?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
Radiofrequency ablation (RFA) is recognized as an effective technique for the treatment of benign thyroid nodules (BTNs), although the long-term results are limited. This study aimed to evaluate the residual vital volume increase, regrowth, and new growth over a 2-year period after RFA among different nodule volume groups.
Subjects and Methods
This retrospective study evaluated 135 patients with 153 BTNs who underwent ultrasound guided RFA. The BTNs were categorized into small (<10 mL), medium (10–30 mL), and large (>30 mL) according to the initial volume of BTNs prior to ablation. The volume changes of each nodule were analyzed at 1, 3, 6, 12 and 24 months after RFA. New growth was defined as the growth in volume not found in the early follow-up on ultrasonography.
Results
The initial ablation ratio of all BTNs was 99.67%. The mean volume reduction ratio (VRR) of BTNs was 85.53% after 2-year follow-up. The small nodule group showed a lower VRR compared to the other two groups at the 1-month follow-up, and there was no difference of VRR at the subsequent follow-ups. The incidence of residual vital volume increase was 4.58%. The overall incidence of regrowth was 3.92% and the mean timing of regrowth was 16.71 months. New growth occurred in 18.95% of patients. No further treatment was required in the majority of cases.
Conclusion
RFA achieved a clinically relevant volume reduction in different sizes of single BTNs which persisted for at least 2 years, thereby preventing the need for retreatment.
Introduction
Benign thyroid nodules (BTNs) are the target of more than half of the population seeking treatment by high-resolution ultrasonography techniques in Asia, Europe, and the United States [Citation1–3]. Owing to the overall positive outcomes, shortened recovery times, and reduced pain involved, minimally invasive procedures such as invasive image-guided thermal ablation offer patients viable alternative treatment modalities to avoid surgery [Citation4]. The methods for generating thermal gradients include radiofrequency, high-intensity-focused ultrasound, microwave, laser, and freezing treatments [Citation5–8]. Among the available options for invasive image-guided thermal ablation, radiofrequency is the most promising in terms of long-term results and efficacy [Citation9,Citation10]. Evidence indicates the reliability and efficacy of radiofrequency ablation (RFA) for BTNs in clinical practice, including for pediatric goiter [Citation11], intrathoracic goiter [Citation12], Warthin tumor [Citation13], soft tissue neoplasm [Citation14], and even for low-risk papillary thyroid microcarcinoma [Citation15] and thyroid follicular neoplasm with low SUV in PET/CT [Citation16]. Meanwhile, RFA guidelines suggest RFA as the first-line treatment for patients with cosmetic issues and symptomatic nonfunctioning solid BTNs [Citation2,Citation17–20].
Although the results of RFA for BTN treatment are convincing, there remain several confounding factors [Citation21] which affect the volume reduction ratio (VRR). Among these factors, the volume of BTNs is one of the most important issues of consideration as it may directly correlate to procedural time, the number of RFA sessions required, cosmetic concerns, compliance and comfort of patients, and initial incomplete ablation [Citation22]. Most importantly, the baseline nodular volume is the primary factor determining the long-term outcome [Citation23], while nodules of different volumes may require further consideration, such as the higher regrowth rate and lower VRR of a BTN with larger baseline nodular volume during follow-up [Citation10,Citation24,Citation25]. Although the differences regarding initial therapeutic success and complication rates for RFA treatment of BTNs of various sizes have been reported [Citation26], data is lacking in terms of the medium-term follow-up results for residual growth or recurrence of BTNs of various sizes.
Regrowth and recurrence rates of BTNs presenting with different nodule volumes after RFA treatment are vague due to a dearth of precise long-term follow-up data. These results and parameters could provide critical information to help patients with BTNs of different nodule volumes determine the need for RFA retreatment. This retrospective study is the first long-term follow-up report of patients with BTNs presenting with different nodule volumes after RFA treatment in Taiwan. We aim to identify the monitoring parameters and provide valuable clinical follow-up data to assist the decision-making process of physicians and patients with regards to the requirement for retreatment.
Materials and methods
This retrospective study was approved by the institutional review board, and all patients provided written informed consent to receive RFA prior to this procedure. The approved protocol number of the Chang Gung Medical Foundation Institutional Review Board is 202200189B0. As this is a retrospective study, informed consent to publish is waived.
