Abstract
Purpose
To evaluate the long-term efficacy of combined radiotherapy (RT) and hyperthermia (HT) in a large mono-institutional cohort of breast cancer (BC) patients affected by recurrent, newly diagnosed non-resectable or high risk resected tumor.
Materials and methods
Records of BC patients treated with RT + HT between 1995 and 2018 were retrospectively analyzed. RT doses of 50–70 Gy concurrent to a twice per week superficial HT were applied. For HT, a temperature between 41 and 42 °C was applied for approximately 1 h. Primary endpoint was local control (LC), secondary endpoints comprised toxicity, overall survival (OS), and progression-free survival (PFS).
Results
A total of 191 patients and 196 RT + HT treatments were analyzed. In 154 cases (78.6%) RT + HT was performed for patients with recurrent BC. Among these, 93 (47.4% of the entire cohort) had received RT prior to RT + HT. Median follow up was 12.7 years. LC at 2, 5, and 10 years was 76.4, 72.8, and 69.5%, respectively. OS at 2, 5, and 10 years was 73.5, 52.3, and 35.5%, respectively. PFS at 2, 5, and 10 years was 55.6, 41, and 33.6%, respectively. Predictive factors for LC were tumor stage, distant metastases, estrogen/progesterone receptor expression, resection status and number of HT fractions. At multivariate analysis tumor stage and receptor expression were significant. No acute or late toxicities higher than grade 3 were observed.
Conclusion
Combined RT + HT offers long-term high LC rates with acceptable toxicity for patients with recurrent, newly diagnosed non-resectable or resected BC at high risk of relapse.
Introduction
One out of every eight women will be diagnosed with breast cancer (BC) during the course of her life [Citation1]. While BC incidence shows a growing trend, mortality decreased during the past years, thanks to improvements in screening, diagnosis, and treatments. Even patients with a detrimental prognosis, i.e., diagnosed with recurrent BC or with metastatic cancer might achieve long-term survival. Approximately half of patients with recurrent BC are alive at 10 years [Citation2] and one third diagnosed with stage IV disease are alive at 5 years [Citation1]. Given the improved long-term prognosis for patients with recurrent and/or high-risk histopathological features, it is essential to develop methods for achieving higher long-term control. In these patients, the parallel application of radiotherapy (RT) and hyperthermia (HT) represents a treatment intensification option aiming to enhance LC [Citation3]. Among the many biological mechanisms underlying the radiosensitizing effect of HT, increased heat-mediated oxygenation appears to be responsible for enhancing RT-induced DNA damage [Citation4]. Furthermore, the anti-tumor immune response seems to be amplified under HT [Citation5]. HT combined with RT for selected high-risk BC patients has been introduced into the international guidelines [Citation6] on the basis of small randomized trials and retrospective studies [Citation7–20].
The aim of this study was to evaluate the long-term results of RT and HT in a large mono-institutional cohort of BC patients affected by recurrent BC, either resected with high risk features or non-resectable.
Materials and methods
Patients’ selection
This retrospective study was approved by the ethics committee of the Medical Faculty of Tübingen (Nr. 142/2020BO2). The requirement for informed consent for publication of this study was waived because of its retrospective nature. The records of BC patients treated with RT and HT between 1995 and 2018 at the Tübingen University Hospital were analyzed. Early outcome data of small subgroups of patients included in the present analysis, who were treated with re-RT + HT for recurrent BC, have been previously published [Citation12,Citation21]. Here, we report on the long-term results of a larger cohort, which includes patients with no irradiation prior to receiving RT + HT and at the first diagnosis of BC. Indications for treatment with HT combined with RT were: recurrent or primary BC resected with close (defined as <5 mm until 2011 and <2 mm from 2012), R1 (microscopic), R2 (macroscopic) resection margins or inoperable or R0 resected but with other high risk features, e.g., multiple recurrences, multiple nodal involvement, inflammatory cancer and loco(regional) progression after adjuvant chemotherapy.
Radiotherapy
For patients with recurrent BC who had previously received RT, target volumes for RT + HT were defined individually as involved-field to cover the site of recurrence. For RT-naïve patients prior to RT-HT, the thoracic wall with or without lymphatic regions, according to nodal involvement, was defined as target volume. If required, for example by positive resection margins or the presence of macroscopic disease, a boost volume was defined. Doses of 50–50.4 Gy in 25–28 fractions were prescribed. A boost was applied sequentially up to 60–60.4 Gy or, in the case of macroscopic disease, 70–70.4 Gy if clinically feasible. Feasibility depended on previous RT-course and constraints of the organs at risk. External beam RT using 6 or 15 MV photons or 4–12 MeV electrons as one or multiple fields, or using an electron-rotation technique, was performed. RT doses of the first RT course for patients treated with RT prior to RT + HT ranged between 40 and 66.4 Gy.
