Abstract
Background
Our study aimed to explore the prognostic value of the aspartate aminotransferase–platelet ratio index (APRI) and to develop a new nomogram for patients with hepatocellular carcinoma (HCC) who experience late recurrence after radiofrequency ablation (RFA). To date, no study has explored the value of APRI for assessing the late recurrence of HCC after RFA.
Materials and Methods
The prognostic value of APRI was evaluated and validated in our multicenter retrospective analysis. A total of 466 HCC patients undergoing RFA were reviewed as a training cohort, and 234 HCC patients were included in the external validation cohort. The nomogram was built based on significant prognostic factors in a multivariate analysis and validated in the external validation cohort.
Results
The cutoff APRI score was 0.78, and it appropriately discriminated between low- and high-risk groups for late recurrence in HCC patients. The cumulative recurrence-free survival rates of the low-risk group were significantly higher than those of the high-risk group (p < 0.001), according to the Kaplan–Meier curves. Late recurrence in HCC patients after RFA was associated with APRI, sex and multiple tumors. The nomogram based on potential risk factors (APRI score, sex and multiple tumors) as indicated by multivariate Cox regression analysis showed good discrimination and calibration in the training and external verification groups.
Conclusions
The APRI score is a feasible independent prognostic factor for the late recurrence of HCC after RFA. The proposed nomogram could aid clinicians in following disease progression and providing tailored therapy for patients.
1. Introduction
Liver cancer was the sixth most commonly diagnosed cancer and the third most common cause of cancer-related deaths worldwide in 2020 [1]. Hepatocellular carcinoma (HCC) accounts for 75%–85% of all primary liver cancers [Citation1]. In China, HCC has the fifth highest incidence and second highest mortality rate of all cancers [Citation2]. Surgical resection, local thermal ablation and liver transplantation are the main curative treatment modalities for early stage HCC [Citation3]. Radiofrequency ablation (RFA) and surgical resection show comparable outcomes, but the long-term prognosis of patients with HCC who undergo RFA is still unsatisfactory because of the high recurrence rate [Citation4–6].
Late recurrence (> 2 years) after treatment is generally considered a multicentric tumor or a de novo second primary HCC [Citation7,Citation8]. The predictive role of prognostic factors in late recurrence has received increasing attention. Previous studies reported that late recurrence could be associated with underlying liver conditions, such as cirrhosis and active hepatitis [Citation7,Citation9,Citation10]. Nevertheless, relatively few studies have attempted to predict late recurrence after RFA using noninvasive clinical indicators [Citation7,Citation11,Citation12].
Increasing evidence indicates that the host inflammatory response is correlated with the occurrence and development of HCC. The aspartate aminotransferase (AST)–platelet (PLT) ratio index (APRI) is a noninvasive marker for assessing liver function in patients and is calculated by dividing the serum AST level by the serum PLT level [Citation13–15]. AST is commonly used to evaluate liver tissue damage, including a wide range of etiologies from hepatitis to fatty liver. PLT is associated with cirrhosis and severe fibrosis [Citation13,Citation14]. As an easy-to-use and accurate biomarker, many recent studies have shown that an elevated APRI is a valuable prognostic marker in HCC patients, which can predict clinical outcomes at a relatively accurate level [Citation16–19]. However, it is unclear whether the APRI score can be extended to determine HCC recurrence after RFA.
The pathogenesis of HCC is mainly based on inflammation, which may also lead to a de novo second primary cancer; that is, the cause of late recurrence. Therefore, we believe that APRI may be associated with late recurrence. To date, no study has explored the value of APRI in patients for assessing the late recurrence of HCC after RFA.
Hence, the aim of this study was to explore the prognostic value of APRI in HCC patients with late recurrence after RFA and to develop an APRI-based nomogram to predict recurrence-free survival (RFS).