Patients
From September 2016 to January 2019, 353 patients underwent RFA for treatment of BTNs at the Kaohsiung Chang Gung Memorial Hospital Medical Center in Taiwan.
Patients with BTNs presented with nodule-related symptoms, cosmetic issues, or were identified incidentally. Patients visited otolaryngologists, surgeons, or internal medicine physicians, and were subsequently transferred to the Radiology Department for assessment of nodular composition by sonography or CT/MRI. Nodules were then classified as solid, predominant solid, predominant cystic, or cyst [Citation27]. Ultrasound guided fine-needle aspiration cytology (FNAC) or core needle biopsy was performed to confirm the benign nature of the nodules. At least two benign cytological results or a single benign cytological result with ultrasound appearance was considered at low risk of malignancy [Citation18]. Patients had no contraindication for surgery, however they refused surgery over concerns of post-treatment scarring, complications, side effects, or thyroid functional changes. The demographic data records of these patients and their follow-up outcomes were retrospectively analyzed.
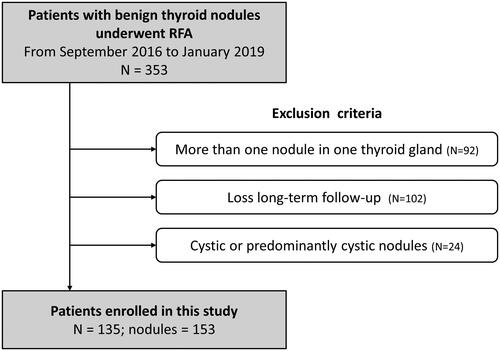
The exclusion criteria were patients with more than one nodule in one thyroid gland, and patients who did not return for each follow-up session (1-, 3-, 6-, 12-, and 24-month). To clarify the efficacy of RFA, patients with cyst or predominant cystic nodules were also excluded to avoid the cofounding effects from ethanol sclerotherapy. After exclusion criteria, a total of 135 patients with 153 BTNs who underwent RFA were enrolled in this study. The flowchart of patient enrollment is shown in . The patients were further divided into three groups, according to the initial volume of BTNs assessed by sonography evaluation: (1) small group, a pre-ablation nodule volume of <10mL; (2) medium group, a pre-ablation nodule volume of 10–30 mL; (3) large group, a pre-ablation nodule volume of >30 mL.
Pre-ablation assessment
All patients were evaluated by clinical examination, blood tests, and ultrasound examination. Prior to the ablation procedure, patients were asked to rate their nodule-related symptoms on a questionnaire including five clinical symptoms (compression, cough, difficulty swallowing, voice change, and pain; range from 0 to 5), and a cosmetic grading score was assessed by the physician (0, no visible or palpable mass; 1, not visible but palpable mass; 2, visible when swallowing only; 3, an easily visible mass) [Citation17]. Laboratory examinations included measurements of serum thyrotropin (TSH), total triiodothyronine (T3), and free thyroxine (T4).
For each nodule, three orthogonal diameters (the largest diameter and two perpendicular diameters) and the proportion of the solid components were measured by sonography. The volume of each nodule was calculated using the following equation: V = abc/6 (where V was the volume, a was the largest diameter, and b and c were the two perpendicular diameters) [Citation28].
Ablation technique
The RFA procedure was performed by a radiologist with over 10 years of experience under ultrasound guidance on an outpatient basis. Patients were in a supine position with their necks fully extended. Prior to ablation, a solution of 2% lidocaine hydrochloride was used as local anesthesia at the puncture site and around the thyroid gland. In accordance with US examination guidelines, the electrode tip size was chosen based on tumor size and status of the surrounding critical structures. An internally cooled electrode (18 gauge, with 5 mm, 7 mm or 1-cm active tip) with RF generator (VIVA, STARmed and M2004, RF Medical) were used. A trans-isthmic approach method and the moving-shot technique were applied in the procedure. To prevent hemorrhage, vessels along the approach route were carefully observed using Doppler ultrasound. An electrode was inserted into the thyroid nodule at the deepest and most remote portion of the nodule. Ablation termination was determined when all visual fields of the nodule had changed to transient hyperechoic zones. Complications during and immediately following the procedure were monitored for proper management. After the RFA procedure, patients were observed for 1–2 h in the hospital, and arrangements for regular post-RFA follow-up were determined.