Hyperthermia
As previously described [Citation12,Citation21], superficial HT was performed according to the ESHO-guidelines [Citation22]. Briefly, a two-arm spiral applicator SA-115 (BSD Medical Corp., currently Pyrexar medical, Salt Lake City, UT mounted on a Plexiglas surface fitted with a bolus filled with deionized water was used. The SA-115 superficial HT applicator has an effective field size of 13 × 10 cm2. The water in the bolus was constantly maintained at a temperature of 39 °C in order to cool the skin surface and avoid skin burning. A temperature increase of 1–2 C within 1 cm below the skin surface is expected. We followed the manufacturer instructions of our HT device and treated targets from the skin surface to a maximum depth of 3 cm below. Six temperature probes, placed between the skin and the water bolus of the HT applicator, were used to monitor the temperature on the target surface. HT was applied twice per week up to 1 h before or after RT. After a 5–15 min preheating phase, a therapeutic temperature between 41 and 42 C was applied for one hour. The target for HT was the initial tumor location according to preoperative images and postoperative clips and/or scar or the macroscopic tumor, if present.
Endpoints and statistical analysis
The primary endpoint was LC. Secondary endpoints were toxicity, overall survival (OS) and progression free survival (PFS). Events (relapse, progression, or death) were timed from the start of RT + HT. Acute and late toxicities were scored according to the CTC AE and the LENT-SOMA/RTOG toxicity criteria, respectively. To define of late toxicity, a cut off of 3 months after end of RT was used. Subgroup analyses according to previous RT course and first diagnosis of BC were performed. Patient-, tumor-, and treatment-related factors were included in a univariate and multivariate analysis. Univariate analysis was performed using the Cox model and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The parameters considered for the univariate analysis were: tumor stage, nodal stage, presence of distant metastases, oestrogen/progesterone (ER/PR) expression, Her2 expression, grading, resection margins, RT dose, average HT temperature, minimal HT temperature, maximal HT temperature, and numbers of HT fractions applied. Proliferation index, in situ tumor component and lymphovascular invasion were not available for the majority of patients and therefore were not included. A log-rank Mantel-Cox test was used for comparison and p-values <0.05 were considered statistically significant. Parameters with p < 0.1 were included in the multivariate analysis. The statistical analysis was performed using GraphPad Prism version 9 for Windows (GraphPad Software, La Jolla, CA, USA ). Multivariate analysis was performed with SPSS version 25 (IBM, Armonk, NY, USA).
Results
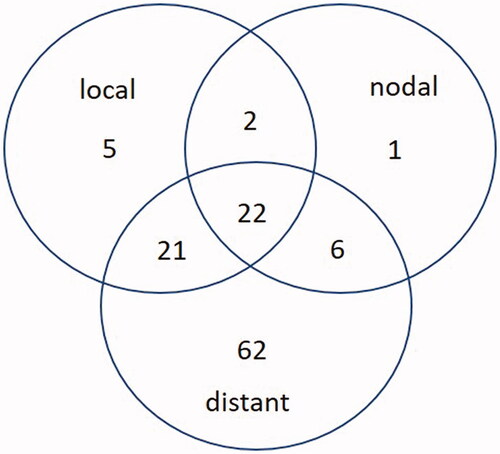
A total of 196 RT + HT treatments were performed on 191 BC patients between 1995 and 2018. The majority of patients (n = 154, 78.6%) were treated for recurrences and 42 (21.4%) for primary BC. Among those with recurrent BC, 93 (47.4% of the entire cohort) had already received RT prior to RT + HT. Patient and treatment characteristics are shown in . The median follow-up was 12.7 years (range, 0.9–22). In total, 111 recurrences were observed. Of these, 50 were local relapses. However, only 10% (n = 5/50) were isolated local relapses ().
Table 1. Patients, tumor, and treatment characteristics at the time of treatment with RT + HT.
