Abstract
Objective
To evaluate the effectiveness of high-intensity focused ultrasound (HIFU) combined with hysteroscopy-guided suction curettage (HGSC) in treating cervical pregnancy.
Materials and methods
This is a retrospective study. Seven patients with cervical pregnancy who visited the Third Xiangya Hospital of Central South University from January 2015 to December 2020 were enrolled in the current study. All seven patients were treated with HIFU under conscious sedation. All of them underwent HGSC at an average of 2 ± 1 days (range: 1–3 days) after HIFU. Before the therapy, the patient's clinical characteristics were collected, including duration of amenorrhea, gravidity and parity, the patient history of cesarean section and miscarriage, and the size of the gestational sac. The levels of β-hCG and hemoglobin in serum were also reviewed. To assess the clinical outcomes of this combined treatment, the suction time of HGSC, bleeding volume, the clearance time of β-hCG, and the time with returning of menstruation were evaluated.
Results
All seven patients (average age: 31 ± 6 years) have experienced amenorrhea (duration range, 48 ± 8 days) before the treatment of HIFU. The average number of pregnancies was four, and the number of deliveries was one. Previous medical history showed six patients had cesarean sections, and five patients have been miscarriages. After HIFU treatment, the fetal heartbeats were stopped in all seven patients based on the diagnosis by doppler ultrasound. The bleeding of gestational tissue decreased significantly. All patients had only mild lower abdominal pain, no fever, intestinal damage, or other complications were reported. The average operation time of operative suction curettage was 21 ± 9 min (range: 9–32 min), and the median bleeding volume was 10 ± 8 mL (range: 2–20 mL). Follow-up observations showed that the menstruations were returned in patients at an average of 38 ± 9 days (range: 30–50 days) after the treatment. The β-hCG decreased from 41773 ± 32242 mIU/mL to 13101 ± 8454 mIU/mL in 29 ± 10 days after surgery.
Conclusion
Based on these results with small subjects, we concluded that HIFU combined with HGSC might be an effective and safe treatment for patients with cervical pregnancy.
Introduction
Cervical pregnancy is an ectopic pregnancy that a gestational sac and fertilized egg implant in the endocervical canal [Citation1,Citation2]. It is a scarce condition with an incidence of 1/8600–1/12400 ectopic pregnancies [Citation3,Citation4]. The causes of cervical pregnancy remain elusive. It has been associated with both a history of the previous curettage and cesarean delivery and fibroids [Citation5]. The use of assisted reproductive technologies has also been considered a risk factor for cervical pregnancies [Citation6]. As most of the cervical tissue is made up of fibrillar connective tissue, when a patient with a cervical pregnancy has an incomplete abortion or undergoes a curettage, a hysterectomy may be required to save the patient’s life because of the heavy bleeding that may occur. It also has an adverse effect on future pregnancies. Therefore, an early diagnosis and proper treatment are important for patients with cervical pregnancy. To reduce bleeding and preserve fertility, pretreatments that can reduce embryonic cardiac activity and vaginal bleeding before suction curettage are conducive. The commonly used preoperative treatment include methotrexate (MTX) and B-scan ultrasonography (USG)-guided injection of MTX and potassium chloride, uterine artery embolization (UAE), and high-intensity focused ultrasound (HIFU).
HIFU is a newly developed medical procedure that uses focused ultrasound energy to ablate lesion areas. By increasing the temperature to 65–100 °C at the lesion site, HIFU precisely causes coagulative necrosis and destroys the target area in a noninvasive and bloodless manner. HIFU is being used widely for the treatment of female reproductive system diseases. HIFU has been proven a feasible and safe preoperative management of cesarean scar pregnancy (CSP) [Citation7]. A retrospective study of 53 patients with CSP showed that HIFU combined with suction curettage was a practical option [Citation8]. Here, we evaluate the feasibility and safety of suction curettage under hysteroscopic guidance combined with HIFU treatment in seven patients with cervical pregnancy in our clinic.
Materials and methods
Ethical considerations
All patients in this study signed an informed consent form. This study was approved by the ethics committee of the Third Xiangya Hospital of Central South University (Protocol approval number: 2022-127).