2. Materials and methods
2.1. Study population
With the approval of the institutional review board at each study center, we retrospectively reviewed 466 patients with HCC who were treated with RFA treatment from January 2001 to December 2016 at the National Cancer Center and Beijing Hospital of Traditional Chinese Medicine. A total of 234 patients treated at the First Hospital of Shanxi Medical University between January 2002 and December 2016 were included in the external validation cohort. All patients received RFA treatment by consensus from an interdisciplinary committee consisting of surgeons, oncologists, radiologists, radiation therapists and pathologists. The Institutional Review Boards of the four hospitals approved this retrospective study (NCC3251). Written informed consent for treatment was obtained from all patients.
The inclusion criteria were as follows: (1) HCC confirmed by histology or noninvasive diagnostic criteria recommended by the European Association for the Study of Liver [Citation20]; (2) Eastern Cooperative Oncology Group performance status score of 0 or 1; (3) solitary tumor ≤ 3 cm or up to three tumors ≤ 3 cm without vascular invasion or extrahepatic metastasis; (4) Child-Pugh class A or B liver function and (5) absent history of HCC therapy. The exclusion criteria were as follows: (1) patients with early recurrence (< 2 years), (2) beyond Barcelona Clinic Liver Cancer A stage (up to three tumors ≤ 3 cm, BCLC A stage), (3) loss to follow-up and (4) history of treatment for HCC before RFA.
2.2. Data collection
We obtained patient baseline information before RFA and collected blood samples when patients first presented to the hospital. Relevant clinical variables were collected from electronic medical records, including age, sex, maximum tumor size, number of tumors, etiology, cirrhosis, Child-Pugh class, albumin-bilirubin (ALBI) grade, serum alpha-fetoprotein (AFP), serum albumin (ALB), AST, alanine aminotransferase, total bilirubin, serum γ-glutamyl transpeptidase (γ-GT) levels and PLT levels. The upper limits of the normal values at our hospital laboratory were used as the cutoff values.
The APRI score was calculated using AST and PLT as follows: APRI score = [(AST (U/L)/upper limit of normal value [U/L]/PLT [×109/L]) × 100. The ALBI score was calculated as follows: ALBI score = log10 bilirubin (U/L) × 0.66 + albumin (g/L) × −0.0852.
2.3. RFA procedure
All RFA procedures were performed percutaneously under ultrasound or computed tomography (CT) guidance by a hepatologist with more than seven years of experience. We first chose ultrasound-guided RFA to reduce the economic burden of cancer care for patients with HCC [Citation21]. The RFA equipment used in this study was a commercial RFA generator (S-1500, MedSphere International, Fremont, CA). The type of electrode used (monopolar or expandable multi-tined electrode; MedSphere International) and the power and time of RFA was determined according to the manufacturer’s standard recommendations and lesion characteristics, such as tumor shape, location and diameter. Decisions regarding electrode selection and ablative technique were discussed in our therapeutic group before procedure. After the administration of 1% lidocaine for local anesthesia, the RFA electrodes were inserted into the target tumor. An ideal ablative margin was achieved by expanding or overlapping ablation to completely cover the tumor and surrounding tumor margin of ≥ 0.5 cm [Citation22]. After lesion ablation, the ablation path was cauterized to avoid tumor seeding and hemorrhage. Heart rate, blood pressure and respiratory saturation were continuously monitored during RFA.
2.4. Follow-up
After RFA, the patients underwent contrast-enhanced CT or MRI (magnetic resonance imaging) to evaluate the technical success rate. The patients were then followed up every three months for the first year and every six months for subsequent years. Each follow-up included the collection of information about the clinical history, chest CT, abdominal contrast-enhanced CT or MRI, and laboratory examinations, such as liver function tests, ALB, AFP and γ-GT.
2.5. Oncological outcomes
The primary outcomes were RFS and overall survival (OS). In accordance with consensus recommendations, RFS was defined as the time between the first RFA treatment and tumor recurrence [Citation23]. OS was defined as the time interval from the first RFA procedure to death from any cause. In this study, patients who were still alive at the last follow-up examination were censored. Tumor recurrence was confirmed using contrast-enhanced medical imaging criteria. Recurrence was divided into local tumor progression, intrahepatic distant progression and extrahepatic progression. Adverse events that occurred within 30 days of the procedure were recorded and graded according to the Society of Interventional Radiology (SIR) guidelines [Citation24].