LC at 2, 5, and 10 years was 76.4, 72.8, and 69.5%, respectively. OS at 2, 5, and 10 years reached 73.5, 52.3, and 35.5%, respectively. PFS at 2, 5, and 10 years was 55.6, 41, and 33.6%, respectively. Tumor stage (p = 0.009, HR 0.33, CI 0.17–0.65), distant metastasis (p = 0.02, HR 0.46, CI 0.19–1.09), ER/PR expression (p < 0.0001, HR 3.31, CI 1.64–6.69), resection status (p = 0.005, HR 0.43, CI 0.21–0.91) and the number of HT fractions (≥10, p = 0.02, HR 1.92, CI 1.05–3.54) were found to be predictive for LC (, ). A trend was shown for tumor grading (p = 0.07, HR 0.57, CI 0.31–1.04), radiation doses (p = 0.18, HR 1.46, CI 0.82–2.57) and minimal HT temperature (p = 0.13, HR 1.56, CI 0.83–2.98). In the multivariate analysis, tumor stage (p = 0.04, HR 0.37, CI 0.15–0.94) and ER/PR expression (p < 0.0001, HR 4.7, CI 2.13–10.36) remained statistically significant ().
Figure 2. Kaplan–Meier curves for local control (A), overall survival (B), progression-free survival (C) and for local control according to resection status (D), number of HT fractions (E) and combined resection status with applied radiation dose (F).
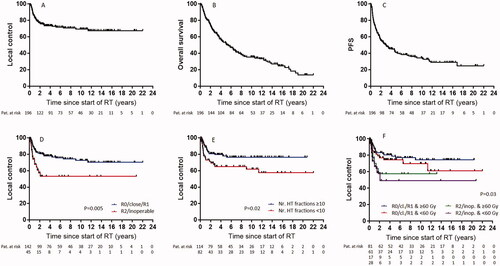
Table 2. Univariate analysis for local control.
Table 3. Multivariate analysis for local control.
A subgroup analysis was performed on the 42 patients who received RT + HT at the time of initial diagnosis of BC. LC at 2 and 10 years was 80.8 and 77.3%, respectively (Supplementary Figure 1A). OS at 2 and 10 years was 66.7 and 37.5% (Supplementary Figure 1B).
Nine patients (4.6%) had no acute skin reaction, 76 (38.6%) experienced CTC AE grade 1 toxicity, 92 (46.7%) had grade 2 and 18 (9.1%) had grade 3 toxicity. No acute toxicity higher than grade 3 was observed. Other reported acute toxicities were fatigue, pain, breast edema, arm edema, none higher than grade 2. Radiation pneumonitis was observed in six cases. In one patient documented by CT findings without symptoms (RTOG grade 1) and in five patients symptoms resolved after steroid therapy (grade 2). Other HT-related, acute side effects were tachycardia and fever, none higher than grade 2. For two patients (1%), no acute toxicity data were available.
Regarding late skin toxicity, in 55 (27.9%) patients no side effects were observed. Forty-five (22.8%) experienced grade 1, 13 (6.6%) grade 2 and 9 (4.6%) grade 3 toxicity. Other late toxicities examined such as fibrosis, telangiectasia, edema, pain and fatigue, did not exceed grade 2. Radiation-induced brachial plexus injury was suspected in one instance. This patient was irradiated 6 years before with 50 Gy and with 59.4 Gy at the time of RT + HT and had an inoperable tumor. One patient had prolonged wound healing problems. Late toxicity data were not available for 75 (38.1%) patients.
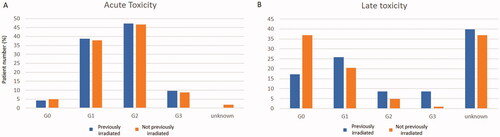
A subgroup toxicity analysis was performed between patients who had already received RT prior to RT + HT (n = 93) and those who had not (n = 103). Acute toxicity was similar between the two groups: grades 0, 1, 2 and 3 were 4.3, 38.7, 47.3, and 9.7%, respectively, for those who had already received RT prior to RT + HT and 4.9, 37.9, 46.6 and 8.7%, respectively, for those who had not (). Instead, patients who had already received RT prior to RT + HT showed higher grade 2 and grade 3 late toxicity compared to those who had not been previously irradiated, that is, 8.6% grade 2 and 8.6% grade 3 if previously irradiated versus 4.9% grade 2 and 1% grade 3 if not (). The difference in high-grade toxicity (defined as grade 3) between the two subgroups was statistically significant (p = 0.004, supplementary figure 2).
