Abstract
Background
Hyperparathyroidism (HPT) is classified into primary HPT (PHPT), secondary HPT (SHPT), tertiary HPT (THPT), and pseudohyperparathyroidism. Parathyroid surgery is generally reserved for patients with symptomatic PHPT and asymptomatic patients who meet the surgical guideline criteria. However, the risk of complications and mortality after parathyroid gland surgery increases with increasing patient age.
Aim
This study aimed to review existing research on laser ablation, radiofrequency ablation, microwave ablation, and high-intensity focused ultrasound in the treatment of HPT and analyze its application prospects.
Conclusions
Thermal ablation is a good alternative treatment for patients with parathyroid hyperplasia who do not meet the criteria or decline surgery. Being a type of minimally invasive treatment, ultrasound-guided thermal ablation has the advantages of easy operation, rapid recovery, and reusability and is used widely.
Introduction
The parathyroid glands are located at the upper and lower poles of the posterior part of the thyroid gland. There are two pairs (four glands) of parathyroid glands that secrete mainly parathyroid hormone (PTH) and regulate the metabolism of calcium and phosphorus.
Hyperparathyroidism (HPT) is classified into primary HPT (PHPT), secondary HPT (SHPT), tertiary HPT (THPT), and pseudohyperparathyroidism [Citation1,Citation2]. PHPT is a primary disease of the parathyroid gland and is common among women, mainly those aged >50 years. The incidence of PHPT differs across sexes and ethnicities, according to a survey conducted in the United States that estimated a PHPT prevalence of 0.86% in the general population [Citation3]. HPT is caused by excessive synthesis and secretion of PTH by one or more of the four parathyroid glands. Based on concurrent normal or high (i.e., exceeding 10.5 mg/dL) serum calcium levels, PHPT is defined as either normo- or hypercalcemic HPT, respectively [Citation4].
The main cause of SHPT worldwide is the deficiency or failure of the kidney to convert vitamin D into its active form (1′-25′-OH-vitamin D). Thus, it represents the most common problem in the treatment of patients with end-stage renal disease, as it increases the risk of mortality [Citation5]. SHPT is characterized by continuously increasing serum PTH levels, parathyroid hyperplasia, and mineral metabolism disorders (e.g., calcium and phosphorus) [Citation6,Citation7]. Furthermore, THPT refers to those with chronic kidney disease (CKD) and SHPT remain hyperparathyroidism after kidney transplantation [Citation8]. It may also be difficult to distinguish from PHPT because serological results may be similar; however, THPT is usually caused by CKD or kidney transplantation, and phosphorus levels may be low, whereas serum calcium, phosphorus, and PTH levels are elevated [Citation9].
Parathyroid surgery is generally reserved for patients with symptomatic PHPT and asymptomatic patients who meet the surgical guideline criteria [Citation4,Citation10]. Surgical removal of the parathyroid glands or parathyroidectomy (PTx) is an alternative in cases of SHPT resistance to medical therapy [Citation11]. For patients with CKD, the longer the duration of CKD, the greater the demand for surgical treatment [Citation12,Citation13]. The National Kidney Foundation Kidney Disease Outcome Quality Initiative guidelines recommend PTx for patients on dialysis with persistent serum intact PTH (iPTH) >800 pg/mL, especially when SHPT results in hypercalcemia and/or hyperphosphatemia that are refractory to medical therapy [Citation14–16].
For patients with a poor cardiopulmonary function who are unsuitable for surgical procedures or who decline surgical procedures, ultrasound-guided ablation of a hyperplastic parathyroid gland (as a type of minimally invasive treatment) has the advantages of being safe, easy to perform, rapid recovery, and reusability. According to a meta-analysis, ablation of the parathyroid glands reduces the risk of hypocalcemia but increases the risk of persistence and recurrence of SHPT [Citation17].
To date, the efficacy and complications of various thermal ablation methods (laser ablation (LA), radiofrequency ablation (RFA), microwave ablation (MWA), and high-intensity focused ultrasound (HIFU)) for the treatment of HPT are unclear. Herein, we summarize and analyze various thermal ablation methods for HPT treatment.
Anatomy of the parathyroid glands and adjacent structures
The average dimensions of each gland are 5 mm × 3 mm × 1 mm (length × width × thickness), and each gland weighs approximately 60 mg [Citation18,Citation19]. The superior gland is relatively fixed and is located in the middle third of the posterior thyroid gland. The final position of the inferior gland changes considerably because of the relatively long descending process, with >50% located at the lower pole of the thyroid gland [Citation20–22]. There are abundant vascular network anastomoses between the parathyroid gland and the pharynx, larynx, trachea, and esophagus. The blood supply to the parathyroid gland mainly comes from the inferior thyroid artery and, in a few cases, from the superior thyroid artery. The venous system of the parathyroid gland is accompanied by the corresponding artery, which flows into the internal jugular vein. Furthermore, the lymphatic drainage of the parathyroid gland is similar to that of the thyroid gland, leading to the deep neck and anterior trachea [Citation23].