Study participants and enrollment
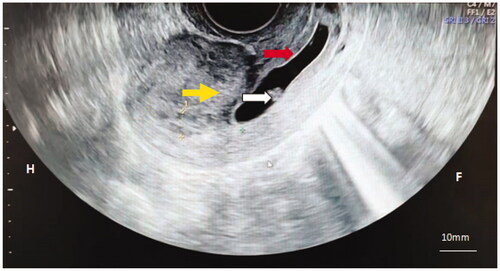
Seven patients with cervical pregnancy treated with HIFU combined with hysteroscopy-guided suction curettage (HGSC) from January 2015 to December 2020 at the Third Xiangya Hospital of Central South University were retrospectively studied. Patient inclusion criteria were as follows (1): diagnosis of cervical pregnancy by B-scan ultrasonography () and hysteroscopy based on the criteria proposed by Paalman and McEli [Citation2] (2) postoperative pathological examination showed villi, which was consistent with the diagnosis of cervical pregnancy (3); successful localization of gestational sac by ultrasound scan (4); stable vital signs after surgery, no vaginal hemorrhages. Exclusion criteria were as follows (1): incomplete medical records (2); heterotypic pregnancy (3); malformation of the female genital tract (4); systematic or pelvic inflammatory disease (5); other severe diseases.
Ultrasound-Guided HIFU ablation
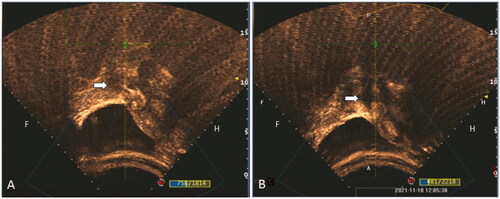
A JC-200 high-intensity focused therapeutic ultrasound system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) was used in HIFU ablation. Pre-operational preparation and HIFU ablation were performed as described below. In brief, after localization of the gestational sac by ultrasound scan, patients maintained a clear liquid diet for three days before the surgery. Before HIFU ablation, all patients took polyethylene glycol electrolyte solution (Wanhe Pharmaceutical Co., Ltd., Shenzhen, China) to clean their bowels. In the evening before the surgery and the next morning of the surgery, two times of enemas were performed. After being inserted with a urinary catheter, patients were placed in a prone position. The HIFU ablation was performed under general analgesia and sedation by giving patients of fentanyl (1 μg/kg, Renfu Co., Ltd., Jiangxi, China) and midazolam (0.02–0.03 mg/kg, Enhua Co., Ltd., Jiangsu, China) via intravenous injection. Sulfur hexafluoride micro-bubble (Bracco, Suisse, Italy) was used as an ultrasound contrast agent to localize the gestational sac and the surrounding blood supply. The treatment was stopped when the blood flow signal disappeared, and the grayscale changed. No apparent contrast agent perfusion was observed in the gestational sac tissue after HIFU treatment (). After ablation, 500 ml of ice-cold saline was used to clean the patient’s bladder. After these procedures, all patients rested in a prone position in bed for 2 h. Antibiotics were given to patients for three days after the HIFU ablation to prevent surgical infections.
Figure 2. Color flow Doppler imaging demonstrated successful removal of the gestational tissue. (A) Contrast-enhanced Ultrasound was performed before HIFU, which shows of obvious perfusion of the gestational sac existed (white arrow). (B) Contrast-enhanced Ultrasound was performed again after HIFU treatment, and it shows no obvious perfusion agent was detected in the gestational sac tissue (white arrow).
Hysteroscopy-guided suction curettage
2 ± 1 days (range: 1–3 days) after HIFU treatment, the patients underwent the suction curettage under the HEOS hysteroscopic guidance (Sopro-comeg Company, Bordeaux, France). After 8 h of fasting, the patient was placed in the lithotomy position and was given intravenous general anesthesia. Then routine disinfection and draping were performed. After inserting the speculum into the vagina, the patient’s cervix was held in place with a clamp. A 0.9% saline (speed: 400 ml/min) was sent through the catheter into the uterus to cause dilation. To determine the depth and angle of the uterus and localize the gestational sac, a rigid hysteroscope with a diameter of 4.5 mm (Olympus) was inserted into the uterus. The cervix was widened by gradually increasing the size of the dilator to 7.5 mm. The 7 mm cavity suction tube was used to scrape and suction the placental contents in the cervix. Hysteroscope was used to check if any contents remained in the uterus. If any pregnancy residues were detected, a clamp was used under the guidance of a hysteroscope with an outer sheath (diameter: 6.5 mm) to remove the remaining tissue. If necessary, an electrosurgical resection was used to remove the residues after re-dilation of the uterus.
