Abstract
Objectives
To investigate the relationship between preoperative CA125 and symptom recurrence in adenomyosis after ultrasound-guided high-intensity focused ultrasound ablation surgery (FUAS).
Methods
A total of 502 adenomyosis patients after FUAS in Affiliated Nanchong Central Hospital of North Sichuan Medical College from June 2017 to March 2021 were reviewed. Factors associated with symptom recurrence of adenomyosis were analyzed by binary logistic regression model. ROC was used to determine the optimal cutpoint. Magnitude of preoperative CA125 relating to timing of symptom recurrence was measured by cox regression and Kaplan-Meier (K-M) curves. Besides, multiple liner regression model was used to identify the impacting factors for preoperative CA125.
Results
Multiple binary logistic analysis showed preoperative CA125 was related to symptom recurrence (OR = 1.002, 95%: 1.000~1.004, p = 0.043). The ROC of preoperative CA125 for recurrence validated 35 U/ml had a high sensitivity (82.5%). Preoperative CA125 was related to timing of symptom recurrence (HR = 2.255, 95%: 1.387–3.667, p = 0.001). K-M curves showed medium recurrence time in preoperative CA125 level >35 U/ml group (38.5 months) was shorter than that in CA125 level ≤35 U/ml group (44.5 months) (p = 0.001). Multiple liner regression analyses showed uterus volume and adenomyotic lesions volume positively correlated to preoperative CA125 level, while age negatively correlated to preoperative CA125 level.
Conclusion
The higher level of preoperative CA125 was related to an earlier onset of symptom recurrence after FUAS.
TWEETABLE ABSTRACT
Preoperative serum CA125 level over 35 U/ml might inform the symptom recurrence of adenomyosis after FUAS.
Introduction
Adenomyosis is a common gynecologic disorder that affect women in 40 s–50s [Citation1]. Treatment of adenomyosis is full of challenge in clinical practice. Until now, hysterectomy has been the most effective treatment for adenomyosis; however, it is not recommendable for women who would like to bear lovely babies. Nowadays, ultrasound-guided high-intensity focused ultrasound ablation surgery (FUAS), as a noninvasive technique, has become a promising option for women with adenomyosis who hope to spare uterus. Although numerous studies have shown that FUAS is safe and effective for the treatment of adenomyosis [Citation2–4], symptom recurrence rate is still high [Citation3,Citation5] and relapse may occur in 12 months after treatment [Citation6]. Liu XF [Citation7] reported that a greater body mass index and a lower mean acoustic intensity of FUAS were associated with a higher recurrence rate of adenomyosis in three years, however, no more studies investigated symptom recurrence after FUAS.
The main symptom recurrence after FUAS were dysmenorrhea and abnormal bleeding [Citation8]. Inflammation was found to be underlying causes of dysmenorrhea [Citation9]. A number of studies show inflammatory mediators, such as TNF-α and IL-1β, are aberrantly expressed in the endometrium of adenomyotic patients, promoting inflammation by activation of NF-κB pathway [Citation10]. However, it is not clear whether inflammation plays a great role in symptom recurrence after FUAS.
Related to inflammation, serum cancer antigen125 (CA125) is a glycoprotein derived from the embryonic celomic epithelium and has been used to distinguish adenomyosis from uterus fibroid [Citation11,Citation12]. CA125 has been used for diagnosis, evaluating clinic outcomes and predicting recurrence of endometriosis and ovarian cancer [Citation13,Citation14]. CA125 also was reported to be a risk factor of recurrence of severe adenomyosis after adenomyrectomy [Citation15]. However, few studies have been conducted with respect to the magnitude of CA125 on symptom recurrence of adenomyosis after FUAS.
Thus, we retrospectively examined the association between preoperative CA125 and symptom recurrence of adenomyosis in an cohort of patients with symptomatic adenomyosis who had FUAS in our center over the last four years.