Discussion
Concurrent HT and RT resulted in durable long-term LC of 70% after a median follow-up of more than 12 years in our cohort of high-risk BC patient. Surgery is the treatment of choice for patients with recurrent BC. However, about one-third of patients experience a relapse after surgery alone [Citation23,Citation24] and approximately 30% of local relapses are inoperable [Citation25]. For patients with recurrent BC, especially if previously irradiated and/or with not R0-resected or non-resectable tumors, the benefit of using HT in addition to RT is well documented [Citation3,Citation6,Citation7,Citation20]. The application of HT in R0-resected recurrent BC or for patients with the first diagnosis of BC at high risk for a local relapse (i.e., aggressive histopathological features and/or not R0-resected) has been described in the literature [Citation11], but with less-clear benefits. We included in our analysis BC patients treated homogenously with RT + HT over nearly three decades. In this group, almost 80% of patients were treated for recurrent BC. As might be expected, among the patients treated at the time they received their first diagnosis, rates of LC and OS were better compared to the whole cohort (80.7 vs. 76.4% and 77.3 vs. 69.5% LC at 2 years and 66.7 vs. 73.5 and 37.5 vs. 35.5% OS at 2 and 10 years, respectively).
Because our primary endpoint was LC, we included patients with metastatic tumors as well, even though the large majority (70%) was M0. Over recent years, our department has narrowed the indications for RT + HT to recurrent BC, especially if the patient was previously irradiated, according to guidelines [Citation6]. Nevertheless, previously we have also treated patients with newly diagnosed inoperable BC or for resected BC at high risk of relapse, e.g., non R0-resected tumors, and we included these patients in the present study as well. The broad use of HT in multiple oncological scenarios, including newly diagnosed high-risk BC [Citation7,Citation11], is rationalized by the fact that HT has been known for decades to be a potent radiosensitizer, with a thermal enhancement ratio of approximately 2.5 [Citation26,Citation27]. As expected, we observed better outcomes in patients with R0-, close- or R1-resected tumors. Nevertheless, out of 45 patients with macroscopic tumors (R2-resected or inoperable), 15 had no local progression 2 years after treatment (), with approximately 60% LC at 2 years in those who received at least 60 Gy (). These results are in line with those of Linthorst et al., who documented a LC of 53% at 1 year in patients with irresectable BC recurrences treated with a palliative schedule of 32 Gy in 8 fractions + HT [Citation16]. Many research groups have reported on the use of hypofractionated RT schedules with doses of approximately 30 Gy to treat BC in combination with HT [Citation9,Citation13–16]. The debate on RT dose is still open, particularly concerning higher doses, such as those we used, which might cause more toxicity, especially in those patients who had already received a RT course.
The higher efficacy of combined RT + HT compared to RT alone for BC patients was documented in a meta-analysis of five small randomized trial conducted more than 30 years ago [Citation7]. In the absence of recent evidence-generating phase III randomized trials comparing RT-HT and RT alone, our data need to be put in context through a comparison with cohorts of patients treated with RT alone. Even considering only instances in which re-irradiation doses of 50 Gy or more were used, i.e., excluding those in which re-irradiation was performed with palliative doses, LC rates vary widely. Merino et al. reported for a cohort of 47 patients treated with re-RT for recurrent BC a LC of 50% at 2 years [Citation28]. LC at 2 years between 60 and 75% has been reported after re-irradiation with brachytherapy [Citation29,Citation30]. Following reirradiation after second breast conservative surgery, LC up to 90% after 5 years has been reported [Citation31–34]. Fattahi et al. recently documented locoregional recurrence-free survival of 74% after 2 years [Citation35]. This wide range of variability might depend on the mixed nature of patients cohorts, especially regarding tumor stage, type of surgery and residual tumor after surgery, where the higher LC rates have been reported after second breast-conserving surgery for early-detected isolated in-breast relapses and R0 resection. Here, we report LCs of 76% after 2 years and 70% after 10 years, with only five isolated local recurrences. Our results are encouraging, considering the nature of our cohorts, that is, including patients with inoperable macroscopic disease and distant metastases. Supporting the importance of patients’ selection criteria in determining outcomes after RT + HT, in a prospective evaluation on a cohort of 20 selected patients treated with re-irradiation and HT, we documented LC of approximately 90% after 2 years [Citation21].
