Abstract
Objective
To examine the effectiveness and safety of thermal ablative methods and myomectomy for the treatment of uterine fibroids.
Materials and methods
We searched EMBASE, PubMed, the Cochrane Central Register of Controlled Trials, Scopus, CINAHL, ClinicalTrials.gov and Web of Science databases through April 2021. Clinical trials comparing the thermal ablative methods and myomectomy for the treatment of uterine fibroids were included.
Results
Thirteen studies including 4205 patients were eligible. The thermal ablative treatment group was associated with less major adverse events (only ultrasound guided high-intensity focused ultrasound) (RR, 0.111 [95% CI, 0.070–0.175], p=.0), shorter duration of hospital stays in observational studies (–0.1497 day, [95% CI, −1.593 to −0.321], p=.0) and in randomized controlled trials (RCTs) (–0.844 day, [95% CI, −0.1.142 to −0.546], p=.0), higher uterine fibroid symptom (UFS) score after operation (0.252 [95% CI, 0.165–0.339]; p=.0), transformed symptom severity (tSS) score after operation (0.515 [95% CI, 0.355–0.674]; p=.0) and quality of life (QoL) score after operation (0.188 [95% CI, 0.093–0.283]; p=.0) in comparison with myomectomy group. No statistically significant difference was found between the thermal ablative treatment group and myomectomy group with respect to reintervention rate and pregnancy rate.
Conclusion
The current data available demonstrate that thermal ablative methods were not inferior to myomectomy in the treatment of uterine fibroids. The findings in this study need to be further confirmed by large RCTs.
Introduction
Uterine fibroids are the most common solid pelvic tumors in women of reproductive age [Citation1], and are often associated with symptoms including heavy menstrual bleeding, abdominal pain and pressure [Citation2]. Prevalence varies from 50% to 77% depending on the method used for diagnosis [Citation3]. There are many modalities for the management of symptomatic fibroids, including abdominal, laparoscopic or hysteroscopic myomectomy, hysterectomy; thermal laser, radiofrequency energy, focused ultrasound, microwave energy [Citation4]; or interventional treatment by uterine artery embolization (UAE) [Citation5].
For patients who prefer to preserve their fertility, uterus-sparing treatments for symptomatic fibroids are better options. In addition to myomectomy, UAE emerged as an alternative therapy during the 1990s. It has been proven to be an effective and safe treatment for women who wish to preserve their uterus [Citation6]. However, the use of UAE in pregnant women remains controversial. A previous meta-analysis found that pregnancies in the UAE group were associated with higher rates of preterm delivery and malpresentation than those in the laparoscopic myomectomy group [Citation7]. Thermal ablative approaches with respect to medical therapies are those which ablate pathological tissues through thermal injury, such as by using devices creating focused ultrasound, radiofrequency energy or microwave energy. These are applicable in various diseases, such as hepatocellular carcinoma, sarcoma, renal cell carcinoma and bone metastases [Citation8–11]. Over the years, thermal ablative approaches for uterine fibroids have been developed and applied effectively [Citation4]. When the local temperature of tissue reaches to 50–52 °C for 4–6 min, irreversible cellular damage is observed [Citation12]. As temperature increases to 60–100 °C, coagulative necrosis occurs due to irreversible damage caused by protein coagulation [Citation13,Citation14]. Different thermal ablative systems for the treatment of uterine fibroids have been invented and refined to treat the maximum amount of fibroid tissue safely, while avoiding damage to the surrounding normal uterine tissue. Some of the systems are laparoscopic radiofrequency ablation system, transcervical radiofrequency ablation system and high-intensity focused ultrasound ablation system guided by ultrasound or MRI [Citation15–18].
As minimally invasive methods for fibroids, thermal ablative treatments are expected to provide better options for patients who want to preserve their uteri, especially those with a desire for fertility. We performed this meta-analysis to examine the effectiveness and safety of thermal ablative methods and myomectomy for the treatment of uterine fibroids.
Methods
The present meta-analysis was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [Citation19].
Eligibility criteria
Articles were included if they: (1) were comparative trials; (2) compared thermal ablative methods and myomectomy for the treatment of uterine fibroids; and (3) were published in English.
Exclusion criteria
Articles were excluded if they were: (1) animal experiments; (2) duplicated trials; (3) trials without comparative groups; (4) reviews; (5) comments; (6) case reports; or (7) publications not in English.
