Abstract
Objectives
This study aimed to determine the performance of Sonazoid-based contrast-enhanced ultrasound (CEUS) in the microwave ablation (MWA) of primary hyperparathyroidism (pHPT).
Methods
Forty patients with pHPT were enrolled and treated with percutaneous ultrasound (US)-guided MWA assisted by CEUS. All patients underwent immediate CEUS examinations following MWA. On post-ablation day 1, patients who did not display a decrease in intact parathyroid hormone (iPTH) levels to the norm were examined by CEUS to evaluate an incomplete ablation. We compared the serum iPTH and calcium levels and the nodule volumes before and after MWA. The complications were evaluated during and after treatment.
Results
Immediately following MWA, CEUS demonstrated complete ablation with all 44 parathyroid nodules. On post-ablation day 1, five nodules in five patients displayed annular enhancement around the ablation zone on CEUS. The average maximum diameters of the nodules and the ablation zone were 1.09 ± 0.28 cm and 1.36 ± 0.23 cm, respectively. An ablation zone larger than the primary lesion (p < 0.05) generated a higher rate of complete ablation. Compared with pre-MWA, serum iPTH and calcium levels were significantly improved. Treatment success was achieved in 38 patients (95%). Hoarseness was a major complication in six patients (15%); however, it improved spontaneously within 1–4 months. We observed two recurrences (2/40, 5%) at 9 months and 11 months following MWA, respectively.
Conclusion
US-guided percutaneous MWA assisted by CEUS for pHPT is an effective and safe therapy. CEUS can avoid operative failure and improve the cure rate.
Introduction
Primary hyperparathyroidism (pHPT) is a common endocrine disorder, and its incidence increases with age. The prevalence of pHPT in patients aged over 65 years and postmenopausal women is 1.5% and 2.1–3.4%, respectively [Citation1]. The disease manifests as hypercalcemia and elevated intact parathyroid hormone (iPTH) levels [Citation2]. Recently, the percentage of individuals with normocalcemic pHPT has increased from 21% to 52.5% owing to the development of biochemical detection methods [Citation3]. Classical pHPT refers to a symptomatic, multi-system disorder characterized by skeletal, renal, gastrointestinal, neurological, and psychiatric manifestations as well as increased mortality [Citation1,Citation4,Citation5]. However, most patients are asymptomatic upon discovery.
Traditional surgical resection is the primary treatment for pHPT [Citation6,Citation7]. However, this surgery is associated with risks, such as general anesthesia, wound infection, and intraoperative hemorrhage, which may be high in older patients, particularly in those with comorbidities [Citation8]. In recent years, researchers have demonstrated the efficacy of ultrasound (US)-guided microwave ablation (MWA), a minimally invasive alternative, in the treatment of pHPT [Citation9–12]. However, approximately 10–20% of the patients do not meet the criteria for treatment success [Citation9,Citation12,Citation13]. A recent retrospective study suggested that small parathyroid nodules (maximum diameter <0.6 cm) and incomplete ablation are the key factors that lead to operative failure [Citation13].
Contrast-enhanced ultrasound (CEUS) substantially improves spatial resolution, accurately locates the lesion, and differentiates small lesions from surrounding tissues. The use of CEUS to assess the ablation area immediately following laser ablation (LA) optimizes the results [Citation14]. However, MWA results in higher thermal efficiency than LA; therefore, the lesion necrosis boundary may display a different CEUS pattern. To our knowledge, no study has reported on the application of the contrast agent Sonazoid in evaluating the therapeutic effect of MWA for pHPT. Therefore, we aimed to determine the performance of Sonazoid-based CEUS and assess if it results in complete ablation in patients with pHPT, thereby increasing the cure rate.
Methods
Patients
This retrospective study protocol was approved by the Ethics Committee of the Gansu Provincial Hospital (No. 2021-243). Written informed consent had been obtained from all patients before each procedure, and the requirement of informed consent was waived for this retrospective investigation. Between August 2018 and February 2020, we reviewed the data of 40 patients with pHPT (12 men and 28 women) treated with MWA.
