Abstract
Objectives
To examine the prognostic value of preoperative alfa-fetoprotein (AFP) density and other clinical factors in patients undergoing percutaneous radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC).
Methods
From January 2010 to December 2018, a total of 543 patients undergoing RFA for HCC meeting the Milan criteria were included at our institution. AFP density was calculated as absolute AFP pre-ablation divided by the total volume of all HCC lesions. The survival rates according to AFP density were estimated using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional-hazards regression analyses were used to assess predictors of overall survival (OS) and progression-free survival (PFS).
Results
The Kaplan–Meier 1-, 3-, and 5-year OS rates were 98.8%, 88.5%, and 70.4%, respectively, for the low AFP density group, and 98.3%, 74.9%, and 49.4%, respectively, for the high AFP density group. The corresponding PFS rates were 78.9%, 56.7%, and 40.9% (low AFP density group), and 63.6%, 40.8%, and 27.5% (high AFP density group). High AFP density was associated with significantly reduced PFS and OS (both p < 0.001). Multivariate analysis suggested that AFP density was a predictor of OS and PFS.
Conclusions
Serum AFP density may serve as a promising predictor of survival in patients with HCC undergoing RFA. High AFP density could identify patients who might be prone to recurrence or progression and need close surveillance.
Introduction
Hepatocellular carcinoma (HCC) is a fatal malignancy and ranks as the fourth leading cause of cancer-related mortality worldwide [Citation1]. During very early or early stage, radical treatment, which includes resection, transplantation, and radiofrequency ablation (RFA), is suggested as the first-line therapeutic approach [Citation2]. In contrast, transarterial chemoembolization (TACE) and systemic treatment such as immune checkpoint inhibitors are recommended for unresectable HCC in the intermediate and advanced stages [Citation3]. RFA, which is characterized by technical ease and minimal invasiveness, has been recommended as the first-line treatment for patients with early-stage HCC by international HCC practice guidelines, including the guidelines of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) [Citation4,Citation5]. However, the high incidence of tumor relapse (>60%) hinders improved survival after RFA [Citation6–8]. Therefore, accurate prediction of postoperative recurrence and survival is needed to guide the development of effective follow-up surveillance program and prevention strategies.
Serum tumor markers, such as prostate-specific antigen (PSA), carcinoembryonic antigen (CEA), and alfa-fetoprotein (AFP), are well-established prognostic biomarkers of increased tumor virulence in prostate cancer, colorectal cancer, and HCC, respectively. They have been shown to be predictive factors of worse tumor phenotype and worse prognosis [Citation9–13]. Moreover, tumor size and tumor number have been proposed as predictors of oncological outcome in various tumors [Citation14,Citation15]. Recently, tumor biomarker density has been described to improve the predictive ability by minimizing the confounders of tumor size and number of lesions [Citation16–18]. For example, PSA density has been shown to be superior to PSA and the Gleason score in predicting poorer prognosis in prostate cancer patients undergoing radical prostatectomy [Citation16]. As shown by Huo et al., CEA density is an independent predictor of overall survival (OS) during percutaneous ablation of pulmonary metastases from colorectal cancer [Citation18]. However, the role of AFP density as a predictor of tumor prognosis in HCC patients undergoing RFA has not been identified.
Therefore, we aimed to examine the prognostic value of pre-ablation AFP density and other clinicopathological factors in patients undergoing percutaneous ablation of HCC lesions.