2.6. Statistical analysis
Data are presented as frequencies (percentages) and compared using the Chi-square test or Mann–Whitney U-test as appropriate. Kaplan–Meier analysis was used to calculate the cumulative incidence rates of OS and RFS. Differences between groups were evaluated using the log-rank test. Univariate and multivariate analyses were performed using Cox regression analysis. For the cutoff values, X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT) was used to determine the optimal cutoff value [Citation25]. The R software package (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses. All statistical tests were two-tailed, and statistical significance was set at p < 0.05. The nomogram was built using significant variables from the Cox regression model. Internal validation was performed using 500 sets of bootstrap samples to test the usefulness of the nomogram. Discrimination was evaluated using the concordance index (C-index). Calibration was performed by plotting the predicted probability of three-, four‐ and five‐year RFS versus observed probability. The predictive value of the model was assessed using time-dependent receiver operating characteristic analyses.
3 Results
3.1. Patient characteristics
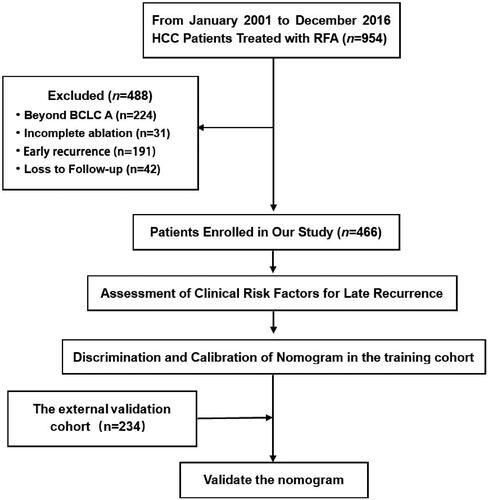
A total of 954 patients with HCC received RFA at the National Cancer Center and the Beijing Hospital of Traditional Chinese Medicine. Among them, 488 patients were excluded because the HCC was beyond the BCLC A stage (n = 224), ablation was incomplete (n = 31), patients with early recurrence (n = 191) and there was loss to follow-up (n = 42). Finally, 466 HCC patients were enrolled in this study. The flowchart of patient selection is shown in .
summarizes the baseline characteristics of the patients in the primary cohort, and they were divided into late recurrence (n = 84) and late recurrence-free (n = 382) groups. There were significant differences between the two groups in terms of APRI, sex and tumor number. Compared with the patients without late recurrence, the proportion of patients with APRI > 0.78 was higher, the proportion of women was lower and the number of multiple tumors was larger among the patients with late recurrence. In our study cohort, most patients with late recurrence had cirrhosis and hepatitis, but the differences were not significant (p = 0.055 and 0.134, respectively) compared with those without late recurrence.
Table 1. Patient demographics of primary cohort.
3.2. Risk factors for late recurrence
Univariate analysis showed various clinical risk factors for late recurrence after RFA (). Multivariate analysis showed that APRI (hazard ratio [HR]: 10.160, 95% CI: 5.622–18.380; p < 0.001), sex (HR: 6.152, 95% CI: 3.175–11.920; p < 0.001) and multiple tumors (HR, 3.100; 95% CI, 2.005–4.793; p < 0.001) were independent risk factors for late recurrence.
Table 2. Cox regression analyses of clinical risk factors for late recurrence in the primary cohort.
3.3. Risk stratification for late recurrence of HCC after RFA based on APRI
To identify the optimal cutoff value for APRI, we applied the X-tile software which was based on a time-dependent method. Our study of the APRI cutoff value (0.78) enabled us to distinguish between poor outcomes and favorable outcomes in patients.