Sources of information
We searched EMBASE, PubMed, the Cochrane Central Register of Controlled Trials, Scopus, CINAHL, ClinicalTrials.gov and Web of Science databases for relevant trials to April 2021. Studies were identified using the following MeSH descriptors: ‘thermal’, ‘ablation’, ‘myolysis’, ‘coagulation’, ‘myomectomy’ and ‘Leiomyoma’.
Study selection
Two investigators performed the literature search and criteria selection independently. Retrieved articles would be included in our meta-analysis if they met the eligible criteria. Any disagreements were resolved by consensus.
Data collection process
Two reviewers extract data independently based on a previously designed Excel form. Reintervention rate was regarded as the primary endpoint. Secondary endpoints included major adverse events, hospital stay, pregnancy rate, uterine fibroid symptom (UFS) score after operation, quality of life (QoL) score after operation and transformed symptom severity (tSS) score after operation. Extracted data included the first author, published time, country, study type, age of patients and type of interventions. The data form included mean (standard deviation (SD)) values and number of events. However, when only the median, median (range) or median (Q1–Q3) values were reported, the estimated mean (SD) values were attained based on the estimated formula reported [Citation20,Citation21].
Risk of bias and applicability
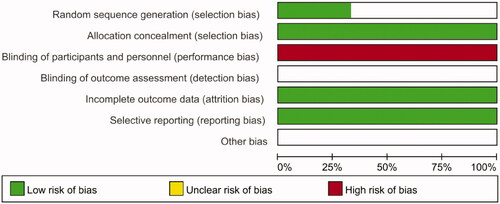
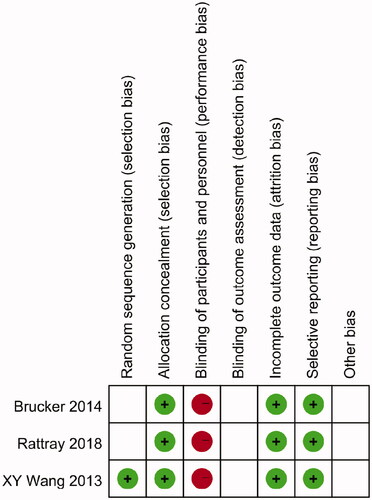
The quality assessment for randomized controlled trials (RCTs) was performed via the Cochrane Collaboration tool, which incorporated six items including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. The quality assessment for observational trials was performed by the Newcastle-Ottawa scale. A score of 7–9 was defined as ‘good’, 4–6 as ‘fair’ and <4 as ‘poor’ [Citation22].
Summary measures
The results were expressed as the risk ratio (RR) or mean difference, with 95% CIs. Standardized mean difference (SMD) with 95% CI was calculated for continuous values.
Synthesis of results
The meta-analysis was performed using STATA software version 14.0 (STATA Corporation, College Station, TX).
Heterogeneity was evaluated by using χ2 and I2 test. Heterogeneity was assumed to be high when p<.1 in the χ2 test and the I2 index was over 50%. A fixed-effects model was used for the meta-analysis if there was no significant heterogeneity; otherwise, a random-effects model was used. Sensitivity analyses were conducted by removing the included studies one by one if heterogeneity was significant [Citation23]. Publication bias was illustrated using a Begg funnel plot [Citation24]. p Values less than .05 were considered statistically significant.
Results
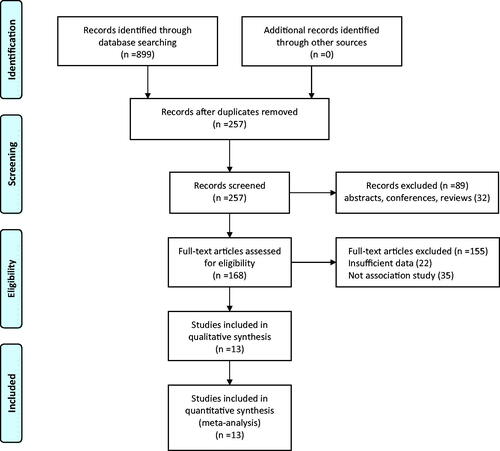
We identified 899 trials from the selected seven databases. A total of 642 trials were excluded based on the inclusion and exclusion criteria. After reviewing the full text of the remaining 257 trials, 10 observational studies [Citation25–34] and three RCTs [Citation35–37] were included finally. They were then selected for subsequent meta-analysis (). Basic information about these 13 trials is shown in .
Table 1. Basic information of all included studies used for meta-analysis.
Risk of bias and concerns regarding applicability
We assessed the three RCTs based on the Cochrane Collaboration risk-of-bias tool ( and ). At the same time, the 10 observational studies were assessed based on the Newcastle-Ottawa scale ().