Follow-up evaluation
Patients were followed up by clinical evaluations and ultrasound examinations at 1, 3, 6, 12, and 24 months after RFA. The thyroid nodule volume, cosmetic and symptom scores were evaluated in the same manner before and after ablation. The VRR was the percentage reduction in volume as assessed by ultrasound imaging and calculated by the equation: ([initial volume – final volume] × 100)/initial volume.
To evaluate the efficacy and recurrence rate of RFA, several parameters were defined, as shown in . The total volume (Vt) of a nodule was divided into an ablated (Va) and a vital portion (Vv). Vt represents the total nodule volume, and Va is the ablated nodule volume presenting as a hypoechoic area without vascularity on ultrasound. Vv is the incompletely treated vital nodule volume, noted on the 1- and 3-month follow-up ultrasounds [Citation29], and can be calculated by the following equation: Vv = Vt – Va. In this study, we defined the new growth portion (Vn) as the new growth volume not identified in the early follow-up ultrasound. The initial ablation ratio (IAR) is an index that measures the amount of ablation after RFA, and is associated with technique efficacy. It is the ratio between the ablated volume (Va) and the total volume (Vt), and is calculated as: IAR = (Va/Vt) × 100 [Citation30].
Figure 2. The definitions of parameters for volume assessment after RFA.
(1) Regrowth was defined as a more than 50% increase in total volume (Vt) compared to the previously reported smallest volume on ultrasonography.
(2) Residual vital volume (Vv) increase was defined as a more than 50% Vv increase compared to the previously reported smallest volume. The Vv was the incompletely treated vital nodule volume, noted on the 1- and 3-month follow-up ultrasounds.
(3) New growth was defined as new growth volume (Vn) not found in the early follow-up on ultrasonography. The size of the Vn was not the major consideration.
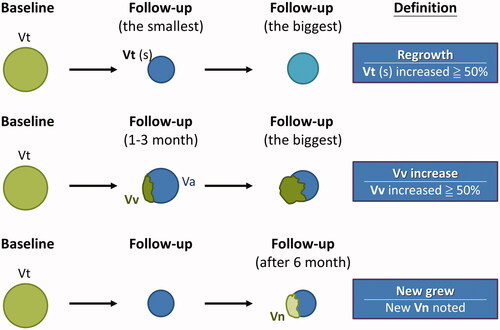
The residual vital volume (Vv) increase was defined as a more than 50% Vv increase compared to the previously reported smallest volume on ultrasonography, while regrowth was defined as a more than 50% Vt increase compared to the previously reported smallest volume [Citation29,Citation31].
Analyses and statistics
Statistical analyses were performed using SPSS, version 22 (SPSS, Inc. Chicago, IL, USA). All data are given as the mean ± standard deviation (SD) or number of nodules (percentage). Demographic characteristics and sonographic results among the BTN subgroups were compared. During the follow-up periods, the volume changes, frequency and timing of Vv increase, regrowth, and new growth of treated nodules were analyzed and compared among the 3 groups. Group comparison for continuous variables data were performed using an analysis of covariance (ANCOVA) model with age and gender as covariates. Standard Chi-Square was used for group comparison of categorical data. Statistical significance was set at p < .05.
Results
Demographic characteristics
Inclusion criteria were met by 135 patients (F:M = 110:25) with 153 BTNs, who were enrolled in this study (). shows the clinical characteristics of all patients based on pre-RFA volume groups. The nodule structure was solid in 45.10% of cases, and predominantly solid in 54.90% of cases. Among the 153 treated BTNs, 63 nodules (41.18%) comprised the small nodular group; 44 nodules (28.76%) comprised the medium nodular group; and the remaining 46 nodules (30.01%) comprised the large nodular group. There were no significant group differences in age or sex distribution, serum T3, T4 or TSH levels.
Table 1. Demographic characteristics of patients before RFA based on nodule volume.
The mean cosmetic score was elevated by the nodular volume, being highest in the large group before RFA (p < .001). The mean symptomatic score before RFA was significantly higher in the large group compared to the small group (p = .007). The RF power, ablation time, and total energy rose as the nodule volume increased (p < .001). The mean total energy delivered per mL pretreatment nodule volume was 2007.02 J, without significant group difference.