Etiology and treatment of HPT
The main function of the parathyroid glands is to secrete PTH and regulate the metabolism of calcium and phosphorus. Most PHPT cases are complicated with a single benign parathyroid adenoma, which is most often identified in postmenopausal women and is the most common cause of hypercalcemia [Citation24]. Diffuse hyperplasia of the parathyroid glands is caused by hypocalcemia, hyperphosphatemia, and vitamin D deficiency in patients with CKD. In patients with CKD, the concentration of intracellular calcium-sensing receptors is reduced, resulting in nodular hyperplasia of the parathyroid glands. Nodular hyperplasia is an advanced stage of hyperplasia that occurs in patients with more severe HPT [Citation25,Citation26].
The earliest treatment for SHPT is drug therapy involving calcitriol, phosphate binders, activators of vitamin D receptors, calcimimetics, or alfacalcidol. However, for refractory SHPT, the effect of these drugs is minimal [Citation27]. According to the guidelines of the Fourth International Workshop on Asymptomatic Primary HPT [Citation28], surgery remains the only effective treatment for HPT.
However, the complications and mortality of elderly patients after PTx have increased. Although minimally invasive PTx relies on positioning technology and intraoperative auxiliary measures to reduce the risk to a certain extent, it remains challenging for patients with mild hypercalcemia and involvement of multiple glands [Citation29,Citation30]. Moreover, for patients whose physical status is not suitable for surgery and those who refuse surgery, ultrasound-guided ablation of parathyroid lesions is very important.
Thermal ablation
Laser ablation (LA)
A laser is a focused beam of light. After an optical fiber is implanted into the tissue, the photons emitted by the laser light source concentrate the heat to the tip of the optical fiber. The local temperature can increase to 100 °C instantaneously so as to achieve the treatment purpose of coagulation and necrosis of local tissue. Compared with other ablation methods, the main advantages of this method are the use of a fine needle, low power, concentrated energy, flexible operation, and accurate positioning [Citation31,Citation32].
Radiofrequency ablation (RFA)
This refers to all electromagnetic energy sources which induce coagulation within the radiofrequency spectrum (3 kHz to 300 GHz), including “radiofrequency” and “microwave” [Citation33]. The use of RFA in the treatment of HPT involves introducing a 17–19 G needle inside the parathyroid gland to be treated under ultrasound guidance. The needle is an electrode that generates high tissue temperature, ranging from 60 to 100 °C, through high-frequency alternating current [Citation34–36]. This temperature is sufficient to dehydrate the cells in the parathyroid gland, denature the protein in the cells, and coagulate and necrotize the cells, which are then absorbed gradually by the body. Before thermal ablation, physiological saline (0.9%) and a 5% aqueous solution of glucose were used to separate the surrounding structures. During RFA, the ionic components in physiological saline can conduct electricity, thereby potentially leading to pyrexia in non-target tissues [Citation37].
Microwave ablation (MWA)
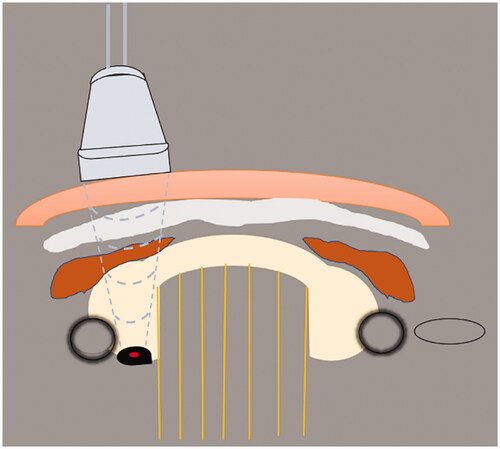
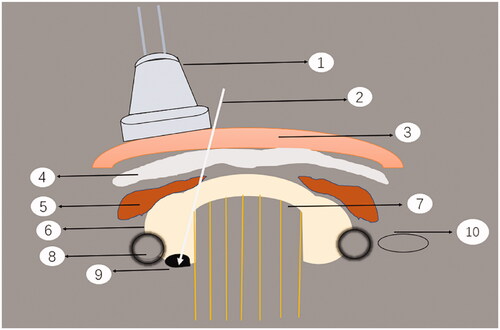
MWA functions within the RF spectrum; therefore, it is a subset of RF ablation but with a different mechanism of heating. Microwaves are a type of electromagnetic wave. When it acts on tissues, the polar molecules of the tissue rotate at a high speed under the action of the microwave electric field to generate heat. In addition, the polarized particles move under the electric field of the microwave, and the particles collide constantly, converting kinetic energy into heat energy, which causes the lesions to coagulate and necrotize instantly owing to the high amount of heat [Citation38]. A schematic diagram of ultrasound-guided percutaneous thermal ablation of HPT is shown in .
Figure 1. Schematic diagram of ultrasound-guided percutaneous thermal ablation of HPT. 1. Linear array probe used for guiding and positioning; 2. ablation needle; 3. skin and subcutaneous soft tissue; 4. fascia tissue; 5. anterior cervical muscles; 6. thyroid gland; 7. trachea; 8. carotid artery; 9. hyperplastic parathyroid gland; 10. internal jugular vein.