Measured outcomes
The pooled results of all measured outcomes are summarized in .
Table 2. The pooled results of all meta-analysis.
Reintervention rate
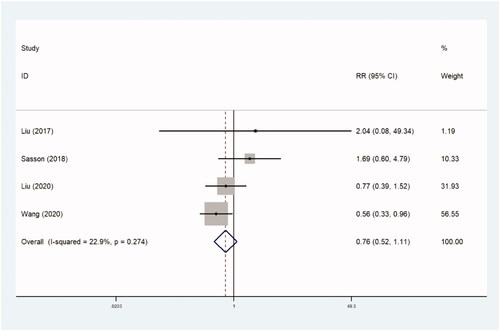
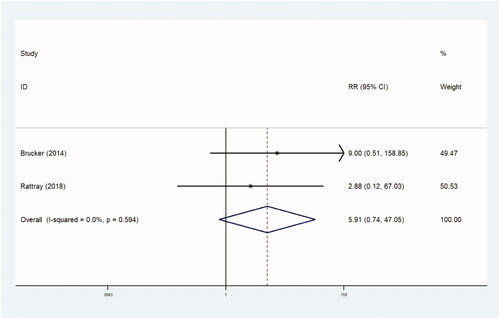
The incidence of reintervention was recorded in four trials comparing 827 patients in observational studies (RR, 0.760 [95% CI, 0.519–1.115], p=.160) ( and ) and two trials comparing 95 patients in RCTs (RR, 5.905 [95% CI, 0.741–47.046], p=.094) ( and ). There were no statistical differences between the two groups.
Table 3a. Reintervention rate (observational studies).
Table 3b. Reintervention rate (RCTs).
Major adverse events
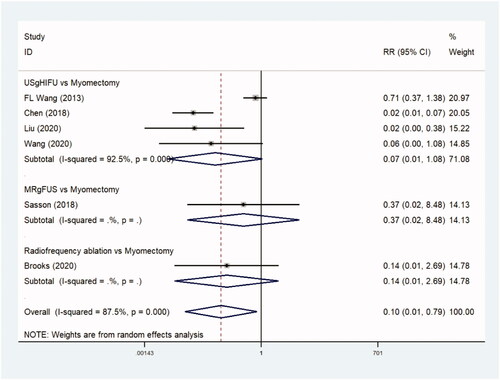
The incidence of major adverse events was reported in six trials comparing 2761 patients in observational studies, and the thermal ablative treatment group was associated with less major adverse events (only ultrasound guided high-intensity focused ultrasound) in comparison with the myomectomy group (RR, 0.111 [95% CI, 0.070–0.175], p=.0) ( and ).
Table 4. Major adverse events.
Hospital stays
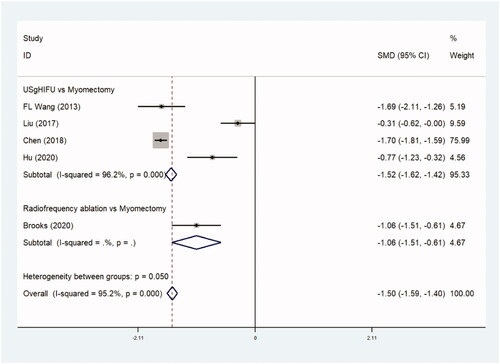
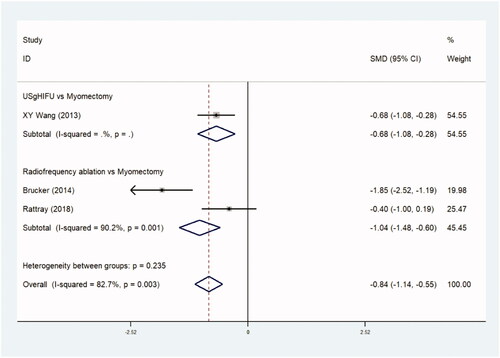
Hospital stays were reported in five trials comparing 2404 patients in observational studies (–0.1497 day, [95% CI, −1.593 to −0.321], p=.0) ( and ) and three trials comparing 195 patients in RCTs. (–0.844 day, [95% CI, −0.1.142 to −0.546], p=.0) ( and ). The duration of hospital stay was shorter in the thermal ablative treatment group than that in the myomectomy group.
Table 5a. Hospital stay (observational studies).
Table 5b. Hospital stay (RCTs).