Treatment outcomes in VRR
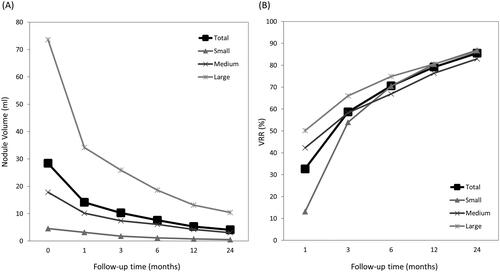
The treatment characteristics of our study patients are summarized in . The mean baseline thyroid nodule volume was 28.44 ± 37.97 mL. For the primary outcome of nodule volume reduction, the VRR was 85.53% at the 24-month follow-up and the individual rates from each subgroup were as follows: 86.90% in the small group; 82.96% in the medium group; and 86.31% in the large group. At the 1-month follow-up, the VRR was significantly higher in the medium (p = .001) and large (p < .001) groups as compared to the small group, while there was no group difference at the other follow-up periods. The nodular volumes and respective changes in total subjects and subgroups are presented in .
Table 2. Treatment outcomes in the subgroups at each follow-up period after RFA.
Regrowth, residual, and new growth
During the follow-up periods, regrowth with a more than 50% increase of Vt occurred in 6 cases (3.92%: 2 in the small group; 4 in the large group). Residual nodule with a more than 50% increase of Vv occurred in 7 cases (4.58%: 2 in the small group; 2 in the medium group; 3 in the large group). New growth occurred in 29 cases (18.95%: 7 in the small group; 10 in the medium group; 12 in the large group). The mean timing of regrowth was 16.71 ± 9.46 months and of new growth was 16.90 ± 7.93 months.
Complications
The overall complication rate was 5.23% (8/153: 1 in the small group; 7 in the large group), all of which were classified as minor complications. All subjects with complications recovered without sequelae. No patient experienced a life-threatening or delayed complication during the follow-up period.
Discussion
The results of this study demonstrate the effectiveness of RFA treatment for BTNs during the 2-year follow-up period. Previous studies have reported VRR results for small to medium BTNs of approximately 79% (23 months) [Citation32] to 93% (4 years) [Citation33], while the VRR in this study achieved approximately 86% at the 2-year follow-up, including the results of the large BTN group. In terms of the BTN group differences identified herein, the small BTN group showed an initially smaller VRR at the 1-month follow-up, although the VRR caught up to the other groups in follow-ups after 3 months, with the VRR at the 2-year follow-up reaching 87%. This may be due to the relatively high average IAR (99.67%), as the IAR is a quantitative indicator of the performance efficacy of the RFA procedure, and is highly correlated with the VRR [Citation32]. The relatively high IAR may contribute to a satisfactory VRR, no matter the baseline volume, but may indicate a potential overtreatment effect which could have influenced the initial VRR presentation here; however, this had no impact on the final VRR with regards to group differences [Citation26].
In terms of residual vital volume increase, a previous study has reported that it may be an early sign of regrowth, based on the 57.4% residual volume increase contributing to an overall regrowth of 24.1% [Citation34]. The residual vital volume increase in this study was 4.58%, while the overall regrowth reached 3.92% with a mean timing of regrowth of 16.71 months. These results could support the notion that the residual vital volume increase could be an early sign of regrowth, together with factors including baseline nodular volume, vascularity, delivered energy, and the number of ablation sessions [Citation23]. Furthermore, the residual vital ratio could be an independent factor and an early quantitative predictor for BTN regrowth after RFA [Citation35], further impacting the VRR results. Other factors affecting VRR, including energy per mL in nodular volume, blocking of peripheral flow, initial nodule volume and margin have been reported in other minimally invasive thermal ablation procedures [Citation36]. These factors exhibited only minor effects in this study, which achieved a nearly complete initial ablation rate, as the VRR reached over 80% regardless of the initial BTN volume. It may thus be concluded that the initial ablation rate could compensate for the effects of the poor VRR predictive factors, at least with regards to the initial BTN volume.
Another phenomenon noted in this study was that no hyperechoic foci nor increased Doppler signal intensity of the BTN after RFA were recorded at follow-ups, up to 6 months in some cases. However, newly onset foci with hyperechoic of increasing Doppler signal intensity change with an increasing trend was indeed noted during the follow-ups after 6 months, and accordingly termed new growth volume (Vn). The new growth volumes for the small, medium, and large groups were 11.11%, 22.73%, and 26.09%, respectively (total: 18.95%), with an average volume of 1.92 mL. Although no significant group differences were noted, there was a higher prevalence in the larger BTN groups. Although new growth was noted in several subjects for whom the IAR reached the maximum noted in this study, no retreatment was necessary due to the relatively low volume of the new growth, and no subjective complaints were reported by the subjects. Several studies have indeed reported that marginal recurrence is often observed with longer follow-up times [Citation37]. One study noted a tendency for marginal recurrence after 3 years [Citation38], while another study reported a low recurrence rate (5.6%) at the 4-year follow-up [Citation33]. Owing to the potential of the new growth phenomenon for the early detection of regrowth and recurrence, this indicator was included in this study to determine the need for RFA retreatment and for continued follow-up.