High-intensity focused ultrasound (HIFU)
HIFU is a type of three-dimensional conformal therapy. It can be used to cut a focus from a point, surface, or body in vivo through external mechanical movement under ultrasound monitoring [Citation39,Citation40]. When high-intensity ultrasound propagates in tissues, it is continuously absorbed by the tissue and transformed into heat. This action causes the tissue temperature to rise rapidly up to 65–100 °C in the focal area. This makes the cells in the tissue necrotize (i.e., a thermal effect). Simultaneously, high-frequency vibration of tissue cells due to ultrasound can cause cytoplasmic flow, protein deformation, cell function change, degradation of DNA macromolecules, and protein denaturation if the elastic limit of the tissue is exceeded (i.e., a mechanical effect). Under the action of ultrasound, microbubbles in biological tissues and liquids undergo rapid and repetitive expansion and contraction. If the sound intensity exceeds the threshold, the cavity will vibrate sharply, and the volume will change drastically or even quench, resulting in a local high temperature and high pressure (i.e., a cavitation effect) [Citation41–44].
The indications for HPT were as follows: (1) patients with CKD-5D whose intact iPTH level is >800 pg/mL [Citation45]; (2) parathyroid hyperplasia detected by ultrasonography (maximum diameter >0.6 cm) [Citation46]; (3) surgical treatment is not successful or available [Citation45]; (4) SHPT and patients who are not suitable for surgery or who refuse surgery [Citation45]; (5) serum iPTH level >500 pg/mL even in the presence of adequate dialysis or drug treatment; or serum iPTH <500 pg/mL combined with hypercalcemia and hyperphosphatemia, with typical clinical symptoms [Citation47]; (6) definite pathological diagnosis before surgery, or there was no pathological diagnosis, but there were typical imaging features of benign SHPT lesions [Citation48]; and (7) ultrasound evaluation has a safe needle entry path [Citation49].
Ultrasound-guided LA for HPT
Web of Science and PubMed were searched systematically for studies in English published before or on August 30, 2021. We used the search terms “hyperparathyroidism” OR “HPT” OR “Hyperplastic parathyroid gland” AND “Laser ablation” OR “LA” OR “thermal ablation.” Five articles were obtained, as discussed below [Citation50–54].
Ultrasound-guided LA for parathyroid adenomas was first reported by Bennedpak et al. [Citation50] in 2001. Studies by Bennedba et al. [Citation50] and Cusano et al. [Citation51] provided case reports that specifically recorded the treatment process of a single patient and were not included in the following comparative analysis. A comparison of the experimental studies of Massimiliano et al. [Citation52], Appelbaum et al. [Citation53], and Adda et al. [Citation54] is presented in .
Table 1. Baseline characteristics of patients undergoing laser ablation for hyperparathyroidism.
Massimiliano et al. and Appelbaum et al. used relatively high energy. After 2 years of follow-up, the recovery of PTH and serum calcium levels was quite different. Massimiliano et al. reported that PTH levels slightly and temporarily decreased, whereas serum calcium levels returned to normal. They postulated that this phenomenon was due to the ability of cells in the parathyroid glands to grow and replicate. After LA, these cells can regenerate and the PTH level does not decrease to normal levels. Conversely, Appelbaum et al. reported that PTH and serum calcium levels recovered in 12 patients, possibly because they also received high-energy LA of normal gland tissue around the parathyroid adenoma, which resulted in results that were inconsistent with those of the study by Massimiliano et al. The energy penetration of LA is limited, and the burning area is clear and has little impact on surrounding tissues; therefore, it does not lead to complications, such as fibrosis and adhesion. Hence, there is little impact on PTx after LA. In the three studies, some patients had transient damage to the vocal cords and abnormal sounds but no long-term complications. However, the sample size was small, and the follow-up duration was short; large cohorts should be observed for longer periods in future studies.
Presently, there are relatively few studies on LA for the treatment of HPT. These studies lack long-term follow-up and evidence-based medical support for the clinical effect of LA in the treatment of HPT. However, as far as the current research is concerned, LA has a clear burning range and a clear burning boundary, but the specific burning range of the lesions needs to be further studied and determined.
Ultrasound-guided RFA for HPT
Web of Science and PubMed were searched systematically for studies in English published before or on August 30, 2021. The search terms used were: “Hyperparathyroidism” OR “HPT” OR “Hyperplastic parathyroid gland,” AND “Radiofrequency ablation” OR “RFA” OR “thermal ablation.”
RFA was first reported by Hänsler et al. [Citation55] in 2002, followed by scattered case reports [Citation56–58]. In recent years, relatively more experimental studies on RFA for the treatment of parathyroid hyperplasia have been conducted. Peng et al. [Citation45] performed RFA on 34 patients with SHPT, of which, 15 patients had four ablated glands, 10 patients had three ablated glands, and nine patients had one or two ablated glands. All patients were followed for 1 year. The levels of iPTH, serum levels of calcium and phosphorus, and clinical symptoms improved regardless of the number of ablated parathyroid glands. However, patients with four ablated parathyroid glands showed a more obvious improvement. Therefore, RFA discovered by ultrasound should be undertaken for parathyroid hyperplasia, and they suggested that a margin of 1 mm should be reserved during RFA because too high or too low iPTH levels can increase the risk of complications after RFA.