Materials and methods
Study populations
Written informed consent for the treatment was obtained from each patient. Informed consent for retrospective studies was waived by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University. The study was conducted in accordance with the recommendations of the Human Subjects Biomedical Research Helsinki Declaration, and the protocol was approved by the institutional ethics committee (decision/protocol number 2020/186). From January 2010 to December 2018, a total of 697 patients with HCC who met the Milan criteria underwent percutaneous ablation as the initial treatment at our institution. The inclusion criteria for this study were as follows: (a) age between 18 and 80 years; (b) compliance with the Milan criteria: single nodule ≤5 cm, or up to three nodules (each ≤3 cm); (c) Eastern Cooperative Oncology Group performance status 0 or 1; (d) liver function classified as Child–Pugh class A or B; (e) no previous treatment of HCC; (f) lack of extrahepatic metastasis or macrovascular invasion; and (g) absence of previous or simultaneous malignancies. The exclusion criteria were as follows: (a) severe coagulopathy (prothrombin activity <40%, or platelet count <40,000/mm3); (b) evidence of hepatic decompensation; and (c) lack of laboratory test data. The flowchart of patient selection and grouping is shown in .
All patients underwent pretreatment evaluation, including medical history, laboratory, and imaging studies. The maximum diameter of the tumor was measured by cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI). HCC was diagnosed by histology or noninvasive diagnostic criteria in accordance with the EASL guidelines [Citation19]. Baseline staging was performed using Child–Pugh, ALBI grade, and BCLC classification systems [Citation20].
RFA protocol
RFA procedures were performed under intravenous moderate sedation and local anesthesia. Vital signs were continuously monitored throughout the procedure. In this study, we used Cool-tip™ RF ablation system (Covidien, Mansfield, Mass, USA), and a 17-gauge needle electrode with a 2- or 3-cm exposed tip. All RFA procedures were done percutaneously under ultrasound guidance. The ablation time and number of ablation points were determined by the number and diameter of the tumors with the purpose to achieve an ablative margin of at least 0.5 cm in the liver tissue surrounding the tumor, except for subcapsular and perivascular portions. Artificial ascites was used for ablating tumors on the liver surface in proximity of the diaphragm or gastrointestinal tract. All of the procedures in this study were performed by two experienced physicians, each with more than 10 years of experience in tumor ablation.
Data collection
All clinically relevant variables were determined prior to RFA and were collected in accordance with the results of previous studies. The variables included age, gender, etiology of HCC, maximum tumor diameter, tumor number, blood platelet (PLT) count, albumin (ALB) level, alanine aminotransferase (ALT) level, aspartic transaminase (AST) level, total bilirubin level (TBIL), γ-glutamyl transpeptidase (γ-GGT) level, and alpha-fetoprotein (AFP) level. The classification systems including albumin–bilirubin (ALBI) ratio, Child–Pugh class, and BCLC stage were also used.
AFP density (ng/mL*mm3)
The radius of each HCC lesion was measured from the axial abdominal CT/MRI. The volume of each HCC lesion was calculated using the following formula: [4 × π × radius3]/3. For multiple lesions, volumes of all of the HCC lesions were added to determine the total HCC lesion burden. AFP density was calculated as AFP value pre-ablation (ng/mL) divided by the total volume of all HCC lesions.
X-tile software was used to calculate the outcome-based optimal cutoff points for AFP density [Citation21]. Statistical significance was assessed using the cutoff score by a standard log-rank method, with P values obtained from a lookup table.
Follow-up and outcomes
Contrast-enhanced CT (CE-CT) or contrast-enhanced MRI (CE-MRI) was performed one month after RFA to assess the effectiveness of the ablation therapy. The treatment was considered successful if imaging showed no contrast enhancement or abnormal washout in or around the ablation area. The patients were followed-up every three months for the first two years and every 4–6 months for subsequent years, as suggested before [Citation22]. The last follow-up date for this study was May 15, 2021. Each visit included a clinical history interview, physical examination, routine blood tests, liver function tests, AFP level, and CE-CT or CE-MRI. In case of local tumor progression, intrahepatic distant recurrence, or extrahepatic recurrence during the follow-up period, corresponding treatments such as resection, RFA, TACE, sorafenib, and conservative treatments were given, based on recurrent tumor characteristics, liver function status, and patient requirement.