The inclusion criteria were as follows: (1) diagnosed with pHPT [Citation6,Citation15,Citation16]; (2) at least one enlarged parathyroid gland clearly displayed on US; (3) increased 99 m Tc-sestamibi (MIBI) radionuclide concentrations in both early and delayed phases; (4) refusal or ineligibility for surgery; and (5) no intractable coexisting morbidity, such as cardiac insufficiency or hypertension.
The exclusion criteria were as follows: (1) abnormal coagulation profile, (2) abnormal vocal cord movement, and (3) malignant suspicious parathyroid tumor on US (irregular shape, non-circumscribed margin, and local invasion).
Clinical data, biochemical tests, routine US, CEUS, MIBI, and technetium 99-m-labeled sestamibi single-photon emission computed tomography (99 m Tc-sestamibi SPECT) were used together for preoperative localization and qualitative diagnosis. Preoperative fine-needle aspiration biopsy helped us identify ectopic glands and was performed in seven patients, with ectopic parathyroid glands and small parathyroid nodules (maximum diameter < 0.7 cm) in four and three patients, respectively.
pHPT diagnosis on US and CEUS was based on the following criteria: (1) enlarged hypoechoic parathyroid glands with clearly defined margins, (2) no suspicion of lymph node metastasis, and (3) enlarged hypoechoic parathyroid glands displaying hyperenhancement in the arterial phase on CEUS.
CEUS examination
All patients underwent US and CEUS using a LOGIQ E9 system (GE Healthcare, Chicago, IL) with a 6–15 MHz linear array probe, and the US contrast agent was Sonovue (Bracco, Milan, Italy).
Before ablation, we used CEUS to observe the size and position of the nodules upon our failure to locate them by routine US. Following ablation, CEUS was used to examine the original ablation zone. Abnormal enhancement inside or around it was used to confirm residual or recurrent lesions. Additional overlapping ablations were performed if required, and the size of the immediate post-ablation zone was measured on CEUS in all patients.
MWA procedure
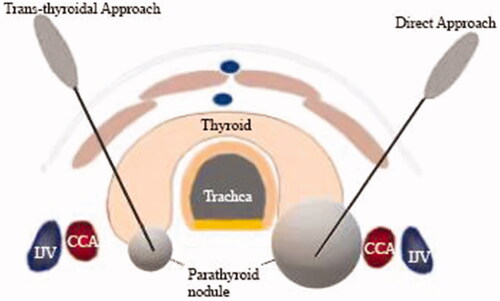
MWA of the parathyroid gland was performed using a cooled MWA antenna (17 G) with a 0.4-cm tip (Intelligent Basic Type Microwave Tumor Ablation System, Nanjing ECO Microwave System, Nanjing, China). The procedure was performed by a single doctor with >12 years of experience in MWA treatment of thyroid and parathyroid nodules. The patients were placed in a supine position with routine disinfection and local anesthesia with 2% lidocaine. A liquid isolation zone was created by injecting normal saline around the parathyroid nodule to avoid thermal injury to adjacent structures, followed by CEUS to observe the size and position of the nodule. Before MWA, the needle-tract route was determined using US or CEUS and two different needle-tract routes (). An MWA antenna was inserted into the nodule within the carotid sheath under real-time US guidance. Single or multipoint overlapping ablation was selected to ablate the nodule according to its volume, and the therapy was terminated upon covering the entire nodule by the hyperechoic zone. CEUS was performed to assess residual lesions; when the nodule showed complete non-enhancement within the nodule, complete ablation was defined, and if there was abnormal enhancement inside or around the ablation site, further ablation was performed immediately. Vital signs were continuously monitored, and the patients were requested to report any discomfort (the MWA was stopped immediately on encountering voice hoarseness until its recovery) during the procedure.