Zeng et al. [Citation59] divided 56 patients with hyperplasia of the four parathyroid glands into two groups and performed RFA. It is considered that RFA for four hyperplastic parathyroid glands at a time is better than ablation of the four parathyroid glands on two occasions, which could save costs and reduce hospitalization. However, the PTH level was reduced to normal in both methods. Sormaz et al. [Citation60] followed up five patients with PHPT for 6 months after RFA. The PTH level remained higher than normal in two patients with residual lesion diameter >30 mm, whereas the PTH level in the other three patients with residual lesion diameter <30 mm was within the normal range.
Li et al. [Citation61] reported that 84% (21/25) of patients with PHPT recovered to normal serum iPTH and calcium levels. One year after RFA, the percentage volume reduction of hyperplastic glands was >70%, and only 20% (5/25) of the patients had mild complications (four cases of mild pain and one case of temporary hypocalcemia).
Ha et al. [Citation62] performed RFA in 11 patients with PHPT and 11 with SHPT: two patients had temporary hypocalcemia and one had permanent hoarseness. During follow-ups of 6 months and 1 year, the size and volume of the parathyroid glands decreased significantly (p < 0.05), the lesions almost disappeared in seven patients, and the levels of PTH and serum calcium returned to normal. Among the eight SHPT patients, five showed persistent HPT within 6 months after RFA and three showed a therapeutic response in terms of serum PTH levels. One study reported that almost all patients had slight postoperative pain; 91.07% (51/57) of PHPT patients had hypocalcemia postoperatively, but the serum calcium level returned to normal after calcium supplementation (i.v.) or after using calcium tablets [Citation59]. A comparison of basic information of HPT patients who underwent RFA is shown in .
Table 2. Baseline characteristics of patients undergoing radiofrequency ablation for hyperparathyroidism.
Currently, RFA is rarely used for parathyroid ablation. Compared with MWA, there is no significant difference in cure rates and postoperative complications [Citation63,Citation64]. According to the existing studies [Citation45,Citation59], for the hyperplastic glands found by imaging, ablation of all hyperplastic glands at one time can not only save costs and reduce hospitalization but can also obtain better therapeutic effects. For patients with a residual lesion diameter of <30 mm, the effect of RFA may be better. Although many patients experience slight pain or discomfort during ablation, no serious complications occur.
Ultrasound-guided MWA for HPT
Web of Science and PubMed were searched systematically for studies in English published before or on August 30, 2021. The search terms used were: “Hyperparathyroidism” OR “HPT” OR “Hyperplastic parathyroid gland,” AND “Microwave ablation” OR “MWA” OR “MW ablation” OR “thermal ablation.”
MWA for PHPT treatment
Fan et al. [Citation65] reported 22 patients with PHPT who underwent MWA. All patients were followed up for 1 year. The serum calcium and PTH levels returned to the normal range in 19 patients, and the nodules disappeared completely in 15 patients. However, during treatment, 15 patients had mild-to-moderate pain or discomfort, and eight patients may have experienced transient sound changes (tone reduction) due to an increase in heat conduction and local pressure. Fan et al. pointed out that if patients with PHPT had normal serum calcium levels, they could be maintained within the normal range after MWA.
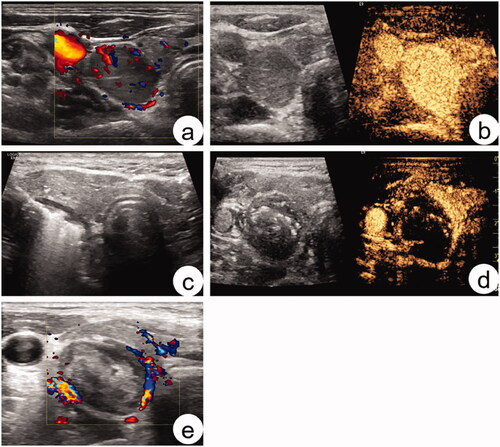
Liu et al. [Citation66] compared MWA with surgical treatment for PHPT. The basic characteristics of the two groups were similar before the MWA. Operation time and blood loss in the MWA group were significantly lower than those in the surgical group (p < 0.01). Six months after the procedure, the PTH and serum calcium levels in 82.1% of MWA patients returned to normal, but there was no significant difference in the cure rate between the two groups. There was no significant difference in the prevalence of complications between the two groups. Ying et al. [Citation46] reported that 11.4% of the patients failed to undergo surgical treatment after MWA (10/88). Ultrasound-guided MWA of a patient with PHPT is shown in .
Figure 2. Sonogram of parathyroid adenoma before and after MWA. A 56-year-old woman was diagnosed with a parathyroid adenoma by ultrasound-guided biopsy before ablation. (a) Sonogram of parathyroid adenoma before MWA: the adenoma was hypoechoic, with a regular shape and clear boundary. Color Doppler flow imaging reveals a slightly rich-colored blood flow in the interior and edge; (b) Before MWA, the adenoma showed uniform and high enhancement in contrast-enhanced ultrasound (CEUS); (c) During ablation. (d) After ablation, there was almost no enhancement in CEUS. (e) One month after MWA, no obvious blood flow was found in the tumor.