Methods
Patients
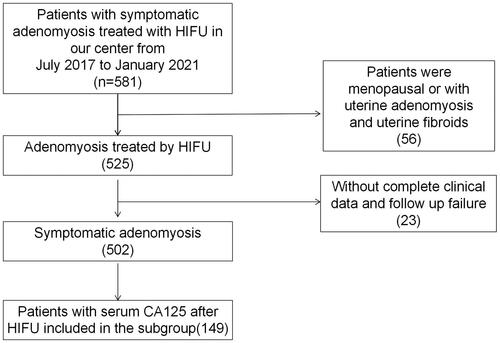
In this cross-sectional study, data of 581 patients diagnosed with symptomatic adenomyosis in the affiliated Nanchong Central Hospital of North Sichuan Medical College from June 2017 to March 2021 were collected. This study was approved by ethics committee of the affiliated Nanchong Central Hospital of North Sichuan Medical College (permit number. 2021104). The informed consent was waived due to the retrospective design. The data of 502 patients with complete clinical data and follow-up were retrospectively analyzed (). Adenomyosis was diagnosed based on magnetic resonance imaging (MRI): the maximal junctional zone thickness (JZmax) ≥12 mm [Citation16]. Symptom recurrence was defined as patient-self-reported the recurrence of dysmenorrhea or menorrhagia after symptomatic relief by FUAS.
Inclusion criteria were patients who received ultrasound-guided FUAS; had complete clinical data from medical record; performed MRI imaging before FUAS [Citation17]; received serum CA125 test before FUAS; had willing to follow up after FUAS.
Exclusion criteria were patients who refused ultrasound-guided FUAS; had no complete clinical data from medical record; had no MRI imaging before FUAS [Citation17]; refused to examine serum preoperative CA125 before FUAS; refused to follow up after FUAS.
Samples and marker assays
Preoperative CA125 was collected from clinical record. Blood samples for preoperative serum CA125 were transported to the laboratory center, measured by two-step immunoassay for the quantitative determination with flexible assay protocols [Citation18], assayed in a central laboratory before FUAS within one day. And the lab personnel were blinded to the clinical data [Citation19].
FUAS procedure
Preparation before FUAS and FUAS procedure conformed to the guildline of Focused Ultrasound Tumor Therapeutic System (Model-JC200, Chongqing Haifu Medical Technology Co., Ltd.) [Citation8,Citation17]. The ablation volume and non-perfused volume (NPV) ratio of adenomyotic lesions were performed by contrast-enhanced ultrasound [Citation17]. Treatment details including total sonication time, average sonication power and gray scale change were recorded by computer. The ablation volume was calculated according to the ellipsoid volume formula V = 4/3πABC [Citation20], in which A, B and C represent the long diameter, wide diameter and thickness diameter of the lesion, respectively. The NPV ratio = NPV/adenomyotic lesion*100%. All patients were required to stay in the hospital for 24 h of observation after FUAS.
Adjuvant therapy after FUAS and follow-up
To consolidate the efficacy of FUAS in the treatment of adenomyosis, three to six cycles of GnRH-a were suggested for the patient whose uteri were greater than 10 weeks of gestation or the length of the uterine cavity were more than 9 cm, then mirena was chosen to insert into the uteri if the patient had no fertility requirement when the uterus volume were suitable [Citation8].
To assess ablation effects and symptom relief, the patients were suggested for regular follow up. From August 2021 to September 2021, 502 patients completed the questionnaire either on the spot or by telephone if they could not come back to our cohort.
Statistical methods
Continuous data are summarized by the mean ± standard deviation and proportion. BMI was categorized as >24 or ≤24 [Citation21]. The Student’s t-test and chi square test were used to identify differences in baseline characteristic between recurrence group and non-recurrence group. A logistic regression mode was used to analyze the relationship between clinicopathological features (age, dysmenorrhea and serum CA125 level before FUAS, high signal intensity on T2WI, massive grayscale change, combined treatment with GnRH-a/mirena) and the symptom recurrence by odds ratios (ORs) with 95% confidence intervals (95% CIs). Areas under the receiver operating characteristic curves (ROCs; MedCalc Software bvba [ver. 15.2.1], Ostend, Belgium) was used to determine the optimal cutpoint of preoperative CA125 level for recurrence. Magnitude of preoperative CA125 relating to timing of symptom recurrence was measured by cox regression with hazard ratio (HR) and 95% CI. K-M curves was applied to estimate the middle recurrence time of different level of preoperative CA125 group for adenomyosis. Linear regression analysis was established to analyze the factors (age, dysmenorrhea, diffuse adenomyosis, massive gray scale change, uterus volume and adenomyotic lesions volume) impacting preoperative CA125. Statistical analysis was completed with SPSS 22.0 (IBM, Armonk, NY), and p < 0.05 was defined as statistically significant.