Follow-up observations
After the HIFU treatment, patients’ vaginal blood loss, abdominal pain, fever, and other complications were recorded. After suction curettage, patients’ vaginal blood loss, serum human chorionic gonadotrophin (HCG) levels on the first day after surgery, time from surgery to returning of menstruation, menstrual blood volume (MBV), and pregnancy were also recorded.
Statistical analysis
Statistical calculations were performed using SPSS 23.0 software (IBM, Armonk, NY, USA). The data were reported as means ± standard deviation (SD).
Results
Patients’ clinical characteristics
The patient clinical characteristics were shown in . Of the seven patients studied in this research, six had uterine scarring, one was primigravida, and five had a cesarean section. Five patients presented with vaginal bleeding for a duration of 7 h to 20 days, and one had lower abdominal pain. Other characteristics are described in .
Table 1. Baseline clinical characteristics of seven patients with cervical pregnancy treated with HIFU combined with HGSC.
Clinical outcomes
HIFU treatment was performed successfully in all patients. Patients only experienced mild lower abdominal pain and coccydynia, which were relieved after bed rest. No complications, such as fever, skin burn, injuries of the intestinal tract, or urinary retention were observed. Ultrasound energy, power, and treatment time were recorded in . All seven patients were treated with suction curettage 1–3 days after the HIFU. No significant cervical pregnancy residues were observed. Bleeding volume and treatment time and the time between HIFU and suction curettage were listed in .
Table 2. Ultrasound parameters of HIFU treatment on seven patients.
Table 3. Clinical parameters of suction curettage for seven patients.
Table 4. Follow-up observations after HIFU and HGSC treatments.
Follow-up observations
As shown by the follow-up data in , β-HCG decreased significantly from 41773 ± 32242 mIU/mL to 13101 ± 8454 mIU/mL in 29 ± 10 days after the combined HIFU and suction curettage treatment. All seven patients experienced vaginal bleeding after suction curettage, but that type of bleeding was stopped within 12 ± 5 days. All seven patients restored normal menstruation 38 ± 9 days after the surgeries. Two patients showed reduced MBV, one of which was diagnosed with intrauterine adhesions and underwent a separation procedure due to the combined fertility requirements, and there was no pregnancy after hysteroscopic surgeries; None of the other six patients had the desire to have children, three patients used contraceptive tools, two patients had not gotten pregnant without protection, and one patient had chosen for an abortion in 2017.
Discussion
Cervical pregnancy (CP) is a rare type of ectopic pregnancy. No standard regimen for CP has been established. Once diagnosed as CP, the patient typically aborts the pregnancy within the trimester. Clinically, doctors will create an individualized treatment for each patient based on gestational age, the thickness of myometrium, serum β-HCG levels, and the patient’s desire for pregnancy. For a patient who is hemodynamically stable and promptly diagnosed with CP, the therapeutic aim is to abort the fetus, remove residual cervical pregnancy, reduce blood flow around the gestational sac to lower the risk of bleeding during suction curettage, and preserve the patient’s fertility.
As a minimally invasive procedure, HIFU has been widely applied in obstetrics and gynecology [Citation9]. It has been proven to be effective preoperative management in the treatment of placenta accreta and cesarean scar pregnancy. HIFU uses focused ultrasound waves to generate heat in the fetus, leading to the coagulation and thermal ablation of the fetus tissue. The high temperature generated by HIFU also causes the occlusion of the small blood vessels around the gestational sac, especially the vessels smaller than 2 mm in diameter in the gestational sac and anterior uterine wall. Because of HIFU’s capacity of controlling hemorrhages, it has been regarded as a safe and effective preoperative treatment before the removal of pregnancy tissue, which can avoid the removal of the uterus and preserve the patient’s fertility [Citation10,Citation11]. In this study, we treated seven patients diagnosed with early cervical pregnancy using HIFU and HGSC. Before the treatment, the color doppler ultrasound showed that the average diameter of the seven patients’ gestational sacs was 2 ± 1 cm. Primitive heartbeat was detected in five patients. After this combined treatment, fetal heartbeat disappeared, and bleeding from pregnancy tissue also decreased significantly. The average time of backing to normal β-HCG level after surgery was 29 ± 10 days (range: 15–40 days). Patients returning to normal menstruation were recorded as 38 ± 9 days (IQR 30–45) after surgeries.