MWA for SHPT of different severity
Wang et al. [Citation67] reported that 14 patients with mild-to-moderate SHPT were treated with MWA and were followed up for 1 year. Compared with those treated with calcitriol alone, the iPTH level in the MWA group was significantly lower than that in the calcitriol group. The prevalence of severe SHPT was significantly lower in the MWA group than in the calcitriol group. The serum levels of calcium and phosphorus in the two groups returned to normal, and there was no significant difference between the two groups. In their study, three patients had transient hypocalcemia and three had transient hoarseness.
Yu et al. [Citation68] reported on seven patients with recurrent SHPT and four patients with persistent SHPT treated with MWA. One patient developed a hematoma during ablation, and six patients developed transient impairment of sound and hypocalcemia. One-year follow-up showed that 81.8% of patients recovered to normal PTH levels after MWA, and clinical symptoms (stomachache, pruritus, myasthenia) were either relieved or completely disappeared.
Zhou et al. [Citation69] performed MWA in 35 patients with CKD-5D complicated by SHPT. At ≥6 months after MWA, the levels of PTH and serum calcium decreased curvilinearly and were significantly lower than those before MWA. Three patients had mild subcutaneous edema; two had a transient abnormal voice and five had a mild cough after swallowing water but recovered in a short time. More isolation fluid (40–60 ml vs. 20 ml) was injected around the lesion to protect surrounding tissues and organs. The authors believe that this was the reason for fewer and less severe complications.
Jiang et al. [Citation70] compared PTx with MWA for SHPT in maintenance hemodialysis (there was no significant difference in the baseline level between the two groups). Six months after the procedure, there was no significant difference in the cure rate of SPTH between MWA and PTx. However, Diao et al. [Citation71] obtained different results when they performed MWA in 26 patients with severe SHPT undergoing maintenance HD. Sixteen patients did not respond to treatment (i.e., there was no significant change in PTH levels or serum calcium and phosphorus levels compared with those at baseline). Therefore, they did not recommend MWA as a first-line treatment for patients with severe SHPT undergoing maintenance HD.
MWA for THPT treatment
Hu et al. [Citation72] reported 92 hyperplastic parathyroid nodules in 23 patients with THPT treated twice with MWA. The patients were followed up for 3 years. All patients had transient hypocalcemia after MWA but recovered after calcium supplementation. Four patients showed transient changes such as slight headache, low back pain, and voice changes. PTH levels and serum calcium and phosphorus levels in all patients were higher than normal before MWA. Although the PTH level remained higher than normal after MWA, it was significantly lower than that before MWA, and the serum levels of calcium and phosphorus returned to normal. That study suggested that MWA for THPT improves the quality of life and clinical symptoms over a long period.
MWA has been proven effective in the treatment of PHPT, SHPT, and THPT. Compared with surgery, MWA has a shorter operation time and less blood loss [Citation65,Citation66], though MWA also increases the difficulty of surgery [Citation46]. Moreover, MWA has achieved a good therapeutic effect for SHPT [Citation48], and the isolation zone formed by injecting more normal saline seems to be more effective in protecting the surrounding important tissues [Citation69]. The efficacy of SHPT in maintenance hemodialysis MWA, however, seems to be debated. MWA for THPT can improve the quality of life and clinical symptoms over a long period [Citation72].
HIFU for HPT
Web of Science and PubMed were searched systematically for studies in English published before or on August 30, 2021. The search terms used were: “Hyperparathyroidism” OR “HPT” OR “Hyperplastic parathyroid gland,” AND “High-intensity focused ultrasound” OR “HIFU” OR “thermal ablation.”
There are several indications for HIFU when treating HPT: serum calcium ≥2.60 mmol/L; cytological diagnosis of parathyroid origin; adenoma depth between the posterior border and skin surface <23 mm; adenoma thickness >8 mm; distance from trachea >3 mm; distance from esophagus and carotid artery >2 mm; no obvious giant calcification <10 mm behind the target; no suspicious cytological examination; age >18 years [Citation73]. A schematic diagram of HIFU for HPT treatment is shown in .
In 2010, Kovatcheva et al. [Citation74] first proposed HIFU for PHPT treatment. Their study involved four patients. All patients underwent a second round of HIFU because of poor effect after the first HIFU treatment. One year after the procedure, PTH levels and serum calcium levels decreased significantly but returned to normal levels in three patients. In 2014, Kovatcheva et al. [Citation73] conducted a prospective study of 13 patients with parathyroid adenoma with HIFU based on a 2010 study. All patients were followed for 1 year. They found that the symptoms of three patients were relieved completely; nine patients achieved good control but one patient did not. Levels of PTH and serum calcium decreased significantly in other patients; three patients had transient damage to the vocal cords, three patients had subcutaneous edema, and two patients had vocal cord damage and subcutaneous edema. The authors believed that the main cause of vocal cord damage was related to the depth of the adenoma and not to the energy dissipated during HIFU. However, the energy dissipated by HIFU is positively correlated with the therapeutic effect.
Ambrosini et al. [Citation75] performed HIFU in four patients with PHPT who did not meet the requirements for surgery. All patients were followed for 1 year. Four patients had mild pain and discomfort, two patients suffered transient hoarseness after the procedure, the serum PTH level of three patients decreased to <50% before the procedure, and the serum calcium level of two patients returned to normal.