Follow-up and outcome assessment
The follow-up consisted of US, CEUS, and blood biochemistry (including serum iPTH and calcium levels), conducted on 1 day, 7 days, 1 month, 6 months, and 12 months following ablation. We calculated the nodule volume according to the formula V = πabc/6, where V represents the volume, a represents the length, and b and c represent the two perpendicular diameters. The volume reduction rate (VRR) was defined as (Vbefore–Vafter)/Vbefore. We used the visual analog scale (VAS) to assess pain levels ranging from 1 (no pain) to 10 (intolerable pain).
Technical success was defined as complete ablation following appropriate treatment, according to the protocol. The criteria for clinical success were defined as normal values of serum iPTH and calcium levels that lasted for a minimum of 6 months following MWA. Operative failure was defined as the failure to normalize serum iPTH and/or calcium levels within 6 months of MWA. Recurrent pHPT was defined as the relapse of hypercalcemia and/or elevated iPTH levels 6 months following MWA [Citation6]. Major and minor complications were defined according to the criteria of the Society of Interventional Radiology.
Statistical analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences software for Windows 22.0 (SPSS Inc., Chicago, IL). Continuous data were presented as the mean ± standard deviation or median and interquartile range. whereas categorical variables are presented as frequencies. The serum calcium and iPTH levels were compared at baseline and at each follow-up using paired-sample t tests and paired-sample Wilcoxon signed-rank tests. Statistical significance was set at p < 0.05.
Results
Patients
We enrolled a total of 40 patients with pHPT comprising 44 parathyroid nodules (four patients with two nodules and the remaining with one nodule). depicts the flowchart of patient selection. Their ages ranged from 21 years to 78 years (mean, 54.9 ± 11.8 years). A direct approach was made in seven patients (7 nodules) with large nodules that laterally projected to the thyroid or within the carotid sheath and upper mediastinum, compared with a trans-thyroidal approach in 33 patients (37 nodules) with small nodules underlying the thyroid gland or within the thyroid. summarizes their baseline clinical and treatment features.
Figure 2. Flow chart of patients included in the study. sHPT: secondary hyperparathyroidism; MIBI: 99 m Tc-sestamibi; pHPT: primary hyperparathyroidism; MWA: microwave ablation; and PTX: parathyroidectomy.
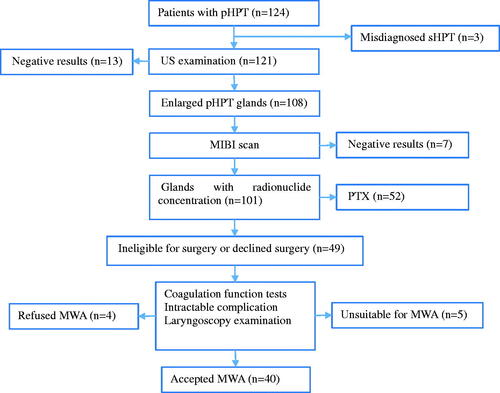
Table 1. Baseline clinical and treatment characteristics of patients with pHPT (n = 40).
MWA results
No enhancement was observed in the 40 patients with 44 nodules on CEUS immediately post-ablation, which was marked as complete ablation. However, on post-ablation day 1, the iPTH level did not decrease to normal in seven patients, and five patients of which revealed an annular enhancement area around the ablation zone on CEUS, thus indicating incomplete ablation. All of them achieved complete ablation after an additional ablation (), thereby leading to a final complete ablation rate of 100%. We measured the size of the immediate post-ablation zone using CEUS in all patients. The average maximum diameter of the nodule before ablation and the ablation zone after the first ablation were 1.09 ± 0.28 cm and 1.36 ± 0.23 cm, respectively. The ablation zone was larger than the primary lesion in 35 patients with 39 nodules, and similar to the size of the primary lesion in five patients with five nodules. Fisher’s exact test revealed a correlation between the size of the ablation zone and complete ablation (p < 0.05); a higher rate of complete ablation was achieved if the ablation zone following the first ablation was larger than the primary lesion. The follow-up duration was extremely short (median: 11.6 months). Compared with pre-MWA, serum iPTH and calcium levels significantly improved post-MWA (). Treatment success was achieved in 38 patients (95%). During the follow-up, we identified local recurrence in two patients (5%), with a recurrence period of 9 months and 11 months (the average recurrence period was 10 months). Moreover, one patient underwent two MWA treatments. The univariate analysis demonstrated a significant correlation between 25-hydroxyvitamin D deficiency and MWA recurrence (p < 0.05).