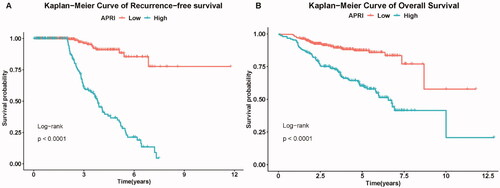
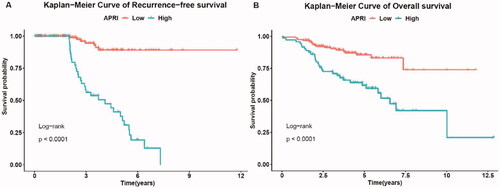
According to the pre-set cutoff points, the low-risk group was significantly associated with longer RFS (p < 0.001). Kaplan–Meier analysis showed significant differences in OS rates between the low-and high-risk groups (p < 0.001).
The three-, four- and five-year RFS rates of the low-risk group were 95.7%, 90.4% and 89.6%, respectively, whereas the three-, four- and five-year RFS rates of the high-risk group were 61.1%, 44.8% and 35.2%, respectively (). The one-, three-, and five-year OS rates of the low-risk group were 98.0%, 89.1% and 86.8%, respectively, whereas the one-, three- and five-year OS rates of the high-risk group were 93.4%, 74.2% and 59.3%, respectively ().
3.4. Construction and validation of the nomogram for late recurrence
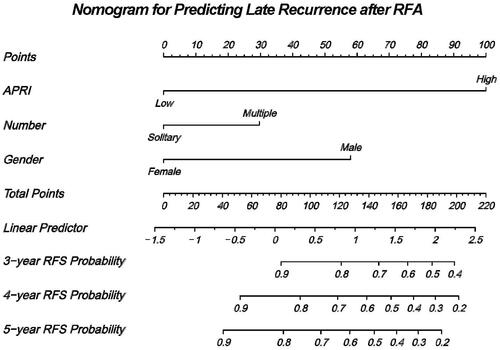
Based on the independent prognostic risk factors (APRI, sex, and multiple tumors) identified in the multivariate analysis, a nomogram was built to assess their association with RFS in patients with HCC after RFA (). Every enrolled HCC patient was assigned one individualized grade, which was the sum of the points from the three prognostic variables.
Figure 3. The nomogram for predicting the three-, four- and five-year RFS after RFA. RFS, recurrence-free survival; RFA, radiofrequency ablation.
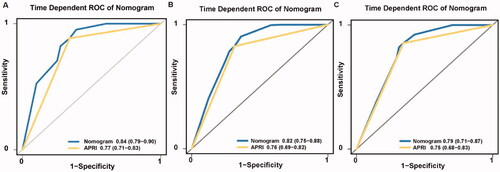
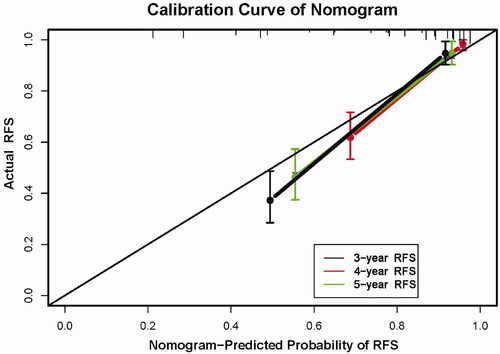
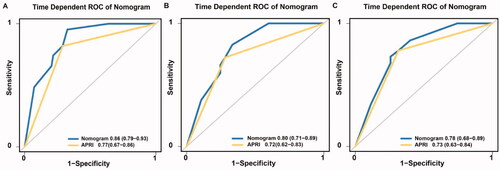
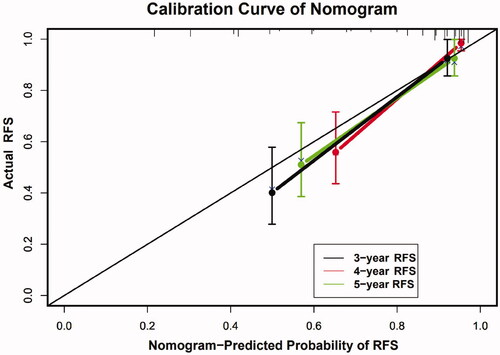
The C-index of the nomogram for predicting RFS probability was 0.808 (95% CI: 0.787–0.829). The time-dependent area under the curve (t-AUC) for predicting the three-, four- and five-year RFS was 0.842 (95% CI: 0.794–0.902) (), 0.820 (95% CI: 0.749–0.881) () and 0.790 (95% CI: 0.710–0.872) (), respectively. The calibration curves plotted with the three-, four- and five-year RFS were well matched with the idealized 45° line (). The x-axis represents the nomogram-predicted probability of RFS, whereas the y-axis shows the actual RFS.