The primary clinical end point was OS, Defined as the time interval from the first RFA treatment to death from any cause or the end of the follow-up. The secondary end point was progression-free survival (PFS), defined as the time interval from the date of the initial RFA to the first recording of recurrence or death. Patients who were still alive at the last follow-up examination were regarded as censored data.
Statistical analysis
Categorical variables were presented as counts and percentages. Continuous variables were reported as mean ± standard deviation, or median with interquartile range. The Kolmogorov–Smirnov test was used to test the distribution pattern. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, and continuous variables were compared using Student’s t test or the Kruskal–Wallis rank-sum test as appropriate. For survival analysis, the optimal cutoff point for AFP density was obtained using X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA), as described previously [Citation21]. The Kaplan–Meier method was used to estimate the OS and PFS rates among groups, and the log-rank test was used to determine the statistical significance. Restricted cubic spline analysis was utilized to graphically display and evaluate nonlinear associations of log relative hazard scale in mortality with AFP density on a continuous scale [Citation23]. Univariate Cox proportional-hazards regression analysis was used to assess prognostic variables for OS and PFS. Variables with a P value <0.10 in the univariate analyses were then tested in the multivariate Cox proportional-hazards regression models. The hazard ratios (HRs) from regression were reported with 95% confidence intervals (CI). All statistical and graphical analyses were performed using R version 3.4.1 (http://www.rproject.org) and MedCalc version 14.10.2 (MedCalc Software, Ostend, Belgium) software. A P value <0.05 was considered statistically significant.
Results
Patients’ characteristics
The baseline characteristics of the study population are summarized in . A total of 543 patients (mean age, 56.5 ± 11.2 years; range, 20–80 years) with 676 HCC lesions (median diameter, 2.4 cm; interquartile range, 1.9–3.0 cm) who had undergone percutaneous RFA as their initial therapy were enrolled in this study. The majority of patients were men (468, 86.2%). Most of the patients (429, 79.0%) had one tumor; 95 patients (17.5%) had two tumors; and 19 patients (3.5%) had three tumors. Diagnosis was made by biopsy in 341 (62.8%) patients. The median value of AFP density was 2.827 (0.680–18.628) ng/mL*cm3. X-tile analysis showed that the optimal AFP density cutoff point was 7.22 ng/mL*cm3, which divided the entire cohort into low AFP density (≤7.22; n = 354, 65.2%) and high AFP density (>7.22; n = 189, 34.8%) groups.
Table 1. Characteristics of the study patients.
Patients with high AFP density tumors tended to have a higher AFP value and smaller tumor diameter and volume (all p < 0.001). There were no significant differences in age, gender, HBV-DNA, ALB, TBIL, ALT, AST, GGT, tumor number, Child–Pugh grade, and ALBI grade between the low and high AFP density groups ().
Recurrence and survival outcomes
In the entire cohort, the median follow-up time was 62 months (95% CI, 55–69 months). At the last follow-up, 200 patients (36.8%) had died, while 282 patients (51.9%) had tumor recurrence. At one month, seven RFA-treated patients were found to have signs of residual tumor. All of the seven lesions with residual tumor were treated again with RFA, and all achieved a complete response after this additional treatment. The other 536 patients all achieved complete ablation as assessed by imaging. The median OS was 83 months (95% CI, 76–91 months), and the 1-, 3-, and 5-year OS rates were 98.7%, 83.8%, and 63.2%, respectively. The median PFS was 38 months (95% CI, 30–43 months), and the 1-, 3-, and 5-year PFS rates were 73.6%, 51.2%, and 36.3%, respectively.
The median follow-up time of the low and high AFP density groups was 62 months (95% CI, 55–71 months) and 61 months (95% CI, 52–76 months), respectively. A total of 110 patients in the low AFP density group died during the follow-up; causes of death were cancer recurrence (n = 83), liver failure (n = 20), cerebral hemorrhage (n = 2), and miscellaneous (n = 5). Ninety patients in the high AFP density group died during the follow-up; their causes of death were cancer recurrence (n = 79), liver failure (n = 9), and miscellaneous (n = 2).