Figure 3. CEUS displaying incomplete ablation in a 46-year-old woman with pHPT. (A) CEUS images immediately after MWA: non-enhancement area covers the pHPT nodule (arrows). (B) Color Doppler images 1 day after MWA: signs of punctate blood flow signals of the margin of the ablation zone (arrows). (C) CEUS images 1 day after MWA: the arterial phase demonstrates an annular enhancement area (arrows) around the ablation zone. (D) US images 1 year after ablation: the VRR of the ablation zone (arrows) is 83.6% ablation zone (arrows). CEUS: contrasted-enhanced ultrasound; pHPT: primary hyperparathyroidism; MWA: microwave ablation; VRR: volume reduction rate; US: ultrasound.
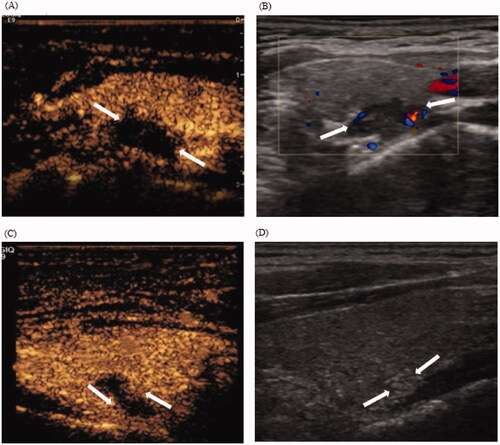
Table 2. Changes in US features and serum biochemical data before MWA and at each follow-up.
Adverse events
Eighteen patients (45%) felt slight pain and burning sensations in the neck during the ablation; however, they were able to tolerate the whole procedure without the need for additional anesthesia, and this pain was rated by all patients as <3 on the VAS. All patients felt neck swelling, which spontaneously resolved following MWA. Two patients (5%) had cough, which resolved within few days after MWA, whereas transient hoarseness was observed in six patients (15%). They achieved complete recovery within 4 months, without the need for clinical treatment. Transient hypocalcemia (4/40, 10%) was reported 1 day post-MWA, which recovered with calcium supplementation within few days. Transient hypoparathyroidism (1/40, 2.5%) occurred after MWA and spontaneously recovered after 1 week. No cases of dysphonia, neck hematoma, local infection, or vital organ injury occurred during the perioperative period ().
Table 3. Complications and side effects following MWA.
Discussion
Our purpose was to determine the performance of Sonazoid-based CEUS as an adjunct to MWA in the treatment of patients with pHPT. This study predominantly included women aged ≥45 years, thus confirming the prevalence of pHPT in postmenopausal women [Citation17]. Sonazoid-based CEUS increased the cure rate of pHPT with MWA to optimize therapy. All patients displayed tolerance and no major complications [Citation18].
The patients with pHPT treated with MWA displayed a clinical success rate of 95%, higher than the previously reported rates of 83–89% [Citation9,Citation12,Citation13]. The present study is comparable to another that reported a parathyroidectomy cure rate of 95–98% [Citation19]. VRRs were 80.2% at the last follow-up, consistent with values ranging from 79% to 89% using MWA [Citation12,Citation20]. Two patients (5%) displayed recurrence, which can be attributed to 25-hydroxyvitamin D deficiency. Cosman et al. [Citation21] suggested that supplementation could safely begin at a dose of 1000–2000 IU/d before ablation in patients with vitamin D deficiency.
The contrast agent Sonazoid consists of several biocompatible materials containing low solution and infusibility gases, such as sulfur hexafluoride. CEUS is a convenient imaging technique that not only guides the operation but also follows residual or recurrent lesions with high accuracy. Currently, CEUS is successfully used to assess the efficacy of thermal ablation in liver tumors, renal cell carcinomas, and benign thyroid nodules [Citation22–24].
