Abstract
Background
Abdominal wall endometriosis(AWE)is an unusual extra-pelvic endometriosis. Currently, multiple treatment modalities are available, but no clear guidelines exist for the management of large AWE.
Materials and methods
We present a 36-year-old female patient with a large AWE lesion who underwent cesarean section due to abnormal fetal position 8 years ago. The mass lesion of AWE located in rectus muscle fascia and rectus muscle with a size of 61 × 25 × 49mm.
Results
HIFU treatment was completed in one session. One day post-HIFU MRI showed the mass was completely ablated. After HIFU treatment, the cyclical abdominal pain disappeared. The mass lesion shrank during follow-up period and disappeared in 1 year after HIFU. No complication was observed after HIFU.
Conclusion
Surgical resection of AWE remains the standard of care. In patients with large AWE lesion located in rectus muscle fascia and rectus muscle where the muscle and fascia must be excised, HIFU treatment should be considered to avoid mesh implantation.
Introduction
Abdominal wall endometriosis (AWE) is an unusual extra-pelvic endometriosis with a reported low incidence of 0.3–3.5%, but extremely impacts reproductive-age women’s qualities of life [Citation1]. The occurrence of AWE is most related to cesarean section delivery or abdominal surgery. The main clinical features of AWE include periodic pain of the mass, which progressively intensifies with menstruation. Surgical resection is the first line treatment for AWE [Citation2]. To reduce the risk of local recurrence, it is often required to remove the tissue at least 1 cm further beyond the margin of the lesion. Therefore, for large masses, especially those located in the muscle or fascia, it often needs mesh placement to maintain structural integrity and strengthen the abdominal wall to avoid the occurrence of postoperative hernia. However, it may result in long-term pain and increase the economic burden of patients. In addition, for obese patients, surgery may cause fat liquefaction, exudation and cavitation, and some of them may need second surgery. Here we report a case with a large AWE who underwent HIFU and was free of AWE related symptoms for 2 years during follow-up period after HIFU.
Case report
A 36-year-old reproductive woman was admitted to our hospital with periodic pain in cesarean section scar for 4 years. The pain was sharp, cramping and non-radiating and her discomfort would worsen during menstruation. Past medical history was significant for a cesarean section due to abnormal fetal position 8 years ago. She had another abdominal surgery for scarred uterus 3 years later, and an AWE mass lesion with 20 mm in diameter was removed. She had a regular menstrual cycle without dysmenorrhea. She didn’t take any medication. Physical exam showed a large non-tender, palpable mass in the lower quadrant of the abdomen underneath the cesarean scar.
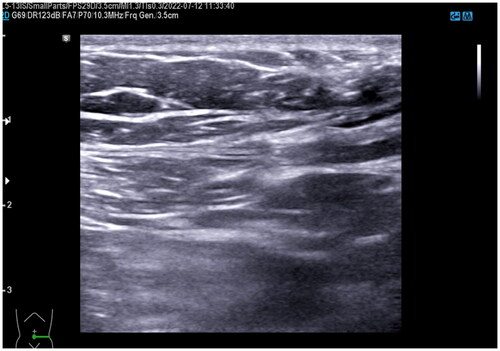
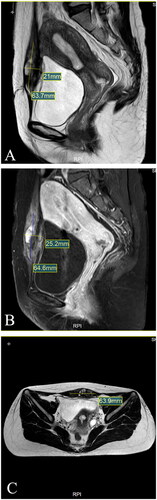
Ultrasound examination showed a 61 × 48 × 19mm hypoechoic mass lesion in the left lower rectus muscle. The echoic of the mass lesion was uneven, with an irregular fluid sonolucent area (). In order to further confirm the depth and size of the lesion, the patient took an MRI examination. MRI showed that the lesion involved rectus abdominis with size of 64.6 × 25.2 × 63.9 mm (). Based on her medical history of multiple cesarean sections and surgical removal of AWE, the cyclic nature of the pain, and the features of ultrasound and MRI images, we deemed that this patient had recurrent AWE.
Figure 1. B-Ultrasound for patient before HIFU (Aug. 17th, 2020). The B-Ultrasound for patient showed the mass is 61.20 × 18.70 × 48.10 mm.