Results
Clinicopathological features
Baseline characteristics of the 502 patients included in this study are summarized in . The median follow-up time were 24 (2-51) months. 133 (26.5%) patients were included in recurrence group, while 369 (73.5%) patients in non-recurrence group. The rates of dysmenorrhea (+), diffuse adenomyosis, uniform gray scale change, treatment with FUAS alone in recurrence group were higher than those in non-recurrence group (p < 0.05). Preoperative serum CA125 levels were higher in recurrence group (117.3 ± 120.3 U/ml) than those in non-recurrence group (81.0 ± 101.3 U/ml) (p = 0.004), while age in recurrence group (42.3 ± 5.4 years) were younger than those in non-recurrence group (44.3 ± 5.1 years) (p < 0.001) ().
Table 1. Clinicopathological features.
Factors influencing symptom recurrence of adenomyosis
In the multivariate logistic regression, age (OR = 0.947, 95% CI 0.907~0.988, p = 0.012), preoperative CA125 (OR = 1.002, 95% CI 1.000~1.004, p = 0.043), dysmenorrhea (+) (OR = 2.167, 95% CI 1.168~4.021, p = 0.013), massive grayscale change (OR = 0.601, 95% CI 0.383~0.944, p = 0.027), and combined treatment with GnRH-a/mirena (OR = 0.491, 95% CI 0.312~0.772, p = 0.002) were associated with symptom recurrence of adenomyosis ().
Table 2. Binary logistic regression analysis of symptom recurrence in patients with adenomyosis.
Identification of preoperative serum CA125 for symptom recurrence
ROC curve of preoperative CA125 for symptom recurrence in adenomyotic patients showed that the optimal cutoff value, sensitivity and specificity, area under the cure of preoperative CA125 were 58.7 U/mL, 65.8%, 58.5%, 0.636, respectively (95% CI: 0.747−0.796, p < 0.001, supplementary Figure). Different cutoff values of preoperative CA125 to predict the symptom recurrence were compared. Cutoff value of 35 U/mL had a higher sensitivity than that of 58.7 U/mL (82.5% vs. 65.8%) (Supplementary table). Hence, preoperative CA125 level of 35 U/mL was validated as sensitive cutoff value to predict recurrence of adenomyosis.
Magnitude of preoperative serum CA125 for symptom recurrence
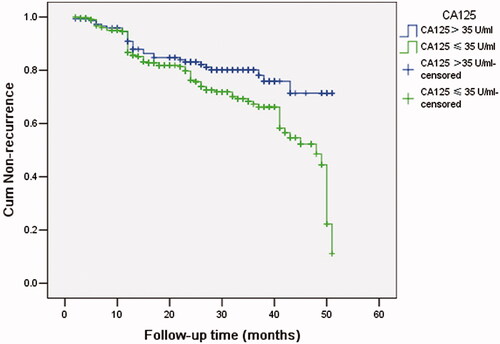
Multivariate cox regression showed that preoperative CA125 was related to timing of symptom recurrence (HR = 2.255, 95%: 1.387-3.667, p = 0.001). K-M curve showed medium recurrence time in preoperative CA125 level > 35 U/ml group (38.5 months) was shorter than that in preoperative CA125 level ≤35 U/ml group (44.5 months) (, p = 0.001).
The factors impacting preoperative serum CA125
Age, preoperative dysmenorrhea, diffuse adenomyosis, uterus volume and adenomyotic lesions volume were performed into linear regression model for preoperative CA125. The analysis showed that before FUAS, uterus volume and adenomyotic lesions volume positively correlated to CA125 level, while age negatively correlated to CA125 level ().
Table 3. Coefficient of multivariable regression model of preoperative CA125.
Discussion
In this cross-sectional study, we established that elevated preoperative CA125 associated with symptom recurrence of adenomyosis after FUAS. We validated that the commonly used preoperative CA125 threshold, 35 U/mL, was sensitive to assess the risk of incidence and timing of symptom recurrence of adenomyosis. Meanwhile, before FUAS, uterus volume, adenomyotic lesions volume positively correlated to serum CA125 level, while age negatively correlated with serum CA125 level.