Regarding treatment tolerance, in our cohort previously irradiated patients showed more grades 2 and 3 late toxicities compared to those not previously irradiated. Nevertheless, we observe no acute or late toxicity higher than grade 3 and the majority of patients developed only grade 1 late toxicity.
A trend toward a better LC according to the applied dose was observed (, ), consistent with our previous results [Citation21]. We observed better LC in patients who received more than 10 HT applications. Since most of our patients were treated with radiation doses of 50 Gy or more (), and in parallel with two sessions of HT per week, patients who received at least 10 HT treatments were those who completed HT treatments as initially prescribed (5 weeks RT plus eventually a sequential boost in the 6th or 7th week). Regarding HT temperature, a trend toward a better LC was observed when a minimal temperature of 38.5 °C (average temperature in the whole cohort) was applied. In a recent paper, Bakker et al. observed that the cumulative equivalent minutes at 43 °C (which incorporates information about median temperature and duration of treatment) correlates with a better locoregional control in patients treated with re-irradiation and HT for breast recurrences [Citation36]. These temperatures were measured with invasive probes. By contrast, the temperatures we recorded were measured with superficial probes placed on the patient´s skin and a temperature approximately 2 °C higher than the surface measurement is to be expected in the target area.
The limitations of our study comprise its retrospective nature, sometimes lacking follow-up and toxicity data, the latter with different classifications over three decades. Here it should be noted that we included patients treated more than 30 years ago. Because of this, although we performed a careful and deep examination of the patients’ records, the quality of some data including pathologic tumor characteristics, superficial temperature measurements as well as, as already mentioned, the follow up and toxicity data, could not be improved. Another limitation arises from the mixed nature of our cohort, including patients with potentially different prognoses, i.e., recurrent and primary BC as well as some with M1-disease was included. A strength of this analysis is the large number of patients and the long-term follow-up.
In conclusion, we could observe in a large cohort of patients with long-term follow up that combined RT + HT offered high LC rates for patients affected by recurrent, non-resectable or resected BC at high risk of relapse, with acceptable toxicity. Further validation, including a prospective evaluation of quality of life and patient-reported outcomes and experience measures (PROMs/PREMs) data is currently ongoing (NCT04878666).
Supplemental Material
Download PDF (42.5 KB)Disclosure statement
Radiation Oncology Tübingen receives financial and technical support from Elekta, Philips, Siemens, Dr. Sennewald Medizintechnik, Kaiku Health, TheraPanacea, PTW and ITV under a research agreement. C.D.C. is supported by the Medical Faculty Tübingen (TüFF). No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34.
- Witteveen A, Kwast AB, Sonke GS, et al. Survival after locoregional recurrence or second primary breast cancer: impact of the disease-free interval. PLOS One. 2015;10(4):e0120832.
- Peeken JC, Vaupel P, Combs SE. Integrating hyperthermia into modern radiation oncology: what evidence is necessary? Front Oncol. 2017;7:132.
- Dewhirst MW, Vujaskovic Z, Jones E, et al. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005;21(8):779–790.
- Hader M, Streit S, Rosin A, et al. In vitro examinations of cell death induction and the immune phenotype of cancer cells following radiative-based hyperthermia with 915 MHz in combination with radiotherapy. Cells. 2021;10(6):1436.
- Interdisziplinaere S3-leitlinie fuer die frueherkennung, diagnostik, therapie und nachsorge des Mammakarzinoms. December 2017. p. 207.
- Vernon CC, Hand JW, Field SB, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International collaborative hyperthermia group. Int J Radiat Oncol Biol Phys. 1996;35:731–744.
- Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23(13):3079–3085.
- Van Der Zee J, De Bruijne M, Mens JW, et al. Reirradiation combined with hyperthermia in breast cancer recurrences: overview of experience in erasmus MC. Int J Hyperthermia. 2010;26(7):638–648.
- Wahl AO, Rademaker A, Kiel KD, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70(2):477–484.
- Welz S, Hehr T, Lamprecht U, et al. Thermoradiotherapy of the chest wall in locally advanced or recurrent breast cancer with marginal resection. Int J Hyperthermia. 2005;21(2):159–167.
- Muller AC, Eckert F, Heinrich V, et al. Re-surgery and chest wall re-irradiation for recurrent breast cancer: a second curative approach. BMC Cancer. 2011;11(1):197.
- Oldenborg S, Griesdoorn V, van Os R, et al. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: size matters. Radiother Oncol. 2015;117(2):223–228.