3.5. Performance of the nomogram in the external validation cohort
A total of 234 patients were included in the external validation cohort, as shown in Supplementary Tables 1 and 2. During the median follow‐up of 48.2 months, 39 patients (16.7%) experienced HCC late recurrence. The three-, four- and five-year RFS rates of the low-risk group were 92.3%, 88.6% and 88.2%, respectively, whereas the three-, four- and five-year RFS rates of the high-risk group were 57.2%, 50.4% and 43.3%, respectively.
The Kaplan–Meier curves showed that the cumulative RFS () and OS () rates were significantly different between the low-risk and high-risk groups (p < 0.001). The discriminative ability of the nomogram on the external dataset was good, based on a C-index of 0.821 (95% CI: 0.793–0.835) and t-AUCs of 0.862 (95% CI: 0.793–0.928) (), 0.803 (95% CI: 0.713–0.893) () and 0.780 (95% CI: 0.682–0.889) (), respectively. The calibration curves for the nomogram demonstrated good agreement ().
Figure 6. Kaplan–Meier curve for the RFS (a) and OS (b) of patients between low-risk and high-risk groups in the external validation cohort. RFS, recurrence-free survival; OS, overall survival.
3.6. Patterns and treatment of late recurrence after RFA
During a 45.5-month median follow-up, 84 (18.0%) patients developed late recurrence, and 19 patients died. Among the 84 patients who developed late recurrence, 62 (73.8%) were first diagnosed with intrahepatic recurrence alone, whereas 22 (26.2%) had intrahepatic and extrahepatic recurrence, which had higher frequencies of multicentric recurrence. No patient had local tumor recurrence or extrahepatic recurrence. During this observation period, 5, 37, 20, and 22 patients with recurrence underwent surgical resection, repeat RFA, transarterial chemoembolization (TACE) and TACE combined with systemic therapy, respectively.
3.7. Complications
In this study, nine patients had major and minor complications according to the SIR classification of complications. The overall complication rate was 1.9%, with major and minor complication rates of 0.7% and 1.2%, respectively. Six patients had low-grade fever, which resolved after treatment with broad-spectrum antibiotics. Two patients had mild perihepatic hemorrhagic collection, and one patient had liver abscesses, which were managed conservatively.
4. Discussion
Late recurrence, which is more likely the consequence of a second primary HCC tumor from de novo carcinogenesis, is associated with underlying liver conditions [Citation7,Citation26]. In our study, late recurrence occurred in 18.0% of the 466 patients who were alive and free of tumor recurrence two years after RFA for HCC. The results of this study showed that APRI, sex and multiple tumors were independent risk factors for late recurrence. This multicenter retrospective study found that the APRI score appropriately discriminated between low- and high-risk groups for late recurrence in HCC patients, and the proposed nomogram had excellent performance in predicting late recurrence of HCC. These results indicate that the nomogram can be used by clinicians to develop individualized follow-up strategies and adjuvant treatments after RFA.