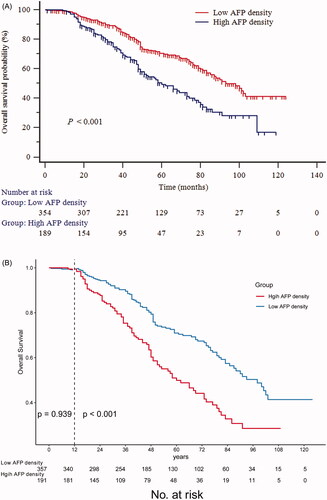
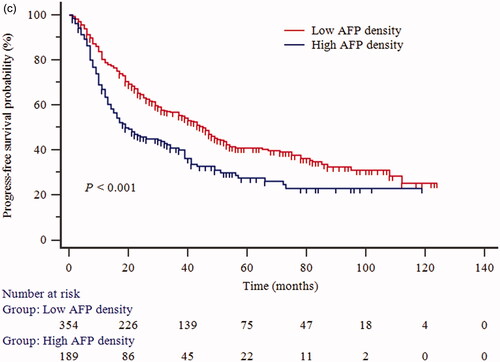
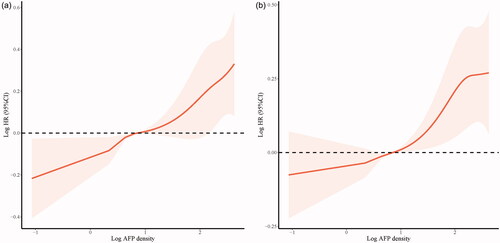
The cumulative OS rates at 1-, 3-, and 5-years in the low AFP density group were 98.8%, 88.5%, and 70.4%, respectively; and 98.3%, 74.9%, and 49.4% in the high AFP density group, respectively (p < 0.001, ). A landmark analysis was performed with a pre-specified landmark set at 1 year. The curves showing the OS in the first year were similar between the low and high AFP density groups (p = 0.939). After the first year, the cumulative OS rate was significantly higher in the low AFP density group than in the high AFP density group (p < 0.001, ). Correspondingly, the 1-, 3-, and 5-year PFS rates in the low AFP density group were 78.9%, 56.7%, and 40.9%, respectively; and 63.6%, 40.8%, and 27.5% in the high AFP density group, respectively (p < 0.001, ). Restricted cubic spline analyses suggested significant positive associations of AFP density with the elevated OS () and PFS ().
Figure 2. Kaplan–Meier survival curves for (a) overall survival (OS); (b) the landmark analysis with a prespecified landmark set at 1 year to provide separate descriptions of OS in the early and late phases; (c) progression-free survival (PFS) of patients who underwent percutaneous radiofrequency ablation of hepatocellular carcinoma.
Univariate and multivariate analyses
Univariate Cox regression analysis suggested that age, PLT, albumin, TBIL, AST, γ-GGT, AFP, AFP density, tumor number, maximum tumor diameter, total tumor volume, Child–Pugh grade, ALBI grade, and BCLC stage were significantly associated with OS. Age, PLT, albumin, TBIL, γ-GGT, AFP, AFP density, tumor number, maximum tumor diameter, total tumor volume, Child–Pugh grade, ALBI grade, and BCLC stage were associated with PFS ().
Table 2. Univariate analysis using Cox regression for overall survival and progress-free survival.