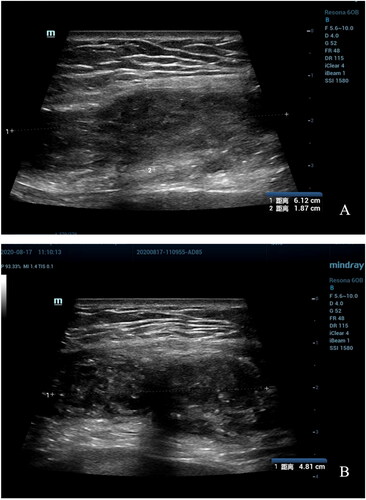
Figure 2. MRI for patient before HIFU (Aug. 22nd, 2020). (A) Sagittal T2-weighted image showed the mass’s size is 63.7 × 21.0 mm; (B) Post-contrast sagittal T1-weighted image showed the mass’s size is 64.6 × 25.2 mm; (C) Axial T2-weighted image showed the mass’s size is 63.9 mm.
Since the mass was large and have involved rectus abdominis and fascia and protruded to bladder, the surgery would cause further trauma and was not the treatment of choice. After a multidisciplinary discussion and a full discussion with the patient, we decided to perform HIFU treatment for this large AWE.
The patient received specific bowel and skin preparation prior to HIFU treatment. Bowel preparation included ingesting liquid food for 2 days, fasting for 12 h pre-HIFU treatment. Skin preparation included shaving, degreasing and degassing the skin of the anterior abdominal wall from the umbilicus to the upper margin of the pubic symphysis. In order to optimize the therapeutic acoustic pathway, a urinary catheter was inserted to control the size of the bladder.
HIFU treatment was performed under conscious sedation using JC200D tumor therapeutic system (Chongqing Haifu Medical Tech. Co. Ltd., Chongqing, China). A transducer, with a 20-cm diameter and a focal length of 15 cm, produced the therapeutic energy required for treatment. An ultrasound imaging probe is situated in the center of the transducer and allows real-time sonographic monitoring during treatment. The transducer is in a water reservoir filled with degassed water.
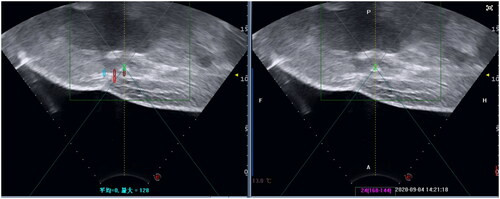
The patient was carefully positioned on the HIFU table with the anterior abdominal wall in contact with degassed water. The ablation boundary depends on intraoperative real-time ultrasonography and preoperative MRI. The treated range was 1 cm larger than the mass lesion (). The volume of ablation is 70 × 60 × 20mm. The ablation began from the largest layer of the lesion. Dotted energy is delivered from the foot side to the head side. The rate of treatment frequency is 1:3 (delivery energy is 1 s, and rest is 3 s). Each treatment time is 50 s, and we cool the skin down and check the skin every 200 s. The average power for point sonication was 152 watts (range: 100–180 W). Significant grey scale change was observed in 36 s of sonication. Ablating to the boundary, we decreased the power delivered to protect normal tissue. The treatment was terminated when the grey scale changed area covered the whole treated area. The total sonication time was 1458s. The mass in the abdomen turned hard to soft after HIFU.
During the procedure of HIFU treatment, she reported mild pain in the treated region, but the pain score was less than 3 points. After HIFU treatment, she also reported mild pain in the treated region. Local edema in treated skin area was observed, ice pack with a towel was applied to the treated area for 30 min. The patient returned to daily routines in two hours after HIFU treatment and the swelling gradually disappeared within 3 days. No other adverse effects related to HIFU treatment, such as blisters or skin burn, were observed.
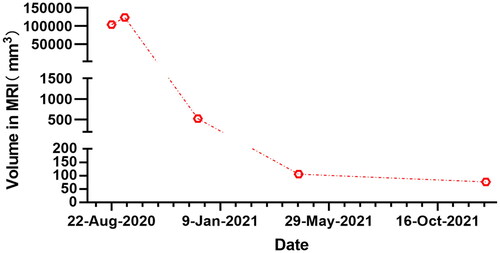
One day after HIFU, MRI showed the AWE mass lesion was completely ablated (). The patient reported the periodic pain of abdominal mass disappeared in the first menstruation after HIFU. Three months after HIFU, the follow-up MRI showed that the size of the mass significantly reduced, and it was 10.9 × 5.8 × 8.3 mm (). Seven months after HIFU, MRI showed the size of mass was 4.4 × 5.0 × 4.8 mm (). We could not touch the mass in abdomen wall anymore. During follow-up, this patient didn’t take any other treatments and medications. Luckily, the lesion was absorbed completely. The abdominal structural integrity didn’t damage. There were not any other abnormalities in 1 year after HIFU (). Recently, she returned to our hospital for another ultrasound examination, and no mass was observed ( and ).