In 2012, Kovatcheva et al. [Citation76] reported five patients with severe SHPT treated with HIFU, and all patients were followed up for 1 year. If the postoperative serum iPTH level continued to increase or there was no shrinkage of the parathyroid tumor, a second HIFU treatment was performed after a 1-month follow-up. During the study, three patients had mild subcutaneous edema, two had transient damage to the vocal cord, and one had temporary difficulty in drinking water. They considered that HIFU could control PTH temporarily and could control serum levels of calcium and phosphorus.
Currently, there are relatively few reports on HIFU in the treatment of HPT; however, HIFU is a non-invasive thermal ablation method with irreplaceable advantages. When HIFU is used in the treatment of HPT, it has relatively strict indications [Citation73] and the follow-up time is short (approximately 1 year). However, its long-term efficacy requires further investigation.
Prospects of ablation for HPT
Thermal ablation is widely used in the treatment of diseases of the liver [Citation77], thyroid gland [Citation78], prostate gland [Citation79], and other tissues, and the postoperative skin scar is not obvious. The parathyroid glands were located on the back of the thyroid gland. Hence, the incision was large, and the postoperative scar was obvious. Ablation technology, a type of minimally invasive surgery, has several advantages.
However, various ablation methods have advantages for HPT treatment. Although percutaneous injection of ethanol was used first and was popular, its complications and therapeutic effect are not ideal, and it is now employed relatively less. LA, RFA, MWA, and HIFU have been widely used for the treatment of HPT in recent years. These methods are surprisingly consistent in reducing serum levels of calcium and phosphorus; however, there are different reports on the degree of reduction in serum PTH levels. There is no unified theory on the ablation boundaries of hyperplastic glands. Some scholars have suggested that the ablation boundary should include the surrounding normal tissues [Citation53], but some researchers believe that 1-mm of proliferative glands should be retained [Citation45]. Studies on the different degrees of parathyroid hyperplasia and the severity of thermal ablation in PHPT and SHPT are scarce, and the number of patients is small. Therefore, there is a lack of high-quality experimental research and analyses that should be addressed in future research. In addition, the follow-up duration after ablation was short, and monitoring of long-term efficacy and long-term complications merits further investigation.
Conclusions
Thermal ablation for patients with parathyroid hyperplasia is a safe and efficacious treatment for both SHPT and PHPT. LA, RFA, MWA, and HIFU have different principles of thermogenesis. When the temperature of local tissues increases markedly, cells undergo necrosis. Compared with surgical treatment, thermal ablation leads to a shorter hospital stay and lower costs. Many patients experience transient complications after ablation; however, for patients who do not meet the surgical indications or refuse surgery, it is an irreplaceable method for the treatment of parathyroid hyperplasia.
Acknowledgement
Thanks to Wenkai Zhang, Yue Du, and Rui Li, for their help and encouragement, and for their great help in my work.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Jawaid I, Rajesh S. Hyperparathyroidism (primary) NICE guideline: diagnosis, assessment, and initial management. Br J Gen Pract. 2020;70(696):362–363.
- Corbetta S, Mantovani G, Spada A. Metabolic syndrome in parathyroid diseases. Front Horm Res. 2018;49:67–84.
- Bilezikian JP, Cusano NE, Khan AA, et al. Primary hyperparathyroidism. Nat Rev Dis Primers. 2016;2:16033.
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet. 2018;391(10116):168–178.
- Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70(2):351–357.
- Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. CJASN. 2015;10(1):98–109.
- Jamal SA, Miller PD. Secondary and tertiary hyperparathyroidism. J Clin Densitom. 2013;16(1):64–68.
- Dulfer RR, Franssen GJH, Hesselink DA, et al. Systematic review of surgical and medical treatment for tertiary hyperparathyroidism. Br J Surg. 2017;104(7):804–813.
- Ahmad R, Hammond JM. Primary, secondary, and tertiary hyperparathyroidism. Otolaryngol Clin North Am. 2004;37(4):701–713.
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968.
- Portillo MR, Rodríguez-Ortiz ME. Secondary hyperparthyroidism: pathogenesis, diagnosis, preventive and therapeutic strategies. Rev Endocr Metab Disord. 2017;18(1):79–95.
- Neves MCD, Rocha LAD, Cervantes O, et al. Initial surgical results of 500 parathyroidectomies for hyperparathyroidism related to chronic kidney disease – mineral and bone disorder. J Bras Nefrol. 2018;40(4):319–325.
- Köberle R, Bendik CF. [Primary hyperparathyroidism]. Ther Umsch. 2020;77(9):433–440.
- National kidney foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201.
- Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6(4):913–921.
- Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update. Ann Intern Med. 2018;168(6):422–430.
- Gong L, Tang W, Lu J, et al. Thermal ablation versus parathyroidectomy for secondary hyperparathyroidism: a meta-analysis. Int J Surg. 2019;70:13–18.
- Hillary S, Balasubramanian SP, et al. Anatomy of the thyroid, parathyroid, pituitary and adrenal glands. Surgery. 2020;38(12):758–762.
- Lopushniak LY, Khmara ТV, Boichuk ОМ, et al. Fetal anatomy of parathyroid glands. Wiad Lek. 2020;73(1):52–57.