Further multivariate analysis determined that AFP density was independently predictive of OS (HR, 1.742; 95% CI, 1.309–2.319; p < 0.001). Age, γ-GGT, and ALBI grade were the other three related variables. The corresponding HRs were 1.583 (95% CI, 1.170–2.142) for age, 1.001 (95% CI, 1.000–1.003) for γ-GGT, and 1.929 (95% CI, 1.384–2.688) for ALBI grade 2 compared with grade 1, and 6.184 (95% CI, 2.128–17.972) for ALBI grade 3 compared with grade 1. The multivariate analysis revealed that two variables, including age (HR, 1.429; 95% CI, 1.118–1.826; p = 0.004) and AFP density (HR, 1.494; 95% CI, 1.186–1.884; p < 0.001), were independent significant risk factors for PFS ().
Table 3. Multivariate analysis using Cox regression for overall survival and progress-free survival.
Discussion
Serum AFP is the most widely used serological marker to establish the diagnosis of HCC, monitor treatment response, and detect disease relapse after curative treatment [Citation12,Citation24–27]. In this study, we tested a new predictive model of AFP density, which combines the AFP level with common factors of tumor size and number. Our findings revealed that AFP density was a valuable prognostic factor and predictive biomarker for OS and PFS. AFP density may be a surrogate serologic end point for individualized identification of patients who would gain clinical benefits from RFA.
AFP has been reported to reflect biological malignancy in early HCC, which is associated with tumor differentiation, vascular invasion, progression, and survival [Citation26,Citation28]. As a large serum glycoprotein, AFP belongs to the class of onco-development proteins [Citation29], and has been considered to continuously reflect the HCC lesion activity and viable burden [Citation25,Citation30–32]. Lesions with high AFP values are characterized by poor differentiation, abundant progenitor features, and enhanced proliferation—features consistent with the prognostic power of AFP; moreover, an increased proportion of tumors that have serum AFP concentrations >400 ng/mL were observed with disease progression [Citation33]. A multicenter study reported that elevated AFP levels were significantly associated with microvascular and macrovascular invasion [Citation34]. Some studies have shown that a high AFP value in tumor tissue is an independent predictor of poor prognosis [Citation35,Citation36]. In the present study, the AFP level was shown to be an independent predictor of recurrence after RFA for HCC and also predicted survival after ablation. However, AFP concentration was not related to tumor burden [Citation37,Citation38]. Our study with a large sample size showed that diameter of the largest tumor was not associated with the AFP level, especially in patients with AFP level ≤1000ng/mL [Citation34]. In this study, we designed a new predictive model of AFP density, which combined the absolute AFP value, tumor size, and number of tumors, for predicting survival. AFP density showed a more significant trend of relationship with PFS (HR, 1.569; 95% CI, 1.252–1.967) and OS (HR, 1.946; 95% CI, 1.472–2.574), compared with AFP levels (PFS: HR, 1.000; 95% CI, 1.000–1.001; OS: HR, 1.000; 95% CI, 1.000–1.001). The results of this study changed the generally accepted view that patients with larger HCC tumor burdens have lower OS than patients with smaller lesions. Among patients with the same AFP levels, those with smaller HCC lesions would actually have worse OS (because of higher AFP density), and those with smaller tumor burden (i.e. high AFP density) would have worse OS compared with patients with larger tumor burden (low AFP density). The clinical implications are enormous, necessitating a change in the way oncologists manage patients with high AFP density. Patients with high AFP density might be prone to recurrence or progression and need close postoperative surveillance [Citation39]. Although it may not be appropriate to exclude patients with poor prognosis from RFA, first adjuvant therapy should be given priority for these patients [Citation40].
