufficiency occurred in one patient who underwent left-sided adrenalectomy. Adrenal function is impaired when more than 90% of adrenal tissue is destroyed [Citation20]. Adrenal ablation preserves adrenal tissue to some extent to decrease the risk of adrenal insufficiency. This could explain why no patients experienced adrenal insufficiency in the ablation group in our study, even those who received bilateral adrenal ablation. Lo et al. also reported one case of bilateral adrenal ablation without any complications or endocrine disorders [Citation38]. Therefore, we consider bilateral adrenal ablation to be safe and feasible.
Although the adrenal gland has hypervascularity, severe hemorrhage did not occur in the ablation group. The favorable hemostatic effect of thermal ablation may account for these effects. Taking advantage of the rich blood supply, Yamakado et al. reported ablation combined with chemoembolization for the treatment of adrenal metastases to improve ablative efficacy and reduce the risk of hemorrhage [Citation39].
SBRT is a noninvasive local treatment for adrenal metastasis with a reported 2-year local control rate and overall survival rate of 63% and 19%, respectively, with limited toxicity. Our study showed similar local control rates of 62.8% and 68.7% at 3 years in both groups [Citation40]. Providing a minimally invasive and well-tolerated therapy is meaningful for inoperable patients. However, a comparative study still needed to include surgical, ablation, and SBRT to provide more evidence to determine the optimal locoregional treatment.
However, there were some limitations to our study. First, this was a retrospective study with some inherent defects, and selection bias was inevitable. Second, patient populations with various primary histopathologies and metastatic burden status were included, which could potentially influence oncologic outcomes. Therefore, a randomized clinical trial between resection and ablation for adrenal metastases from specific tumor subtypes is needed.
In conclusion, thermal ablation could provide similar local control of adrenal metastases and less comorbidities than surgical resection. In clinical practice, thermal ablation is a feasible and safe therapeutic option.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Glomeset D. The incidence of metastasis malignant tumors to the adrenals. Am J Cancer. 1938;32:57–61.
- Abrams HL, Spiro R, Goldstein NJC. Metastases in carcinoma. Analysis of 1000 autopsied cases. Cancer. 1950;3(1):74–85.
- Beland MD, Mayo-Smith WW. Ablation of adrenal neoplasms. Abdom Imaging. 2009;34(5):588–592.
- Wansaicheong G, Goh J. Adrenal metastases. 2008. Available from: http://emedicine.com
- Gittens PR Jr, Solish AF, Trabulsi EJ, editors. Surgical management of metastatic disease to the adrenal gland. Seminars Oncol. 2008. Elsevier.5.
- Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol. 2002;56(1):95–101.
- Kim SH, Brennan MF, Russo P, et al. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer: Interdisciplinary Int J Am Cancer Soc. 1998;82(2):389–394.
- Paul CA, Virgo KS, Wade TP, et al. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol. 2000;17(1):181–187.
- Lo CY, van Heerden JA, Soreide JA, et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83(4):528–531.
- Holy R, Piroth M, Pinkawa M, et al. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187(4):245–251.
- Ahmed KA, Barney BM, Macdonald OK, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36(5):509–513.
- Maki DD, Haskal ZJ, Matthies A, et al. Percutaneous ethanol ablation of an adrenal tumor. AJR Am J Roentgenol. 2000;174(4):1031–1032.
- Xiao Y-Y, Tian J-L, Li J-K, et al. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008;190(1):105–110.
- Hokotate H, Inoue H, Baba Y, et al. Aldosteronomas: experience with superselective adrenal arterial embolization in 33 cases. Radiology. 2003;227(2):401–406.
- Carrafiello G, Lagana D, Recaldini C, et al. Imaging-guided percutaneous radiofrequency ablation of adrenal metastases: preliminary results at a single institution with a single device. Cardiovasc Intervent Radiol. 2008;31(4):762–767.
- Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97(3):554–560.
- Welch BT, Atwell TD, Nichols DA, et al. Percutaneous image-guided adrenal cryoablation: procedural considerations and technical success. Radiology. 2011;258(1):301–307.
- Li X, Fan W, Zhang L, et al. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer. 2011;117(22):5182–5188.
- Wolf FJ, Dupuy DE, Machan JT, et al. Adrenal neoplasms: effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81(8):1717–1723.
- Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation-preliminary results. Radiology. 2004;231(1):225–230.
- Hasegawa T, Yamakado K, Nakatsuka A, et al. Unresectable adrenal metastases: clinical outcomes of radiofrequency ablation. Radiology. 2015;277(2):584–593.
- Ning L, Kong Y, Pan T, et al. Survival benefits of computed tomography-guided thermal ablation for adrenal metastases from hepatocellular carcinoma. Int J Hyperthermia. 2019;36(1):1003–1011.
- Sacks D, McClenny TE, Cardella JF, et al. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9):S199–S202.
- Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Rodriguez MA, Kumar SK, De C. Hypertensive crisis. Cardiol Rev. 2010;18(2):102–107.
- Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005;173(2):519–525.
- Strong VE, D’Angelica M, Tang L, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol. 2007;14(12):3392–3400.
- Frenk NE, Daye D, Tuncali K, et al. Local control and survival after image-guided percutaneous ablation of adrenal metastases. J Vasc Interv Radiol. 2018;29(2):276–284.
- Welch BT, Callstrom MR, Carpenter PC, et al. A single-institution experience in image-guided thermal ablation of adrenal gland metastases. J Vasc Interv Radiol. 2014;25(4):593–598.
- Zhou K, Pan J, Yang N, et al. Effectiveness and safety of CT-guided percutaneous radiofrequency ablation of adrenal metastases. BJR. 2018;91(1085):20170607.
- Zerrweck C, Caiazzo R, Clerquin B, et al. Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann Surg Oncol. 2012;19(11):3621–3626.
- Liu S, Chu C, Kong A, et al. Radiofrequency ablation compared with laparoscopic adrenalectomy for aldosterone-producing adenoma. Br J Surg. 2016;103(11):1476–1486.
- Yang M-H, Tyan Y-S, Huang Y-H, et al. Comparison of radiofrequency ablation versus laparoscopic adrenalectomy for benign aldosterone-producing adenoma. Radiol Med. 2016;121(10):811–819.
- Atwell TD, Wass CT, Charboneau JW, et al. Malignant hypertension during cryoablation of an adrenal gland tumor. J Vasc Interv Radiol. 2006;17(3):573–575.
- Yamakado K, Takaki H, Uchida K, et al. Adrenal radiofrequency ablation in swine: change in blood pressure and histopathologic analysis. Cardiovasc Interv Radiol. 2011;34(4):839–844.
- Gulla N, Patriti A, Fabbri B, et al. Surgical technique and haemodynamic changes in adrenalectomy for secreting neoplasia. Personal experience and review of the literature. Minerva Chir. 2003;58(1):87–92.
- Sudheendra D, Wood BJ. Appropriate premedication risk reduction during adrenal ablation. J Vasc Interv Radiol. 2006;17(8):1367–1368.
- Lo W-K, Shankar S, Morrison PR, et al. Percutaneous CT-guided radiofrequency ablation of symptomatic bilateral adrenal metastases in a single session. J Vasc Interv Radiol. 2006;17(1):175–179.
- Yamakado K, Anai H, Takaki H, et al. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol. 2009;192(6):W300–W5.
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative‐intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer: Interdisc Int J Am Cancer Soc. 2008;112(3):650–658.