- Melo C, Pinheiro S, Carvalho L, et al. Identification of parathyroid glands: anatomical study and surgical implications. Surg Radiol Anat. 2015;37(2):161–165.
- Taterra D, Wong LM, Vikse J, et al. The prevalence and anatomy of parathyroid glands: a meta-analysis with implications for parathyroid surgery. Langenbecks Arch Surg. 2019;404(1):63–70.
- Spartalis E, Giannakodimos A, Athanasiadis DI, et al. The potential role of carbon nanoparticles in lymph node tracing, recurrent laryngeal nerve identification and parathyroid preservation during thyroid surgery: a systematic review. Curr Pharm Des. 2021;27(21):2505–2511.
- Fancy T, Gallagher D, 3rd, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am. 2010;43(2):221–227.
- Insogna KL. Primary hyperparathyroidism. N Engl J Med. 2018;379(11):1050–1059.
- Tokumoto M. [The pathophysiology of secondary hyperparathyroidism]. Clin Calcium. 2016;26(6):821–829.
- Lee HJ, Seo UH, Kim WY, et al. Calcium-sensing receptor and apoptosis in parathyroid hyperplasia of patients with secondary hyperparathyroidism. J Int Med Res. 2013;41(1):97–105.
- Mizobuchi M, Ogata H, Koiwa F. Secondary hyperparathyroidism: pathogenesis and latest treatment. Ther Apher Dial. 2019;23(4):309–318.
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569.
- Hamdy NA. Parathyroid gland: is parathyroidectomy safe and beneficial in the elderly? Nat Rev Endocrinol. 2009;5(8):422–423.
- Komaba H, Shiizaki K, Fukagawa M. Pharmacotherapy and interventional treatments for secondary hyperparathyroidism: current therapy and future challenges. Expert Opin Biol Ther. 2010;10(12):1729–1742.
- Mauri G, Nicosia L, Della Vigna P, et al. Percutaneous laser ablation for benign and malignant thyroid diseases. Ultrasonography. 2019;38(1):25–36.
- Gough-Palmer AL, Gedroyc WM. Laser ablation of hepatocellular carcinoma-a review. World J Gastroenterol. 2008;14(47):7170–7174.
- Ahmed M, Brace CL, Lee FT, Jr, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;258(2):351–369.
- Dinerman JL, Berger RD, Calkins H. Temperature monitoring during radiofrequency ablation. J Cardiovasc Electrophysiol. 1996;7(2):163–173.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Laeseke PF, Sampson LA, Brace CL, et al. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol. 2006;186(5 Suppl):S249–S254.
- Kim C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Future Oncol. 2018;14(17):1755–1764.
- Sanghvi NT, Hawes RH. High-intensity focused ultrasound. Gastrointest Endosc Clin N Am. 1994;4(2):383–395.
- Zaltieri M, Massaroni C, Cauti FM, et al. Techniques for temperature monitoring of myocardial tissue undergoing radiofrequency ablation treatments: an overview. Sensors. 2021;21(4):1453.
- Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42(10):1087–1093.
- Izadifar Z, Izadifar Z, Chapman D, et al. An introduction to high intensity focused ultrasound: systematic review on principles, devices, and clinical applications. JCM. 2020;9(2):460.
- Elhelf IAS, Albahar H, Shah U, et al. High intensity focused ultrasound: the fundamentals, clinical applications and research trends. Diagn Interv Imaging. 2018;99(6):349–359.
- Ter HG. HIFU tissue ablation: concept and devices. Adv Exp Med Biol. 2016;880:3–20.
- Peng C, Zhang Z, Liu J, et al. Efficacy and safety of ultrasound-guided radiofrequency ablation of hyperplastic parathyroid gland for secondary hyperparathyroidism associated with chronic kidney disease. Head Neck. 2017;39(3):564–571.
- Ying W, Zhen-Long Z, Xiao-Jing C, et al. A study on the causes of operative failures after microwave ablation for primary hyperparathyroidism. Eur Radiol. 2021;31(9):6522–6530.
- Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17(3):247–288.
- Wei Y, Zhuo L, Yu M. A, et al. Expert consensus on thermal ablation for the treatment of secondary hyperparathyroidism (2021 edi-tion). Journal of China-Japan Friendship Hospital. 2021;35(4):195–202.
- Isakova T, Nickolas TL, Denburg M, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751.
- Bennedba KFN, Karstrup S, Hegedüs L. Ultrasound guided laser ablation of a parathyroid adenoma. Br J Radiol. 2001;74(886):905–907.
- Cusano NE, Lee JA, Wetmore RF, et al. Primary hyperparathyroidism after laser therapy to the larynx. Endocr Pract. 2013;19(5):888–890.
- Andrioli M, Riganti F, Pacella CM, et al. Long-term effectiveness of ultrasound-guided laser ablation of hyperfunctioning parathyroid adenomas: present and future perspectives. AJR Am J Roentgenol. 2012;199(5):1164–1168.
- Appelbaum L, Goldberg SN, Ierace T, et al. US-guided laser treatment of parathyroid adenomas. Int J Hyperthermia. 2020;37(1):366–372.