Though no major complications were identified in this study, several minor complications were noted. The complication rate of the small BTNs was 1.59%, consistent with a previous report of a low complication rate of 3.6% [Citation33]. However, a borderline significantly elevated minor complication rate of 15.2% was noted in the large BTN group, accounting for 7 of the 8 overall minor complications in this study. Though not statistically significant, the higher minor complication rate for larger BTNs should be noted, and invasive management may be required if the initial maximum diameter is greater than 4.5 cm [Citation39].
There are several limitations to the present study which should be considered. First, there is no consensus regarding the definition of long-term follow-up. Most BTN RFA studies titled as ‘long-term’ have a follow-up period of between 2 and 5 years, as it is generally challenging to ensure that subjects adhere to the follow-up schedule for longer than a period of 2 years. The number of lost follow-ups may significantly impact the relevance and credibility of study conclusions. Second, the clinical symptoms scale in this study uses a simplified combination of clinical symptoms but not a 10 cm visual analog scale. Third, no enhanced ECHO follow-up data were recorded for some of the earlier patients included in this study due to a lack of enhancement agents available in Taiwan at the time. Follow-up sonography without enhancement could influence the results of the residual vital volume data, particularly regarding the data for new growth. It is recommended that the follow-up be continued for patients with new growth to provide further insight into the causal relationship between the new growth phenomenon and recurrence, which currently remains unclear.
Conclusion
This long-term retrospective study further demonstrates the effectiveness of RFA for the treatment of BTNs of various sizes. Our results reveal that near complete initial BTN ablation yields satisfactory volume reduction results with a relatively low regrowth rate. The new growth phenomenon and volume are factors which should be noted as possible indicators for the early detection of regrowth and recurrence. Furthermore, our study indicates that while RFA treatment for large BTNs is effective, this may be accompanied by elevated rates of new growth and minor complications.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kant R, Davis A, Verma V. Thyroid nodules: advances in evaluation and management. Am Fam Physician. 2020;102(5):298–304.
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
- Yi KH. The revised 2016 korean thyroid association guidelines for thyroid nodules and cancers: differences from the 2015 American thyroid association guidelines. Endocrinol Metab. 2016;31(3):373–378.
- Hussain I, Zulfiqar F, Li X, et al. Safety and efficacy of radiofrequency ablation of thyroid nodules-expanding treatment options in the United States. J Endocr Soc. 2021;5(8):1.
- Pacella CM. Correction to letter: image‑guided thermal ablation of benign thyroid nodules. J Ultrasound. 2018;21(1):79–0285.
- Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44(1):14–36.
- Cheng Z, Liang P. Advances in ultrasound-guided thermal ablation for symptomatic benign thyroid nodules. Adv Clin Exp Med. 2020;29(9):1123–1129.
- Cesareo R, Manfrini S, Pasqualini V, et al. Laser ablation versus radiofrequency ablation for thyroid nodules: 12-Month results of a randomized trial (Lara II study). J Clin Endocrinol Metab. 2021;106(6):1692–1701.
- Deandrea M, Trimboli P, Garino F, et al. Long-term efficacy of a single session of RFA for benign thyroid nodules: a longitudinal 5-Year observational study. J Clin Endocrinol Metab. 2019;104(9):3751–3756.
- Bernardi S, Giudici F, Cesareo R, et al. Five-year results of radiofrequency and laser ablation of benign thyroid nodules: a multicenter study from the Italian minimally invasive treatments of the thyroid group. Thyroid. 2020;30(12):1759–1770.
- Lin AN, Lin WC, Cheng KL, et al. Radiofrequency ablation a safe and effective treatment for pediatric benign nodular thyroid goiter. Front Pediatr. 2021;9:753343.