Compared with other conventional preoperative treatments such as local/systematic administration of MTX and uterine artery embolization, HIFU excels at precise lesion localization, minimal damage to the surrounding area, and shorter length of hospital stays [Citation12]. Furthermore, HIFU has not been reported about any risk of embolism syndrome so far. The conventional MTX administration has a failure rate of 14.3% or higher with a pretreatment β-hCG level greater than 5000 mIU/mL [Citation13]. This treatment also requires close monitoring of serum β-hCG to ensure a sufficient response. Some patients who underwent MTX treatment required emergency uterine artery embolization, even hysterectomy due to uncontrolled bleeding. A case report showed a 42-year-old woman with cervical pregnancy who suddenly suffered massive vaginal bleeding on day 6, after receiving a combination of intrafetal potassium chloride and systemic MTX injection. The bleeding could not be controlled by Bakri SOS balloon tamponade, but this patient eventually survived after a hysterectomy [Citation14]. In another study, a patient with CP underwent secondary UAE after systemic MTX and UAE due to the reoccurrence of active vaginal bleeding [Citation15]. In addition to its application in controlling acute uterus bleeding, UAE is also used to prevent massive vaginal bleeding before an obstetrical procedure [Citation16]. However, UAE can also cause severe complications. For instance, the embolization material can fall into vital organs, potentially causing urinary tract infection, uterine necrosis, and pulmonary embolism [Citation17–19]. It may also affect the patient’s reproductive function. Although some researchers believe UAE does affect ovary function [Citation20,Citation21], Sheikh proposed that gelatin/embolization particles could reach the ovarian capillaries through the anastomoses between the uterine artery and the ovarian artery, leading to decreased ovarian artery supply [Citation22]. The UAE may affect endometrial blood flow, resulting in necrosis of basal layer cells of the endometrium. It has also been reported to cause intrauterine adhesions, reduced menstruation volume, and even amenorrhea [Citation23]. In a follow-up study, Li found that, of 139 patients with CSP, 83 (59.7%) experienced reduced menstruation volume, and two (1.4%) had amenorrhea. They also found that the patients with decreased MBV were less likely to become pregnant [Citation24]. The patients who had intrauterine adhesions after UAE treatment were more likely to have miscarriages even after a successful separation procedure. Several clinical cases of uterine necrosis after UAE have also been documented [Citation25]. In this study, the patients after HIFU and HGSC only experienced mild lower abdominal pain and coccydynia, which were relieved after bed rest. No severe complications such as fever, skin burn, injuries of the intestinal tract, or urinary retention were observed. In addition, all seven patients had normal menstruation occurring 38 ± 9 days after the surgery, and two patients experienced reduced MBV, one of which was later diagnosed with intrauterine adhesions. Among the seven patients, two had not gotten pregnant without protection. But these two patients did not seek further treatment due to a lack of desire to become pregnant.
In conclusion, HIFU is a safe and effective preoperative procedure for CP patients who are promptly diagnosed and do not experience massive vaginal bleeding. However, the small number of patients is one of the limitations of this study. In addition, the availability of a control group for comparative analysis and the use of a prospective study method would provide better advice on the effectiveness, safety, and standardization of the treatment of cervical pregnancy.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Parente JT, Ou CS, Levy J, et al. Cervical pregnancy analysis: a review and report of five cases. Obstet Gynecol. 1983;62(1):79–82.
- Paalman RJ, Mc ET. Cervical pregnancy; review of the literature and presentation of cases. Am J Obstet Gynecol. 1959;77(6):1261–1270.
- Ushakov FB, Elchalal U, Aceman PJ, et al. Cervical pregnancy: past and future. Obstet Gynecol Surv. 1997;52(1):45–59.
- Vela G, Tulandi T. Cervical pregnancy: the importance of early diagnosis and treatment. J Minim Invasive Gynecol. 2007;14(4):481–484.