Our results were in agreement with the finding of Sheth S [Citation22], high preoperative CA125 level suggesting larger the adenomyotic lesions volume, especially in the outer lesion of myometrium, higher the chance of its coexistance with adhesion and endometrisis in patients of adenomyosis. On this occasion, patients might be in a high level of inflammation. When preoperative CA125 level of adenomyosis patients was more than 35 U/mL, especially more than 58.7 U/mL, the symptom recurrence rate of adenomyosis was high, especially combined with severe dysmenorrhea, hyperintensity on T2WI and massive grayscale change during FUAS procedure. On this occasion, a sufficient sonication energy and average sonication power without causing safety troubles was suggested to decrease the opportunity to relapse. Besides, combined treatment with GnRH-a or mirena might be a beneficial choice for decreasing the recurrence. Given the pathogenesis of adenomyosis, FUAS alone might not guarantee therapeutic effect and prevent symptom recurrence in patients with adenomyosis, especially diffuse adenomyotic lesions with high preoperative CA125 level [Citation8]. Therefore, 3-6 cycles of GnRH-a after FUAS were administered, and treatment combined with mirena after 4-6 cycles of injection was recommended if patients had no desire to get pregnant in short time.
In recent years, several studies have established that inflammation plays an important role in pathogenesis and progression of adenomyosis [Citation23]. Firstly, variants of genes are involved in the processes of adenomyosis, resulting in hyperestrogenism, progesterone resistance and inflammatory reactions [Citation23]. Secondly, production of supraphysiologic estrogen plays an important role in endometrium through E3 ubiquitin ligase HECW1-mediated Scribble degradation. Then, inflammatory reactions were stimulated as a result of activation of the tissue injury and repair (TIAR) mechanism [Citation24]. Cell proliferation, migration, and invasiveness of endometrial lead to the development of adenomyosis by increasing expression of inflammatory mediators, like interleukin 10 and tumor necrosis factor [Citation23–25].
As an inexpensive and easily measured mediator, serum CA125 is a high polymer glycoprotein molecular cell-surface glycoprotein deriving from the embryonic celomic epithelium of normal tissue types. First of all, inflammatory reactions, peritoneal irritation or peritoneal stretch altering endothelial permeability might simulate preoperative serum CA125 to reach the circulation, thereby resulting in high level of preoperative CA125 [Citation26]. Secondly, the ectopic endometrium is confirmed to secrete significantly higher preoperative CA125 level than did in normal endometrium of adenomyotic patients [Citation27]. And high preoperative serum CA125 level may facilitate ectopic endometrium migration and adhesion in the surrounding myometrium during adenomyotic development [Citation28], resulting in a high pain score and symptom recurrence. While after FUAS, the level of preoperative CA125 decreased [Citation7], which may represent the decreasing of inflammatory burden.
Our study also has some limitations. Firstly, we did not collect CA125 right after FUAS to analyze the dynamic changes of CA125 before and after FUAS to investigate the relationship with the recurrence of adenomyosis, and the cross-sectional design can not make the conclusion that preoperative CA125 is one of the reason for symptom recurrence of adenomyosis, although our analysis confirmed that preoperative CA125 was related to symptom recurrence, which might be of interest to clinicians for predicting the outcome after FUAS and making related preventive strategies. Secondly, the cross-sectional design means a few patients were excluded for incomplete data and failing to follow-up for they lived in different cities far away from our hospital, although we tried our best to follow up every patient. Thirdly, the time of symptom recurrence was collected from patients-self-reported recurrence of dysmenorrhea or menorrhagia, and dysmenorrhea and menorrhagia were recorded as yes or no, not by severity level of dysmenorrhea and menorrhagia in this study. These may lead to bias. However, multicenter study to elaborate the role of preoperative CA125 in recurrence of adenomyosis might support more evidence by Numerical Rating Scale (NRS).
Conclusions
In summary, our results preliminarily showed that elevated preoperative CA125 was a risk factor for symptom recurrence of adenomyosis. When preoperative CA125 level was over 35 U/ml, combined treatment with GnRH-a and mirena may decrease the opportunity of symptom recurrence of adenomyosis.
Supplemental Material
Download PDF (159.7 KB)Acknowledgements
The authors thank Yan Wang, an English professor from Chongqing Medical University, who has done a thorough review and correction of the English of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approach. Fertil Steril. 2018;109(3):406–417.
- Jeng CJ, Ou KY, Long CY, et al. 500 Cases of high-intensity focused ultrasound (HIFU) ablated uterine fibroids and adenomyosis. Taiwan J Obstet Gynecol. 2020;59(6):865–871.