- Chiang PL, Lin WC, Chen HL, et al. Efficacy and safety of single-session radiofrequency ablation for intrathoracic goiter: preliminary results and short-term evaluation. Int J Hyperthermia. 2021;38(1):976–984.
- Cha CH, Luo SD, Chiang PL, et al. Long-term outcomes of radiofrequency ablation for treatment of cystic Warthin tumors versus solid Warthin tumors. Int J Environ Res Public Health. 2021;18(12):6640.
- Lin WC, Tai YF, Chen MH, et al. Ultrasound-guided moving shot radiofrequency ablation of benign soft tissue neoplasm. Medicina. 2021;57(8):830.
- Lim LS, Lin WC, Chiang PL, et al. One year follow-up of US-Guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: the first experience in Taiwan. J Formos Med Assoc. 2021;14(21):00472–00471.
- Lin WC, Tung YC, Chang YH, et al. Radiofrequency ablation for treatment of thyroid follicular neoplasm with low SUV in PET/CT study. Int J Hyperthermia. 2021;38(1):963–969.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655.
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. 2019;36(1):376–382.
- Ha EJ, Baek JH, Che Y, et al. Radiofrequency ablation of benign thyroid nodules: recommendations from the Asian conference on tumor ablation task force. Ultrasonography. 2021;40(1):75–82.
- Ha EJ, Baek JH, Che Y, et al. Radiofrequency ablation of benign thyroid nodules: recommendations from the Asian conference on tumor ablation task force – secondary publication. J Med Ultrasound. 2021;29(2):77–83.
- Negro R, Trimboli P. Thermal ablation for benign, non-functioning thyroid nodules: a clinical review focused on outcomes, technical remarks, and comparisons with surgery. Electromagn Biol Med. 2020;39(4):347–355.
- Ahn HS, Kim SJ, Park SH, et al. Radiofrequency ablation of benign thyroid nodules: evaluation of the treatment efficacy using ultrasonography. Ultrasonography. 2016;35(3):244–252.
- Sim JS, Baek JH. Long-Term outcomes of thermal ablation for benign thyroid nodules: the issue of regrowth. Int J Endocrinol. 2021;2021(9922509):9922509.
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol. 2019;180(1):79–87.
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab. 2015;100(2):460–466.
- Lin WC, Kan NN, Chen HL, et al. Efficacy and safety of single-session radiofrequency ablation for benign thyroid nodules of different sizes: a retrospective study. Int J Hyperthermia. 2020;37(1):1082–1089.
- Andrioli M, Carzaniga C, Persani L. Standardized ultrasound report for thyroid nodules: the endocrinologist’s viewpoint. Eur Thyroid J. 2013;2(1):37–48.
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol. 2008;18(6):1244–1250.
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017;33(8):905–910.
- Bernardi S, Cavallaro M, Colombin G, et al. Initial ablation ratio predicts volume reduction and retreatment after 5 years from radiofrequency ablation of benign thyroid nodules. Front Endocrinol. 2020;11:582550.
- Mauri G, Pacella CM, Papini E, et al. Image-guided thyroid ablation: proposal for standardization of terminology and reporting criteria. Thyroid. 2019;29(5):611–618.
- Sim JS, Baek JH, Cho W. Initial ablation ratio: quantitative value predicting the therapeutic success of thyroid radiofrequency ablation. Thyroid. 2018;28(11):1443–1449.
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044–1049.
- Shemerovskiĭ KA, Ovsiannikov VI. [The role of the beta-adrenoreceptors in the smooth-muscle reactions of the stomach, pyloric sphincter and duodenum to serotonin administration]. Fiziol Zh SSSR Im I M Sechenova. 1988;74(12):1786–1793.
- Yan L, Luo Y, Xie F, et al. Residual vital ratio: predicting regrowth after radiofrequency ablation for benign thyroid nodules. Int J Hyperthermia. 2020;37(1):1139–1148.
- Liu LH, Yang BB, Liu Y, et al. Factors related to the absorption rate of benign thyroid nodules after image-guided microwave ablation: a 3-year follow-up. Int J Hyperthermia. 2022;39(1):8–14.
- Park HS, Baek JH, Park AW, et al. Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol. 2017;18(4):615–623.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010;20(11):1253–1261.
- Chen WC, Luo SD, Chen WC, et al. The importance of nodule size in the management of ruptured thyroid nodule after radiofrequency ablation: a retrospective study and literature review. Front Endocrinol. 2021;12:776919.