- Adda G, Scillitani A, Epaminonda P, et al. Ultrasound-guided laser thermal ablation for parathyroid adenomas: analysis of three cases with a three-year follow-up. Horm Res. 2006;65(5):231–234.
- Hänsler J, Harsch IA, Strobel D, et al. Treatment of a solitary adenoma of the parathyroid gland with ultrasound-guided percutaneous Radio-Frequency-Tissue-Ablation (RFTA). Ultraschall Med. 2002;23(3):202–206.
- Carrafiello G, Laganà D, Mangini M, et al. Treatment of secondary hyperparathyroidism with ultrasonographically guided percutaneous radiofrequency thermoablation. Surg Laparosc Endosc Percutan Tech. 2006;16(2):112–116.
- Machi J. Radiofrequency ablation for hyperparathyroidism: can it be a new treatment? Surg Laparosc Endosc Percutan Tech. 2006;16(2):116.
- Xu SY, Wang Y, Xie Q, et al. Percutaneous sonography-guided radiofrequency ablation in the management of parathyroid adenoma. Singapore Med J. 2013;54(7):e137–e140.
- Zeng Z, Peng CZ, Liu JB, et al. Efficacy of ultrasound-guided radiofrequency ablation of parathyroid hyperplasia: single session vs. two-session for effect on hypocalcemia. Sci Rep. 2020;10(1):6206.
- Sormaz IC, Poyanlı A, Açar S, et al. The results of ultrasonography-guided percutaneous radiofrequency ablation in hyperparathyroid patients in whom surgery is not feasible. Cardiovasc Intervent Radiol. 2017;40(4):596–602.
- Li X, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation for the treatment of primary hyperparathyroidism: an efficacy and safety study. Endocr Pract. 2021;27(12):1205–1211.
- Ha EJ, Baek JH, Baek SM. Minimally invasive treatment for benign parathyroid lesions: treatment efficacy and safety based on nodule characteristics. Korean J Radiol. 2020;21(12):1383–1392.
- Wei Y, Peng CZ, Wang SR, et al. Microwave ablation versus radiofrequency ablation for primary hyperparathyroidism: a multicenter retrospective study. Int J Hyperthermia. 2021;38(1):1023–1030.
- Wei Y, Peng CZ, Wang SR, et al. Effectiveness and safety of thermal ablation in the treatment of primary hyperparathyroidism: a multicenter study. J Clin Endocrinol Metab. 2021;106(9):2707–2717.
- Fan BQ, He XW, Chen HH, et al. US-guided microwave ablation for primary hyperparathyroidism: a safety and efficacy study. Eur Radiol. 2019;29(10):5607–5616.
- Liu F, Yu X, Liu Z, et al. Comparison of ultrasound-guided percutaneous microwave ablation and parathyroidectomy for primary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):835–840.
- Wang G, Liu S, Liu X, et al. Microwave ablation: an effective treatment for mild-to-moderate secondary hyperparathyroidism in patients undergoing haemodialysis. Int J Hyperthermia. 2017;33(8):1–952.
- Yu MA, Yao L, Zhang L, et al. Safety and efficiency of microwave ablation for recurrent and persistent secondary hyperparathyroidism after parathyroidectomy: a retrospective pilot study. Int J Hyperthermia. 2016;32(2):180–186.
- Zhuo L, Zhang L, Peng LL, et al. Microwave ablation of hyperplastic parathyroid glands is a treatment option for end-stage renal disease patients ineligible for surgical resection. Int J Hyperthermia. 2019;36(1):29–35.
- Jiang B, Wang X, Yao Z, et al. Microwave ablation vs. parathyroidectomy for secondary hyperparathyroidism in maintenance hemodialysis patients. Hemodial Int. 2019;23(2):247–253.
- Diao Z, Wang L, Li D, et al. Efficacy of microwave ablation for severe secondary hyperparathyroidism in subjects undergoing hemodialysis. Ren Fail. 2017;39(1):140–145.
- Hu Z, Han E, Chen W, et al. Feasibility and safety of ultrasound-guided percutaneous microwave ablation for tertiary hyperparathyroidism. Int J Hyperthermia. 2019;36(1):1129–1136.
- Kovatcheva R, Vlahov J, Stoinov J, et al. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–2058.
- Kovatcheva RD, Vlahov JD, Shinkov AD, et al. High-intensity focused ultrasound to treat primary hyperparathyroidism: a feasibility study in four patients. AJR Am J Roentgenol. 2010;195(4):830–835.
- Ambrosini CE, Cianferotti L, Picone A, et al. High-intensity focused ultrasound as an alternative to the surgical approach in primary hyperparathyroidism: a preliminary experience. J Endocrinol Invest. 2011;34(9):655–659.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. High-intensity focussed ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant. 2012;27(1):76–80.
- Puijk RS, Ruarus AH, Scheffer HJ, et al. Percutaneous liver tumour ablation: image guidance, endpoint assessment, and quality control. Can Assoc Radiol J. 2018;69(1):51–62.
- Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(1):37–44.
- Valerio M, Cerantola Y, Eggener SE, et al. New and established technology in focal ablation of the prostate: a systematic review. Eur Urol. 2017;71(1):17–34.