Pregnancy rate
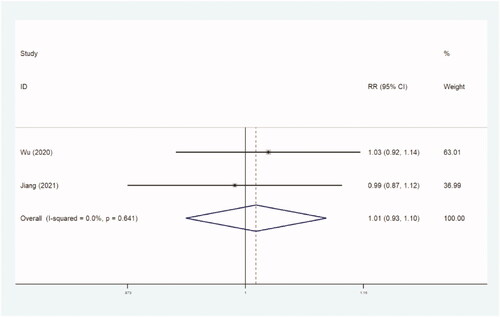
Two studies with 1002 women reported the outcome of pregnancy rate. There was no significant difference between the two groups in incidence of pregnancy rate (RR, 1.012 [95% CI, 0.933–1.098] p=.796) ( and ).
Table 6. Pregnancy rate.
Assessment of treatment outcomes
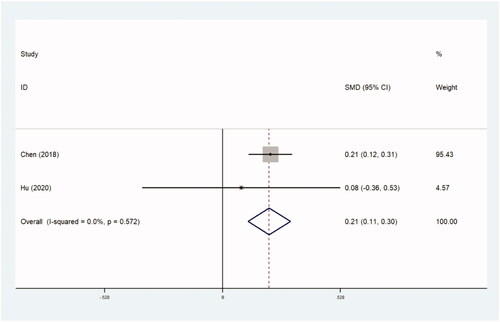
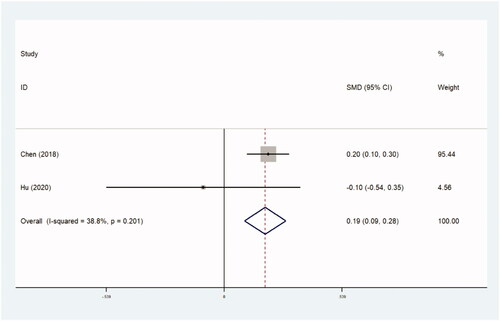
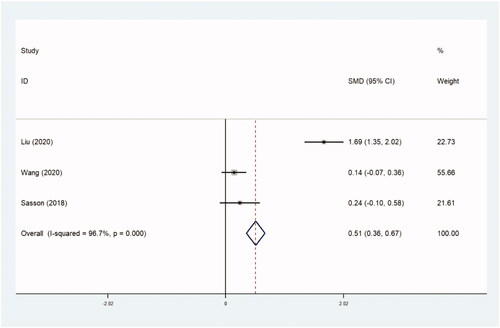
The assessment of treatment outcomes included UFS scores, QoL scores and tSS scores after operation. UFS scores and QoL scores were obtained using the UFS and QoL questionnaires [Citation38]. Both scores ranged from 0 to 100. A higher score on the UFS questionnaire indicates more serious symptoms, and on the QoL questionnaire indicates a better QoL. The tSS score was measured using the following formula: transformed score=(raw score – 8)/32 × 100. Three trials with 2363 women compared UFS scores (0.252 [95% CI, 0.165–0.339]; p=.0) ( and ), two trials with 2017 patients compared QoL scores (0.188 [95% CI, 0.093–0.283]; p=.0) ( and ), and three trials with 694 women compared tSS scores (0.515 [95% CI, 0.355–0.674]; p=.0) ( and ). Higher UFS, tSS and QoL scores after operation were found in the thermal ablative treatment group than those in the myomectomy group.
Table 7. UFS scores.
Table 8. QoL scores.
Table 9. tSS scores.
Discussion
Uterine fibroids are common in women of reproductive age. For these patients, pharmacotherapy may not produce satisfactory results, and there is an urgent to find an optimal therapeutic option. Myomectomy has long been considered the standard treatment for symptomatic fibroids in patients who desire to preserve their fertility. Abdominal, laparoscopic or hysteroscopic myomectomies are the main surgical modalities in use. Nevertheless, thermal ablative methods have proven to be effective alternatives to traditional myomectomy. The goal of this meta-analysis was to examine the comparative efficacy and safety of thermal ablative methods and myomectomy for the treatment of uterine fibroids.
The modalities of thermal ablative treatment for uterine fibroids vary in clinical practice. The result of therapy after percutaneous magnetic resonance (MR) image-guided laser ablation was found to be comparable to hysterectomy for the management of menorrhagia caused by fibroids [Citation39]. Berman et al. [Citation40] reported a significant improvement in symptoms by using radiofrequency volumetric thermal ablation in patients with fibroids. Zhang et al. [Citation41] found that fibroids could shrink by 93.1% without complications and fever, after percutaneous microwave thermal ablation. The safety and efficacy of focused ultrasound guided by ultrasound or MRI have been confirmed for the treatment of fibroids [Citation42,Citation43]. In the relevant 13 studies, we included, focused ultrasound therapy was used in 10 trials, and radiofrequency therapy was used in the rest.