- Notter M, Piazena H, Vaupel P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: a retrospective study of 73 patients. Int J Hyperthermia. 2016;33:227–236.
- Linthorst M, van Geel AN, Baaijens M, et al. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol. 2013;109(2):188–193.
- Linthorst M, Baaijens M, Wiggenraad R, et al. Local control rate after the combination of re-irradiation and hyperthermia for irresectable recurrent breast cancer: results in 248 patients. Radiother Oncol. 2015;117(2):217–222.
- Dharmaiah S, Zeng J, Rao VS, et al. Clinical and dosimetric evaluation of recurrent breast cancer patients treated with hyperthermia and radiation. Int J Hyperthermia. 2019;36(1):986–992.
- Datta NR, Puric E, Klingbiel D, et al. Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2016;94(5):1073–1087.
- Li G, Mitsumori M, Ogura M, et al. Local hyperthermia combined with external irradiation for regional recurrent breast carcinoma. Int J Clin Oncol. 2004;9(3):179–183.
- Kapp DS, Cox RS, Barnett TA, et al. Thermoradiotherapy for residual microscopic cancer: elective or post-excisional hyperthermia and radiation therapy in the management of local-regional recurrent breast cancer. Int J Radiat Oncol Biol Phys. 1992;24(2):261–277.
- De-Colle C, Weidner N, Heinrich V, et al. Hyperthermic chest wall re-irradiation in recurrent breast cancer: a prospective observational study. Strahlenther Onkol. 2019;195(4):318–326.
- Dobsicek Trefna H, Schmidt M, van Rhoon GC, et al. Quality assurance guidelines for interstitial hyperthermia. Int J Hyperthermia. 2019;36(1):277–294.
- Beck TM, Hart NE, Woodard DA, et al. Local or regionally recurrent carcinoma of the breast: results of therapy in 121 patients. J Clin Oncol. 1983;1(6):400–405.
- Dahlstrom KK, Andersson AP, Andersen M, et al. Wide local excision of recurrent breast cancer in the thoracic wall. Cancer. 1993;72(3):774–777.
- Petrella F, Radice D, Borri A, et al. Chest wall resection and reconstruction for locally recurrent breast cancer: from technical aspects to biological assessment. Surgeon. 2016;14(1):26–32.
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys. 1989;16(3):535–549.
- Overgaard J. Hyperthermia as an adjuvant to radiotherapy. Review of the randomized multicenter studies of the European Society for Hyperthermic Oncology. Strahlenther Onkol. 1987;163(7):453–457.
- Merino T, Tran WT, Czarnota GJ. Re-irradiation for locally recurrent refractory breast cancer. Oncotarget. 2015;6(33):35051–35062.
- Harms W, Krempien R, Hensley FW, et al. Results of chest wall reirradiation using pulsed-dose-rate (PDR) brachytherapy molds for breast cancer local recurrences. Int J Radiat Oncol Biol Phys. 2001;49(1):205–210.
- Niehoff P, Dietrich J, Ostertag H, et al. High-dose-rate (HDR) or pulsed-dose-rate (PDR) perioperative interstitial intensity-modulated brachytherapy (IMBT) for local recurrences of previously irradiated breast or thoracic wall following breast cancer. Strahlenther Onkol. 2006;182(2):102–107.
- Arthur DW, Winter KA, Kuerer HM, et al. Effectiveness of Breast-Conserving surgery and 3-Dimensional conformal partial breast reirradiation for recurrence of breast cancer in the ipsilateral breast: the NRG oncology/RTOG 1014 phase 2 clinical trial. JAMA Oncol. 2020;6(1):75–82.
- Hannoun-Levi JM, Resch A, Gal J, et al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO breast cancer working group. Radiother Oncol. 2013;108(2):226–231.
- Kauer-Dorner D, Potter R, Resch A, et al. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother Oncol. 2012;102(1):96–101.
- Trombetta M, Julian TB, Werts DE, et al. Long-term cosmesis after lumpectomy and brachytherapy in the management of carcinoma of the previously irradiated breast. Am J Clin Oncol. 2009;32(3):314–318.
- Fattahi S, Ahmed SK, Park SS, et al. Reirradiation for locoregional recurrent breast cancer. Adv Radiat Oncol. 2021;6(1):100640.
- Bakker A, Tello Valverde CP, van Tienhoven G, et al. Post-operative re-irradiation with hyperthermia in locoregional breast cancer recurrence: temperature matters. Radiother Oncol. 2022;167:149–157.