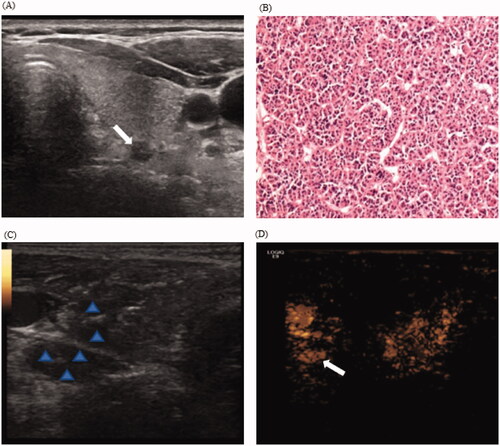
Small parathyroid nodules are the key factor that lead to MWA failure [Citation13]. In our study, US did not detect three parathyroid nodules (maximum diameter <0.7 cm) in three patients following the creation of the liquid isolation zone. The liquid diffused unevenly and caused nodular changes, leading to echogenicity of the liquid consistent with that of the parathyroid nodules, both of which were hypoechoic. However, the injection of the contrast agent enhanced the parathyroid nodules, in contrast to the surrounding tissues (). Moreover, we could accurately locate the nodules and successfully ablate them. In our study, CEUS immediately following MWA demonstrated complete ablation in all patients. However, there was an annular enhancement area around the ablation zone in five patients with nodules on post-ablation day 1, thus suggesting incomplete ablation, which can be attributed to the following reasons: first, the ablation process may lead to temporary occlusion of the microvessels, thereby resulting in a blood supply that did not accurately reflect the lesions [Citation25]. Second, the intensity of the reflected echoes decreases significantly with depth, i.e. deep lesions or those in obese patients may appear to have reduced vasculature [Citation26]. Third, after creating the isolation zone and performing ablation, the gas interposition following ablation and tissue carbonization may also interfere with the quality of CEUS evaluation. On post-ablation day 1, the image quality substantially improved with an improvement in the ablation area, which better reflected the actual blood supply to the lesion than that immediately after ablation. These patients underwent additional overlapping ablations for complete ablation; these nodules had no residual or recurrence lesions during the follow-up. The measurement of perfusion defect area indicated that an ablation range greater than the primary nodule volume would likely indicate complete ablation. Therefore, our results suggested that an evaluation of the ablation zone with CEUS on post-ablation day 1, but not immediate post-ablation, accurately reflected lesion perfusion and achieved complete ablation. In agreement with previous research, the treatment was tolerated by all patients [Citation12,Citation18,Citation27].
Figure 4. CEUS displaying hidden nodule during hydrodissection in a 55-year-old woman with pHPT. (A) Routine US display a hypoechoic pHPT nodule with maximum diameter of 0.5 cm; (B) Preoperative nodule FNA biopsy indicates a parathyroid adenoma with the formation of microfollicles (H&E stain, ×200). (C) During hydrodissection, nodular changes in the surrounding tissues and the pHPT nodule are hypoechoic (arrow). (D) The injection of contrast agents display the uniform enhanced parathyroid nodule (arrows). CEUS: contrasted-enhanced ultrasound; pHPT: primary hyperparathyroidism; FNA: fine-needle aspiration.
The small sample size was a major limitation of our study. This necessitates extensive prospective studies to determine the findings of the present research. On confirming our results with a larger patient population, MWA protocols could be modified to include CEUS in every ablation procedure. Incomplete ablation could be accurately evaluated by CEUS on post-ablation day 1; simultaneously, CEUS could detect shadow nodules during the MWA procedure.
In conclusion, US-guided percutaneous MWA assisted by Sonazoid-based CEUS for pHPT is an effective and safe therapy. CEUS could detect small nodules and assist treatment response evaluation, besides facilitating subsequent additional MWA, thus eventually improving the cure rate.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
Reference
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178.
- Applewhite MK, Schneider DF. Mild primary hyperparathyroidism: a literature review. Oncologist. 2014;19(9):919–929.