Ji et al. [Citation44] showed that high-intensity focused ultrasound treatment resulted in fewer clinical complications and adverse events than treatment with mifepristone, myomectomy or hysterectomy for uterine fibroids. Wang et al. [Citation45] reported that high-intensity focused ultrasound was associated with higher risk of reintervention and shorter duration of hospital stay than myomectomy. Lin et al. [Citation46] found an improved QoL and low-risk of reintervention after laparoscopic radiofrequency ablation for symptomatic fibroids. Though some systematic reviews have compared the efficacy of individual thermal ablation methods with other treatments for uterine fibroids, limited data can be found comparing thermal ablative methods and myomectomy for the treatment of uterine fibroids. We believe that our study makes a significant contribution to the literature because it clarifies the advantages of the thermal ablation procedures compared to myomectomy.
In our meta-analysis, 13 trials comprising 4205 patients were included. This study compared two thermal ablation methods, focused ultrasound and radiofrequency ablation with myomectomy. There was no significant difference in the reintervention rate between the thermal ablative treatment and myomectomy group, which differs from a previous meta-analysis comparing only focused ultrasound ablation with myomectomy [Citation45]. In addition, fewer major adverse events and shorter duration of hospital stay were found in the thermal ablative treatment group, which is consistent with the previous meta-analysis [Citation45]. The results showed no significant differences in pregnancy rate between the thermal ablative treatment and myomectomy groups. In the procedures of thermal ablative treatment, unlike myomectomy, the leiomyomas are left in situ, so the UFS and tSS scores after operation may be higher in the thermal ablative treatment group than those in the myomectomy group. Nevertheless, the differences in absolute scores between the baseline assessed pre-operatively and the endpoint of observation after operation in the ablative treatment group could be higher than those in the myomectomy group [Citation26]. This indicates that symptoms caused by fibroids improved much more in the thermal ablative treatment group than in the myomectomy group, which could explain why the QoL score was higher in the ablative treatment group. However, large-scale randomized trials are needed to validate the findings.
There are some limitations for our meta-analysis. First, Begg’s funnel plot test was fairly powerful when meta-analyses with 75 articles were included [Citation24], and it might be with relatively low power when used to identify publication bias in this study. Second, there were limited RCTs were included. Non-RCTs bring selection bias owing to their nonrandomized unblinded nature. Third, significant heterogeneity was found in some of the outcomes. This may be caused by included observational studies and small single-center studies. Moreover, the current study failed to be registered in PROSPERO system, and it might bring a small deviation to our meta-analysis. However, the present meta-analysis was performed strictly in accordance with the PRISMA guidelines.
In conclusion, based on this study and available evidence, thermal ablative methods were found to be not inferior to myomectomy in the treatment of uterine fibroids. But there is still lack of large-scale RCTs. Those results need further validation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22(4):571–588.
- Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet. 2015;131(2):117–122.
- Lethaby A, Vollenhoven B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin Evid. 2007;2007:0814.
- Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia. 2015;31(3):272–279.
- Stewart JK. Uterine artery embolization for uterine fibroids: a closer look at misperceptions and challenges. Tech Vasc Interv Radiol. 2021;24(1):100725.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98(1):29–34.
- Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191(1):18–21.
- Gkentzis A, Oades G. Thermal ablative therapies for treatment of localised renal cell carcinoma: a systematic review of the literature. Scott Med J. 2016;61(4):185–191.
- Moynagh MR, Kurup AN, Callstrom MR. Thermal ablation of bone metastases. Semin Intervent Radiol. 2018;35(4):299–308.
- Thompson SM, Schmitz JJ, Schmit GD, et al. Image-Guided thermal ablative therapies in the treatment of sarcoma. Curr Treat Options Oncol. 2017;18(4):25.
- Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what's new in 2019. Chin Clin Oncol. 2019;8(6):58.
- Goldberg SN, Gazelle GS, Halpern EF, et al. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3(3):212–218.
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–331.
- Solbiati L, Ierace T, Goldberg SN, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202(1):195–203.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Lyon PC, Rai V, Price N, et al. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschall Med. 2020;41(05):550–556.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the SONATA pivotal trial. J Gynecol Surg. 2019;35(6):345–349.