Figure 4. MRI for patient in 1st day after HIFU (Sept. 04th, 2020). (A) Sagittal T2-weighted image showed the mass’s size is 79.0 × 22.4 mm; (B) Post-contrast sagittal T1-weighted imae showed the mass’s size is 70.4 × 20.1 mm; (C) Axial T2-weighted image showed the mass’s size is 70mm.
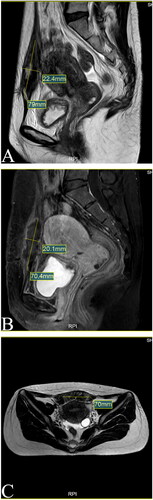
Figure 5. MRI for patient in 3rd month after HIFU (Dec. 11st, 2020). (A) Sagittal T2-weighted image showed the mass’s size is 10.6 × 5.8 mm; (B) Post-contrast sagittal T1-weighted image showed the mass’s size is 10.9 × 5.5 mmI; (C) Axial T2-weighted image showed the mass’s size is 8.3 mm.
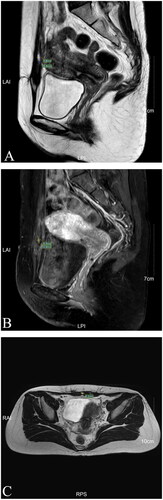
Figure 6. MRI for patient in 7th month after HIFU (Apr. 20th, 2021). (A) Sagittal T2-weighted image showed the mass’s size is 4.4 mm; (B) Post-contrast sagittal T1-weighted image showed the mass’s size is 5mm; (C) Axial T2-weighted image showed the mass’s size is 4.8 mm.
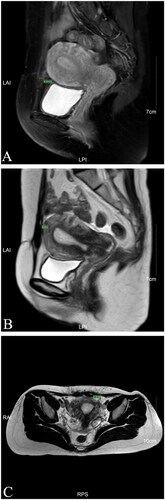
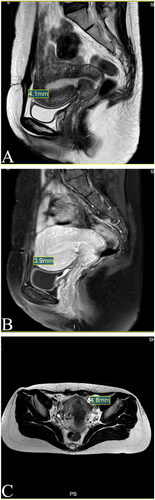
Figure 7. MRI for patient in 1st year after HIFU (Dec. 17th, 2021). (A) Sagittal T1-weighted image showed the mass’s size is 4.1 mm; (B) Post-contrast sagittal T1-weighted image showed the mass’s size is 3.9 mm; (C) Axial T2-weighted image showed the mass’s size is 4.8 mm.
Discussion
AWE was the most common abdominal wall mass found in women between ages 18 and 55 [Citation3]. AWE should be suspected in female patients with a history of cesarean sections, cyclic pain and an abdominal mass. Based on its position within the abdominal wall layers, AWE can be in the subcutaneous tissue only, infiltrating the abdominal rectus muscle fascia, or in the abdominal rectus muscle, below the fascia [Citation4]. Here we present a case with AWE involved rectus abdominis and fascia and protruded to bladder.
Surgical excision remains the criterion standard for the treatment of AWE. If the lesion extends to deeper tissues and the size of the lesion is large, such as muscle, peritoneum, or aponeurosis, mesh or aponeurotic muscle flag to cover the defect is needed [Citation4]. Implanting mesh may result in long-term pain and increase the economic burden on patients. Complications of mesh implantation (including mesh erosion, infection, and poor healing) can increase the risk of secondary surgery. In addition, for obese patients, surgical excision may cause fat liquefaction, exudation, and cavitation, and some may need a second surgery. A previous study comparing HIFU and surgery shows that surgery’s major adverse events (including incision healing delayed and lung infection) are higher than in HIFU [Citation5].
Medical treatment of AWE has been proven to be ineffective. Standard of care of AWE remains surgical excision with free margins. Because the involvement of the anterior rectus fascia and the size of the lesion was large, mesh reconstruction was needed if we perform surgery. After a full discussion with the patient, she decided to choose HIFU treatment.