In our study, γ-GGT was identified as a prognostic marker of survival after RFA, which is consistent with previous reports [Citation41]. γ-GGT is a transferase that is mainly distributed in the cytoplasm of hepatocytes and in the intrahepatic bile duct epithelium. Accumulating evidence has demonstrated that γ-GGT is not only a basic parameter indicative of liver function, but also an important tumor biomarker associated with tumor formation, tumor cell proliferation, and apoptosis [Citation42]. Our study also showed that high ALBI grade was a predictor of tumor recurrence after RFA. In HCC, the ALBI grade is superior to the Child–Pugh class in assessing liver function reserve [Citation43]. In addition, recent studies have demonstrated that ALBI grade is associated with higher levels of AFP and more advanced tumor stage, which correlates with malignant biological behavior and poor prognosis [Citation44]. We speculated that due to the direct development of HCC without liver cirrhosis, patients in the high AFP density group may exhibit more aggressive tumor behavior, as indicated by AFP density or the ALBI grade. However, this speculation requires further validation, and the underlying mechanism should be explored in future biological studies. Besides these biomarkers, the neutrophil-to-lymphocyte ratio, albumin-to-alkaline phosphatase ratio, and γ-GGT-to-lymphocyte ratio have also been reported to be promising indicators in patients with HCC undergoing percutaneous RFA [Citation45–47].
Our study had several limitations. First, as a retrospective and single-center research study, potential selection bias was inevitable. Second, this study was conducted on an Asian cohort, and 86.4% of the patients had HBV infection. Therefore, when validated externally, these findings may not be directly applicable to patients from the United States and Europe, as HCV infection and alcoholism are major causes of HCC in those regions. Furthermore, the current cutoff value for AFP density, derived using the X-tile software, warrants further validation. Fourth, the tumor volume calculated in this study was based on the assumption that the tumor shape is spherical, since only transverse sections of the patients’ abdominal CT or MRI scans were possible to attain. Future studies examining AFP density by measuring three dimensions on medical imaging are warranted. As only 62.8% patients had histological examination, the relationship between AFP density and histological features was not further evaluated.
In conclusion, we validated the AFP density as an independent prognostic factor for determining survival in patients with HCC meeting the Milan criteria and undergoing RFA. The present results also suggest that high AFP density could be used in identifying patients who might be prone to recurrence or progression and need close surveillance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491.
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693.
- Jacome AA, Castro ACG, Vasconcelos JPS, et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: a Meta-analysis. JAMA Netw Open. 2021;4(12):e2136128.
- European association for the study of the liver. Electronic address eee, European association for the study of the L. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750.
- Abu-Hilal M, Primrose JN, Casaril A, et al. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12(9):1521–1526.
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58(4):724–729.
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912.
- Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28(17):2810–2816.
- Loeb S, Bruinsma SM, Nicholson J, et al. Active surveillance for prostate cancer: a systematic review of clinicopathologic variables and biomarkers for risk stratification. Eur Urol. 2015;67(4):619–626.
- Konishi T, Shimada Y, Hsu M, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and Colon cancer outcome. JAMA Oncol. 2018;4(3):309–315.
- Yamamoto K, Imamura H, Matsuyama Y, et al. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45(12):1272–1282.
- Snyder RA, Hu CY, Cuddy A, Alliance for Clinical Trials in Oncology Network Cancer Surveillance Optimization Working Group, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–2115.
- Kim CG, Lee HW, Choi HJ, et al. Development and validation of a prognostic model for patients with hepatocellular carcinoma undergoing radiofrequency ablation. Cancer Med. 2019;8(11):5023–5032.
- Kiriyama S, Uchiyama K, Ueno M, et al. Triple positive tumor markers for hepatocellular carcinoma are useful predictors of poor survival. Ann Surg. 2011;254(6):984–991.
- Sfoungaristos S, Perimenis P. PSA density is superior than PSA and gleason score for adverse pathologic features prediction in patients with clinically localized prostate cancer. Can Urol Assoc J. 2012;6(1):46–50.
- Deniffel D, Healy GM, Dong X, et al. Avoiding unnecessary biopsy: MRI-based risk models versus a PI-RADS and PSA density strategy for clinically significant prostate cancer. Radiology. 2021;300(2):369–379.
- Huo YR, Glenn D, Liauw W, et al. Evaluation of carcinoembryonic antigen (CEA) density as a prognostic factor for percutaneous ablation of pulmonary colorectal metastases. Eur Radiol. 2017;27(1):128–137.