- Zhao L, Liu JM, He XY, et al. The changing clinical patterns of primary hyperparathyroidism in Chinese patients: data from 2000 to 2010 in a single clinical center. J Clin Endocrinol Metab. 2013;98(2):721–728.
- Minisola S, Gianotti L, Bhadada S, et al. Classical complications of primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(6):791–803.
- Ejlsmark-Svensson H, Bislev LS, Lajlev S, et al. Prevalence and risk of vertebral fractures in primary hyperparathyroidism: a nested case-control study. J Bone Miner Res. 2018;33(9):1657–1664.
- Wilhelm SM, Wang TS, Ruan DT, et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968.
- Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341(17):1249–1255.
- Egan RJ, Scott-Coombes DM. The surgical management of sporadic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32(6):847–859.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Wei Y, Peng CZ, Wang SR, et al. Microwave ablation versus radiofrequency ablation for primary hyperparathyroidism: a multicenter retrospective study. Int J Hyperthermia. 2021;38(1):1023–1030.
- Wei Y, Peng L, Li Y, et al. Clinical study on safety and efficacy of microwave ablation for primary hyperparathyroidism. Korean J Radiol. 2020;21(5):572–581.
- Ying W, Zhen-Long Z, Xiao-Jing C, et al. A study on the causes of operative failures after microwave ablation for primary hyperparathyroidism. Eur Radiol. 2021;31(9):6522–6530.
- Jiang T, Chen F, Zhou X, et al. Percutaneous ultrasound-guided laser ablation with contrast-enhanced ultrasonography for hyperfunctioning parathyroid adenoma: a preliminary case series. Int J Endocrinol. 2015;2015:1–6.
- Zini M, Attanasio R, Cesareo R, Italian Association of Clinical Endocrinologists, et al. AME position statement: primary hyperparathyroidism in clinical practice. J Endocrinol Invest. 2012;35(7 Suppl):2–21.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Palmér M, Jakobsson S, Akerström G, et al. Prevalence of hypercalcaemia in a health survey: a 14-year follow-up study of serum calcium values. Eur J Clin Invest. 1988;18(1):39–46.
- Wei Y, Peng LL, Zhao ZL, et al. Complications encountered in the treatment of primary and secondary hyperparathyroidism with microwave ablation – a retrospective study. Int J Hyperthermia. 2019;36(1):1264–1271.
- Machado NN, Wilhelm SM. Diagnosis and evaluation of primary hyperparathyroidism. Surg Clin North Am. 2019;99(4):649–666.
- Wei Y, Peng CZ, Wang SR, et al. Effectiveness and safety of thermal ablation in the treatment of primary hyperparathyroidism: a multicenter study [J]. J Clin Endocrinol Metab. 2021;106(9):2707–2717.
- Cosman F, de Beur SJ, LeBoff MS, National Osteoporosis Foundation, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381.
- Lekht I, Gulati M, Nayyar M, et al. Role of contrast-enhanced ultrasound (ceus) in evaluation of thermal ablation zone. Abdom Radiol (NY). 2016;41(8):1511–1521.
- Li X, Liang P, Yu J, et al. Role of contrast-enhanced ultrasound in evaluating the efficiency of ultrasound guided percutaneous microwave ablation in patients with renal cell carcinoma. Radiol Oncol. 2013;47(4):398–404.
- Zhao CK, Xu HX, Lu F, et al. Factors associated with initial incomplete ablation for benign thyroid nodules after radiofrequency ablation: first results of ceus evaluation. CH. 2017;65(4):393–405.
- Qin X, Wang B, Li B, et al. Value of contrast-enhanced ultrasonography in radiofrequency ablation of secondary hyperparathyroidism. Ren Fail. 2021;43(1):445–451.
- Cokkinos DD, Antypa EG, Skilakaki M, et al. Contrast enhanced ultrasound of the kidneys: what is it capable of?. Biomed Res Int. 2013;2013:595873.
- Ye J, Huang W, Huang G, et al. Efficacy and safety of us-guided thermal ablation for primary hyperparathyroidism: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):245–253.