To our knowledge, this study is the first to explore the relationship between APRI and the prognosis of late recurrence after RFA. We found that the APRI score did not only provide a risk stratification for late recurrence into low-risk and high-risk groups, it was also identified as an independent factor of late recurrence in HCC patients after RFA, which further supports the hypothesis that late recurrence is a multicentric tumor or a de novo second primary HCC. Increasing evidence indicates that the host inflammatory response is correlated with the occurrence and development of HCC. APRI is a noninvasive diagnostic index for liver cirrhosis and has proven to be a valuable prognostic marker in patients with HCC [Citation16–18]. Recent studies have shown that APRI is associated with both tumor recurrence and OS in patients with HCC undergoing RFA [Citation27–29]. This may be because AST reveals the degree of liver cirrhosis and liver tissue damage, which are the main contributing factors to liver carcinogenesis [Citation19,Citation28–30]. Thus, repeated cycles of necrosis and hepatocyte regeneration may result in recurrent lesions in the residual liver after treatment [Citation16]. PLTs are considered to play a role in tumor immunity and the microenvironment and are also thought to facilitate and regulate tumor angiogenesis [Citation31,Citation32]. Therefore, APRI, a combination of AST and PLT, reflects the prognosis of HCC late recurrence from liver reserve, inflammatory and immune levels.
In addition, the male sex and multiple tumors were also independent risk factors for late recurrence in our study. Men have a higher risk of HCC recurrence than women. Sex hormones may be responsible for this sex difference [Citation33,Citation34]. The difference in the patients could be attributed to the multicentric origins of HCC, which was similar to the higher incidence of HCC in men than in women. This result suggests that men might require closer surveillance during the late follow-up period. Moreover, the number of tumors was also associated with a higher risk of HCC late recurrence in our study, which is in line with the results of previous studies that highlighted the importance of tumor burden [Citation7,Citation8].
We created a novel nomogram based on potential risk factors (APRI score, sex and multiple tumors) identified by multivariate Cox regression analysis to predict RFS. The nomogram could generate individualized risk estimations for RFS after RFA and was feasible and reliable as indicated by the calibration plots and t-AUCs. A prognostic model derived from pretreatment clinical variables may improve the chance of patients undergoing curative treatment. For example, when a patient’s nomogram score reaches 160 points, the patient may have a 60% risk of late HCC recurrence within five years.
Compared with patients without late recurrence, the proportion of patients with APRI > 0.78 was higher, the proportion of males was higher and the number of multiple tumors was larger among the patients with late recurrence. In addition, most patients with late recurrence had cirrhosis and hepatitis, which is consistent with previous studies that showed that late recurrence was more likely to be caused by multicentric tumors or de novo cancer formation from underlying liver function abnormalities [Citation7]. Although the differences were not significant, our results should prompt future studies with a larger number of cases. Six patients had low-grade fever, which is a post-ablation syndrome. A recent study indicated that prolonged post-ablation fever was an independent predictive factor for tumor recurrence and may reflect the degree of hepatic cell damage after locoregional therapy [Citation35]. Post-ablation fever was not related to late recurrence in our study, but it provided a clue regarding the need for timely intervention and follow-up.
Our study had several limitations. First, because of the inherent nature of the retrospective study design, selection bias cannot be completely avoided. Second, most cases of HCC result from hepatitis B virus infection in China. Hence, some patients with other etiologies, such as hepatitis C virus, alcoholic liver disease and nonalcoholic fatty liver disease, could change the heterogeneity of the study population. Third, since we only used the APRI baseline to explore the prognostic value in HCC patients with late recurrence, important patient information could have been during the follow-up period.
5. Conclusions
This retrospective study demonstrated that the APRI score is a feasible independent prognostic factor for the late recurrence of HCC after RFA. The proposed nomogram could aid clinicians in following disease progression and help physicians make appropriate clinical decisions.
Supplemental Material
Download PDF (430.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Zhang SS, Zheng RS, Zeng HM, et al. Cancer incidence and mortality in China, 2015. JNCC. 2021;1(1):2–11.
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019; 16(10):589–604.
- Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242(2):158–171.