- Zaher S, Gedroyc WM, Regan L. Patient suitability for magnetic resonance guided focused ultrasound surgery of uterine fibroids. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):98–102.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- McGrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605.
- Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–262.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Brooks EA, Singer AM, Delvadia DR, et al. The CHOICES study: facility level comparative cost, resource utilization, and outcomes analysis of myomectomy compared to transcervical fibroid ablation. Clinicoecon Outcomes Res. 2020;12:299–306.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364.
- Hu L, Zhao JS, Xing C, et al. Comparison of focused ultrasound surgery and hysteroscopic resection for treatment of submucosal uterine fibroids (FIGO type 2). Ultrasound Med Biol. 2020;46(7):1677–1685.
- Jiang Z, Li Q, Li W, et al. A comparative analysis of pregnancy outcomes of patients with uterine fibroids after high intensity focused ultrasound ablation and laparoscopic myomectomy: a retrospective study. Int J Hyperthermia. 2021;38(1):79–84.
- Liu X, Tang J, Luo Y, et al. Comparison of high-intensity focused ultrasound ablation and secondary myomectomy for recurrent symptomatic uterine fibroids following myomectomy: a retrospective study. BJOG. 2020;127(11):1422–1428.
- Liu Y, Ran W, Shen Y, et al. High-intensity focused ultrasound and laparoscopic myomectomy in the treatment of uterine fibroids: a comparative study. BJOG. 2017;124(Suppl. 3):36–39.
- Mohr-Sasson A, Machtinger R, Mashiach R, et al. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am J Obstet Gynecol. 2018;219:375.e1–375.e7.
- Wang F, Tang L, Wang L, et al. Ultrasound-guided high-intensity focused ultrasound vs laparoscopic myomectomy for symptomatic uterine myomas. J Minim Invasive Gynecol. 2014;21(2):279–284.
- Wang Y, Liu X, Wang W, et al. Long-term clinical outcomes of US-guided high-intensity focused ultrasound ablation for symptomatic submucosal fibroids: a retrospective comparison with uterus-sparing surgery. Acad Radiol. 2021;28(8):1102–1107.
- Wu G, Li R, He M, et al. A comparison of the pregnancy outcomes between ultrasound-guided high-intensity focused ultrasound ablation and laparoscopic myomectomy for uterine fibroids: a comparative study. Int J Hyperthermia. 2020;37(1):617–623.
- Brucker SY, Hahn M, Kraemer D, et al. Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet. 2014;125(3):261–265.
- Rattray DD, Weins L, Regush LC, et al. Clinical outcomes and health care utilization pre- and post-laparoscopic radiofrequency ablation of symptomatic fibroids and laparoscopic myomectomy: a randomized trial of uterine-sparing techniques (TRUST) in Canada. Clinicoecon Outcomes Res. 2018;10:201–212.
- Wang X, Qin J, Wang L, et al. Effect of high-intensity focused ultrasound on sexual function in the treatment of uterine fibroids: comparison to conventional myomectomy. Arch Gynecol Obstet. 2013;288(4):851–858.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99(2):290–300.
- Hindley JT, Law PA, Hickey M, et al. Clinical outcomes following percutaneous magnetic resonance image guided laser ablation of symptomatic uterine fibroids. Hum Reprod. 2002;17(10):2737–2741.
- Berman JM, Guido RS, Garza Leal JG, et al. Three-year outcome of the halt trial: a prospective analysis of radiofrequency volumetric thermal ablation of myomas. J Minim Invasive Gynecol. 2014;21(5):767–774.
- Zhang J, Feng L, Zhang B, et al. Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment—a clinical study. Int J Hyperthermia. 2011;27(5):510–516.
- Kim YS, Bae DS, Park MJ, et al. Techniques to expand patient selection for MRI-guided high-intensity focused ultrasound ablation of uterine fibroids. AJR Am J Roentgenol. 2014;202(2):443–451.
- Wang W, Wang Y, Wang T, et al. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol. 2012;22(11):2553–2558.
- Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181–1188.
- Wang Y, Geng J, Bao H, et al. Comparative effectiveness and safety of high-intensity focused ultrasound for uterine fibroids: a systematic review and meta-analysis. Front Oncol. 2021;11:600800.
- Lin L, Ma H, Wang J, et al. Quality of life, adverse events, and reintervention outcomes after laparoscopic radiofrequency ablation for symptomatic uterine fibroids: a meta-analysis. J Minim Invasive Gynecol. 2019;26(3):409–416.