- European association for the study of the L, European Organization for R, treatment of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
- Llovet JM, Di Bisceglie AM, Bruix J, Panel of Experts in HCC-Design Clinical Trials, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259.
- Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection–propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919.
- Harrell F. Regression modelling strategies: with applications to linear models, logistic regression, and survival analysis. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2010.
- Bruix J, Sherman M, American Association for the Study of Liver Diseases American association for the study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022.
- Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27(3):446–452.
- Shim JH, Han S, Lee YJ, et al. Half-life of serum alpha-fetoprotein: an early prognostic index of recurrence and survival after hepatic resection for hepatocellular carcinoma. Ann Surg. 2013;257(4):708–717.
- Memon K, Kulik L, Lewandowski RJ, et al. Alpha-fetoprotein response correlates with EASL response and survival in solitary hepatocellular carcinoma treated with transarterial therapies: a subgroup analysis. J Hepatol. 2012;56(5):1112–1120.
- Fujioka M, Nakashima Y, Nakashima O, et al. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34(6):1128–1134.
- Gillespie JR, Uversky VN. Structure and function of alpha-fetoprotein: a biophysical overview. Biochim Biophys Acta. 2000;1480(1-2):41–56.
- Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229.
- Yamamoto K, Imamura H, Matsuyama Y, et al. Significance of alpha-fetoprotein and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol. 2009;16(10):2795–2804.
- Lee YK, Kim SU, Kim DY, et al. Prognostic value of alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. BMC Cancer. 2013;13:5.
- Montal R, Andreu-Oller C, Bassaganyas L, et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br J Cancer. 2019;121(4):340–343.
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of milan criteria. Gastroenterology. 2012;143(4):986–994.
- Bai DS, Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7(1):12870.
- Toyoda H, Kumada T, Kaneoka Y, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol. 2008;49(2):223–232.
- Singal AG, Hoshida Y, Pinato DJ, et al. International liver cancer association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160(7):2572–2584.
- Cabibbo G, Maida M, Genco C, et al. Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. WJH. 2012;4(9):256–261.
- Hatzaras I, Bischof DA, Fahy B, et al. Treatment options and surveillance strategies after therapy for hepatocellular carcinoma. Ann Surg Oncol. 2014;21(3):758–766.
- Bhangui P, Allard MA, Vibert E, et al. Salvage versus primary liver transplantation for early hepatocellular carcinoma: Do both strategies yield similar outcomes? Ann Surg. 2016;264(1):155–163.
- Ma H, Zhang L, Tang B, et al. gamma-Glutamyltranspeptidase is a prognostic marker of survival and recurrence in radiofrequency-ablation treatment of hepatocellular carcinoma. Ann Surg Oncol. 2014;21(9):3084–3089.
- Wu SJ, Lin YX, Ye H, et al. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg. 2016;36(Pt A):143–151.
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558.
- Pinato DJ, Kaneko T, Saeed A, et al. Immunotherapy in hepatocellular cancer patients with mild to severe liver dysfunction: Adjunctive role of the ALBI grade. Cancers (Basel. 2020;12(7):1862.
- Chen TM, Lin CC, Huang PT, et al. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol. 2012;27(3):553–561.
- Zhang F, Lu SX, Hu KS, et al. Albumin-to-alkaline phosphatase ratio as a predictor of tumor recurrence and prognosis in patients with early-stage hepatocellular carcinoma undergoing radiofrequency ablation as initial therapy. Int J Hyperthermia. 2021;38(1):1–10.
- Li S, Xu W, Liao M, et al. The significance of Gamma-Glutamyl transpeptidase to lymphocyte count ratio in the early postoperative recurrence monitoring and prognosis prediction of AFP-Negative hepatocellular carcinoma. JHC. 2021;ume 8:23–33.