- Ogihara M, Wong LL, Machi J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: long-term outcomes. HPB. 2005;7(3):214–221.
- Sugo H, Ishizaki Y, Yoshimoto J, et al. Salvage hepatectomy for local recurrent hepatocellular carcinoma after ablation therapy. Ann Surg Oncol. 2012;19(7):2238–2245.
- Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;1154(3):209–217.
- Zheng J, Chou JF, Gönen M, et al. Prediction of hepatocellular carcinoma recurrence beyond milan criteria after resection: validation of a clinical risk score in an international cohort. Ann Surg. 2017;266(4):693–701.
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207.
- Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. WJG. 2015;21(4):1207–1215.
- Lin C, Lin C, Wang C, et al. The ALBI grade is a good predictive model for very late recurrence in patients with hepatocellular carcinoma undergoing primary resection. World J Surg. 2020;44(1):247–257.
- Chok K, Chan S, Cheung T, et al. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35(9):2058–2062.
- Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782–789.e4.
- Sonneveld MJ, Brouwer WP, Chan HL, et al. Optimisation of the use of APRI and FIB-4 to rule out cirrhosis in patients with chronic hepatitis B: results from the SONIC-B study. Lancet Gastroenterol Hepatol. 2019;4(7):538–544.
- Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501.
- Lai L, Su T, Liang Z, et al. Development and assessment of novel predictive nomograms based on APRI for hepatitis B virus-associated small solitary hepatocellular carcinoma with stereotactic body radiotherapy. J Cancer. 2020;11(22):6642–6652.
- Maegawa FB, Shehorn L, Aziz H, et al. Association between noninvasive fibrosis markers and postoperative mortality after hepatectomy for hepatocellular carcinoma. JAMA Netw Open. 2019;2(1):e187142.
- Zhang TT, Ye SS, Liang J, et al. Prognostic value of non-invasive fibrosis indices post-curative resection in hepatitis-B-associated hepatocellular carcinoma patients. Exp Biol Med. 2020;245(8):703–710.
- Zhu GQ, Wang K, Wang B, et al. Aspartate aminotransferase-to-platelet ratio index predicts prognosis of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. CMAR. 2018;11:63–79.
- EASL-EORTC Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943.
- Huo J, Aloia TA, Xu Y, et al. Comparative effectiveness of computed tomography- versus ultrasound-guided percutaneous radiofrequency ablation among medicare patients 65 years of age or older with hepatocellular carcinoma. Value Health. 2019;22(3):284–292.
- Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Intervent Radiol. 2018;29(2):268–275.e1.
- Puijk RS, Ahmed M, Adam A, et al. Consensus guidelines for the definition of time-to-Event end points in image-guided tumor ablation: results of the SIO and DATECAN Initiative. Radiology. 2021;301(3):533–540.
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology. 2014;273(1):241–260.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259.
- Wang MD, Li C, Liang L, et al. Early and late recurrence of hepatitis B virus-associated hepatocellular carcinoma. Oncologist. 2020;25(10):e1541–e1551.
- Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011; Jun23(6):528–536.
- Chung HA, Kim JH, Hwang Y, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol. 2016;22(1):57.
- Guo C, Liang H, Yuan W, et al. Analysis on the value of soluble intercellular adhesion molecule-1 (sICAM-1), alpha fetoprotein (AFP), and aspartate aminotransferase/platelet ratio index (APRI) in predicting the prognostic survival of patients with primary liver cancer after radiofrequency ablation. Ann Palliat Med. 2021;10(4):4760–4767.
- Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95.
- Ho-Tin-Noé B, Carbo C, Demers M, et al. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175(4):1699–1708.
- Placke T, Örgel M, Schaller M, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–448.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576.
- White DL, Thrift AP, Kanwal F, et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812–820.e5.
- Ho PH, Teng W, Lin CC, et al. Prolonged post-ablation fever may predict one-year tumor recurrence in hepatocellular carcinoma after radiofrequency ablation. Int J Hyperthermia. 2020;37(1):1008–1015.