- Ahlers J, Goswami K, Quenby S, et al. Evidencen based management if cervival ectopic pregnancy. BJOG Int J Obste Gynaecol. 2013;120:553–554.
- Guan Y, Ma C. Clinical outcomes of patients with heterotopic pregnancy after surgical treatment. J Minim Invasive Gynecol. 2017;24(7):1111–1115.
- Liu CN, Tang L, Sun Y, et al. Clinical outcome of high-intensity focused ultrasound as the preoperative management of cesarean scar pregnancy. Taiwan J Obstet Gynecol. 2020;59(3):387–391.
- Zhu X, Deng X, Wan Y, et al. High-intensity focused ultrasound combined with suction curettage for the treatment of cesarean scar pregnancy. Medicine. 2015;94(18):e854.
- He S, Xue M, Jiang J. Early versus late hysteroscopic resection after high-intensity focused ultrasound for retained placenta accreta. Int J Hyperthermia. 2021;38(1):257–262.
- Huang L, Du Y, Zhao C. High-intensity focused ultrasound combined with dilatation and curettage for cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2014;43(1):98–101.
- Wu F, Chen WZ, Bai J, et al. Tumor vessel destruction resulting from high-intensity focused ultrasound in patients with solid malignancies. Ultrasound Med Biol. 2002;28(4):535–542.
- Zhang PR, Gao HX. Clinical efficacy of high-intensity focused ultrasound combined with methotrexate in treatment of cesarean scar pregnancy and the effect on serum β-HCG. Maternal and Child Health Care of China. 2017;32:4294–4296.
- Dilday E, Douglas C, Brennan K. Single-dose intramuscular methotrexate for treatment of cervical ectopic pregnancy: a case report. Case Rep Womens Health. 2021;31:e00340.
- Saeng-anan U, Sreshthaputra O, Sukpan K, et al. Cervical pregnancy with massive bleeding after treatment with methotrexate. BMJ Case Rep. 2013. DOI:10.1136/bcr-2013-200440
- Elmokadem AH, Abdel-Wahab RM, El-Zayadi AA, et al. Uterine artery embolization and methotrexate infusion as sole management for cesarean scar and cervical ectopic pregnancies: a single-center experience and literature review. Can Assoc Radiol J. 2019;70(3):307–316.
- Ko HK, Shin JH, Ko GY, et al. Efficacy of prophylactic uterine artery embolization before obstetrical procedures with high risk for massive bleeding. Korean J Radiol. 2017;18(2):355–360.
- Qiu J, Fu Y, Huang X, et al. Acute pulmonary embolism in a patient with cesarean scar pregnancy after receiving uterine artery embolization: a case report. Ther Clin Risk Manag. 2018;14:117–120.
- Toor SS, Jaberi A, Macdonald DB, et al. Complication rates and effectiveness of uterine artery embolization in the treatment of symptomatic leiomyomas: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199(5):1153–1163.
- Hamoda H, Tait P, Edmonds DK. Fatal pulmonary embolus after uterine artery fibroid embolisation. Cardiovasc Intervent Radiol. 2009;32(5):1080–1082.
- T, El Shamy S, AK, Amer AA, Mohamed, et al. The impact of uterine artery embolization on ovarian reserve: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99(1):16–23.
- McLucas B, Voorhees WD, Snyder SA. Anti-Mullerian hormone levels before and after uterine artery embolization. Minim Invasive Ther Allied Technol. 2018;27(3):186–190.
- Sheikh GT, Najafi A, Cunier M, et al. Angiographic detection of utero-ovarian anastomosis and influence on ovarian function after uterine artery embolization. Cardiovasc Intervent Radiol. 2020;43(2):231–237.
- Karlsen K, Hrobjartsson A, Korsholm M, et al. Fertility after uterine artery embolization of fibroids: a systematic review. Arch Gynecol Obstet. 2018;297(1):13–25.
- Li X, Niu H, Li J, et al. Clinical assessment of uterine artery embolization combined with curettage when treating patients with cesarean scar pregnancy: a retrospective study of 169 cases. J Obstet Gynaecol Res. 2020;46(7):1110–1116.
- Mutiso SK, Oindi FM, Hacking N, et al. Uterine necrosis after uterine artery embolization for symptomatic fibroids. Case Rep Obstet Gynecol. 2018;2018:9621741.