- Marques ALS, Andres MP, Kho RM, et al. Is high-intensity focused ultrasound effective for the treatment of adenomyosis? A systematic review and meta-analysis. J Minim Invasive Gynecol. 2020;27(2):332–343.
- Guo Y, Duan H, Cheng J, et al. Gonadotrophin-releasing hormone agonist combined with high-intensity focused ultrasound ablation for adenomyosis: a clinical study. Bjog. 2017;124(Suppl 3):7–11.
- Liu X, Wang W, Wang Y, et al. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Medicine (Baltimore). 2016;95(3):e2443.
- Zhang X, Zou M, Zhang C, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol. 2014;83(9):1607–1611.
- Liu XF, Huang LH, Zhang C, et al. A comparison of the cost-utility of ultrasound-guided high-intensity focused ultrasound and hysterectomy for adenomyosis: a retrospective study. BJOG. 2017;124(Suppl 3):40–45.
- Li X, Zhu X, He S, et al. High-intensity focused ultrasound in the management of adenomyosis: long-term results from a single center. Int J Hyperthermia. 2021;38(1):241–247.
- Upson K, Missmer SA. Epidemiology of adenomyosis. Semin Reprod Med. 2020;38(2-03):89–107.
- Feng T, Wei S, Wang Y, et al. Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-Catenin signaling pathway. Biomed Pharmacother. 2017;94:231–237.
- Kil K, Chung JE, Pak HJ, et al. Usefulness of CA125 in the differential diagnosis of uterine adenomyosis and myoma. Eur J Obstet Gynecol Reprod Biol. 2015;185:131–135.
- Sasamoto N, Babic A, Rosner BA, et al. Predicting circulating CA125 levels among healthy premenopausal women. Cancer Epidemiol Biomarkers Prev. 2019;28(6):1076–1085.
- Han AR, Lee TH, Kim S, et al. Risk factors and biomarkers for the recurrence of ovarian endometrioma: about the immunoreactivity of progesterone receptor isoform B and nuclear factor kappa B. Gynecol Endocrinol. 2017;33(1):70–74.
- Sun J, Cui XW, Li YS, et al. The value of 18F-FDG PET/CT imaging combined with detection of CA125 and HE4 in the diagnosis of recurrence and metastasis of ovarian cancer. Eur Rev Med Pharmacol Sci. 2020;24(13):7276–7283.
- Yu W, Liu G, Liu C, et al. Recurrence-associated factors of laparoscopic adenomyomectomy for severely symptomatic adenomyoma. Oncol Lett. 2018;16(3):3430–3438.
- Reinhold C, McCarthy S, Bret PM, et al. Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology. 1996;199(1):151–158.
- Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221.
- Harter P, Sehouli J, Lorusso D, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380(9):822–832.
- Tang Y, Hu HQ, Tang YL, et al. Preoperative LMR and serum CA125 level as risk factors for advanced stage of ovarian cancer. J Cancer. 2021;12(19):5923–5928.
- Quinn SD, Vedelago J, Kashef E, et al. Measurement of uterine fibroid volume: a comparative accuracy and validation of methods study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):161–165.
- Yang P, Chou C, Chang C, et al. Changes in peripheral mitochondrial DNA copy number in metformin-treated women with polycystic ovary syndrome: a longitudinal study. Reprod Biol Endocrinol. 2020;18(1):69.
- Sheth SS, Ray SS. Severe adenomyosis and CA125. J Obstet Gynaecol. 2014;34(1):79–81.
- Zhai J, Vannuccini S, Petraglia F, et al. Adenomyosis: Mechanisms and pathogenesis. Semin Reprod Med. 2020;38(2-03):129–143.
- Jin Z, Liu H, Xu C. Estrogen degrades scribble in endometrial epithelial cells through E3 ubiquitin ligase HECW1 in the development of diffuse adenomyosisdagger. Biol Reprod. 2020;102(2):376–387.
- Xu X, Cai X, Liu X, et al. Possible involvement of neuropeptide and neurotransmitter receptors in adenomyosis. Reprod Biol Endocrinol. 2021;19(1):25.
- Seeber B, Sammel MD, Fan X, et al. Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril. 2008;89(5):1073–1081.
- Kobayashi H, Ida W, Terao T, et al. Molecular characteristics of the CA 125 antigen produced by human endometrial epithelial cells: comparison between eutopic and heterotopic epithelial cells. Am J Obstet Gynecol. 1993;169(3):725–730.
- Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–428.