As a noninvasive treatment, HIFU has been widely used to treatment different types of solid tumors [Citation6–8]. The procedure of HIFU treatment is performed under ultrasound guidance, so it can be used to ablate the tumor lesion precisely and selectively. HIFU causes coagulation necrosis of the target mass lesion without damaging to the surrounding tissues that do not want to be treated.
Recently, HIFU treatment has been used to treat AWE. Several studies have shown that HIFU is a remarkable choice for the treatment of AWE [Citation8–10]. Luo et al. have treated patients with AWE, the periodic pain disappeared in all patients, and the size of mass gradually decreased and disappeared in 16 patients after HIFU treatment. The effectiveness of HIFU or HIFU combined medication is comparable to surgery [Citation9]. Zhu et al. compared 28 patients treated with surgery (average diameter of lesion was about 26.8 mm) and 23 patients treated with HIFU (average diameter of lesion was about 27.0 mm) in another study, the results also showed that the effect of HIFU was comparable to surgery [Citation10].
Although the previous studies have demonstrated that HIFU is effective in treating AWE, it has never been reported that HIFU treats such a large volume of AWE. The lesion in the patient we treated was 2–3 times larger than previously reported in other studies, but it was absorbed entirely in 1 year. This patient refused to accept surgery due to recurrence of AWE. Meantime, surgical trauma is relatively large for a large and deeply invading lesion. During following-up, patient reported periodic pain disappeared and the mass in abdomen gradually shrank. Compared with surgery, HIFU has less trauma and fewer complications, but with similar results. Beneficially, by contrast with surgery, HIFU do not cause adhesion, therefore, repeated HIFU treatment for AWE recurrence is feasible.
The application of HIFU in the treatment of such a large lesion has rarely been reported. During the procedure, ultrasonic input was decreased when the grey scale changes was observed to protect skin and subcutaneous tissue. The grey scale changes indicate necrosis. Tissue with necrosis will enhance the ability of absorbing energy that drives surrounding tissue to absorb energy. Therefore, we can use low energy to make lesion coagulation necrosis. The previous study has demonstrated that Absorption of necrotic tissue is mainly completed by macrophage phagocytosis. As blood supply in subcutaneous tissue is richer than that in superficial tissue. Therefore, the large abdominal endometriosis in deep location can also gain the same effect of HIFU treatment for uterine fibroids and adenomyosis.
Conclusion
Surgical resection remains the standard of care in the management of AWE. However, HIFU treatment should be considered in patients with AWE who are more likely need mesh implantation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Carsote M, Terzea DC, Valea A, et al. Abdominal wall endometriosis (a narrative review). Int J Med Sci. 2020;17(4):536–542.
- Welch BT, Ehman EC, VanBuren WM, et al. Percutaneous cryoablation of abdominal wall endometriosis: the Mayo clinic approach. Abdom Radiol. 2020;45(6):1813–1817.
- Yarmish G, Sala E, Goldman DA, et al. Abdominal wall endometriosis: differentiation from other masses using CT features. Abdom Radiol. 2017;42(5):1517–1523.
- Grigore M, Socolov D, Pavaleanu I, et al. Abdominal wall endometriosis: an update in clinical, imagistic features, and management options. Med Ultrason. 2017;19(4):430–437.
- Zhao L, Deng Y, Wei Q, et al. Comparison of ultrasound-guided high-intensity focused ultrasound ablation and surgery for abdominal wall endometriosis. Int J Hyperthermia. 2018;35(1):528–533.
- Sehmbi AS, Froghi S, Oliveira de Andrade M, et al. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB. 2021;23(2):187–196.
- Feril LB, Fernan RL, Tachibana K. High-Intensity focused ultrasound in the treatment of breast cancer. Curr Med Chem. 2021;28(25):5179–5188.
- Diana M, Schiraldi L, Liu Y-Y, et al. High intensity focused ultrasound (HIFU) applied to hepato-bilio-pancreatic and the digestive system-current state of the art and future perspectives. Hepatobiliary Surg Nutr. 2016;5(4):329–344.
- Luo S, Zhang C, Huang JP, et al. Ultrasound-guided high-intensity focused ultrasound treatment for abdominal wall endometriosis: a retrospective study. BJOG. 2017;124(Suppl 3):59–63.
- Zhu X, Chen L, Deng X, et al. A comparison between high-intensity focused ultrasound and surgical treatment for the management of abdominal wall endometriosis. BJOG. 2017;124(Suppl 3):53–58.