Abstract
The mitochondria are central to skeletal muscle metabolic health. Impaired mitochondrial function is associated with various muscle pathologies, including insulin resistance and muscle atrophy. As a result, continuous efforts are made to find ways to improve mitochondrial health in the context of disuse and disease. While exercise is known to cause robust improvements in mitochondrial health, not all individuals are able to exercise. This creates a need for alternate interventions which elicit some of the same benefits as exercise. Passive heating (i.e., application of heat in the absence of muscle contractions) is one potential intervention which has been shown to increase mitochondrial enzyme content and activity, and to improve mitochondrial respiration. Associated with increases in mitochondrial content and/or function, passive heating can also improve insulin sensitivity in the context of type II diabetes and preserve muscle mass in the face of limb disuse. This area of research remains in its infancy, with many questions yet to be answered about how to maximize the benefits of passive heating and elucidate the mechanisms by which heat stress affects muscle mitochondria.
1. Introduction
Skeletal muscle is important for mobility, blood glucose management, thermogenesis, and various other processes involved in health and longevity [Citation1]. The mitochondria, which are the primary site of ATP production in muscle, are of central importance in the regulation of skeletal muscle mass and overall health [Citation2]. Impairments in mitochondrial respiration or reductions in mitochondrial enzyme content are associated with various metabolic conditions like insulin resistance and muscle atrophy [Citation3,Citation4]. Fortunately, targeting the mitochondria in clinical interventions may prevent, or at least slow the progression of various muscle-related pathologies [Citation5,Citation6].
Exercise training provides a powerful stimulus for increases in mitochondrial content and improved quality of existing mitochondria [Citation7,Citation8]. Consequently, exercise may be prescribed in clinical situations to improve mitochondrial and whole-muscle health, and to combat disease or harmful side effects of medical interventions like chemotherapy or prescribed bed rest [Citation9–11]. Exercise acutely induces various types of stress on skeletal muscle including mechanical, energetic, oxidative, hypoxic, and heat stress, which activate signaling pathways leading to improved muscle health [Citation12,Citation13]. Unfortunately, many clinical situations prevent individuals from exercising and alternate strategies are needed to preserve mitochondrial and whole muscle health [Citation14–16].
One potential approach to obtain some of the same adaptations as exercise is to periodically apply low levels of heat stress to skeletal muscle, like that which would occur during exercise. During intense exercise, internal muscle temperature becomes elevated, in some cases exceeding 40 °C [Citation17,Citation18]. This heat stress is thought to be a potential player in exercise-induced adaptations [Citation19]. When applied passively (i.e., without muscle contraction), heat has been shown to stimulate increases in mitochondrial enzyme content and respiratory capacity in C2C12 muscle cells [Citation20,Citation21], as well as in animals and humans [Citation22–25]. Therefore, passive heating is a potential therapeutic intervention that could improve skeletal muscle metabolism and protect muscle from pathologies associated with mitochondrial dysfunction. The objectives of this review are: 1) to provide a brief overview of the role of the mitochondria in the context of health and disease, 2) to provide an update on the evidence for heat-induced mitochondrial biogenesis in muscle as well as the mechanisms involved, and 3) to review the effects of different heating modalities used in animals and humans as well as potential clinical applications based on currently published literature.
2. Mitochondrial function in health and disease
2.1. Muscle atrophy and insulin resistance are associated with mitochondrial dysfunction
Functional impairments in the mitochondria are associated with many different skeletal muscle-related pathologies. For example, muscle atrophy caused by cancer cachexia [Citation3,Citation26,Citation27], aging [Citation28–31], chemotherapy treatment [Citation32–34], and prescribed bed rest [Citation35–39] is associated with, and in some cases is preceded by, mitochondrial impairments [Citation3,Citation26,Citation34]. Aside from muscle atrophy, insulin resistance and diabetes have also been linked to mitochondrial dysfunction [Citation40,Citation41]. Interestingly, for many years it has been suggested that mitochondrial dysfunction causes insulin resistance [Citation4,Citation42,Citation43]. However, this notion has been widely debated and likely involves several complex mechanisms [Citation40,Citation41,Citation44,Citation45].
Regardless of the mechanism responsible for the development of insulin resistance or muscle atrophy, interventions which target the mitochondria have been shown to limit negative effects of these pathologies as well as other muscle-related disorders [Citation5,Citation46–48]. For example, reducing reactive oxygen species (ROS) generation from mitochondria has shown some therapeutic promise. While ROS act as important signaling molecules in the cell, chronically high levels of ROS are thought to play a role in the development of various mitochondria-related muscle pathologies. SS-31 is a peptide that reduces mitochondrial ROS and limits muscle atrophy during disuse, chemotherapy treatment, and cancer cachexia [Citation34,Citation49,Citation50]. Reducing mitochondrial ROS with SS-31 can also delay the onset of insulin resistance in rodents fed a high-fat diet [Citation48]. While it can be debated whether mitochondria play a causal role in the development of these pathologies, approaches that improve mitochondrial health may be therapeutic.
2.2. Mitochondrial structure and function
The mitochondria are double-membrane organelles which are the primary site of ATP synthesis in the cell. Skeletal muscle contains two subtypes of mitochondria: intermyofibrillar and subsarcolemmal, which are different from each other in function and morphology [Citation51]. Though not well defined, both subtypes of mitochondria likely have distinct roles within the cell due to their locations and structure [Citation12,Citation51,Citation52]. Mitochondrial ‘function’ is a term often used synonymously with mitochondrial respiratory capacity, or ATP production. While the mitochondria perform more functions than just ATP production, including calcium sequestration [Citation53], cell signaling [Citation54], and the regulation of apoptosis [Citation55], for the purpose of this review we will define mitochondrial function as mitochondrial respiratory capacity or mitochondrial enzyme activity.
Oxidative phosphorylation is the process by which the majority of ATP is produced in muscle cells. This process involves a series of redox reactions which result in electrons being transferred through protein complexes (referred to as complexes I-IV), ultimately reacting with molecular oxygen. These redox reactions are coupled with the transfer of protons (H+ ions) out of the matrix, resulting in an increase in membrane potential. Protons then flow down a gradient and drive the production of ATP, catalyzed by ATP synthase. In response to changes in energy demand, like muscle disuse or endurance exercise training, skeletal muscle is able to increase or decrease its capacity to perform oxidative phosphorylation via changes in the density of mitochondrial enzymes in existing mitochondria and/or alteration of mitochondrial volume [Citation3,Citation7].
2.3. Measuring mitochondrial content and function
Mitochondrial content is often expressed as the percent volume of a cell that is occupied by mitochondria, or as the total mitochondrial mass present in a muscle sample. Cross-sectional images of muscle fibers obtained via transmission electron microscopy are often considered the ‘gold-standard’ measure for mitochondrial content, determined by the percent of the surface area in the image composed of mitochondria [Citation56]. However, there are clear limitations to this technique because mitochondria are three-dimensional and non-uniform in structure [Citation57]. Recent improvements in imaging techniques, like three-dimensional focused ion beam scanning electron microscopy, resolve this limitation through improved characterization of mitochondrial volume and morphology. However, this equipment is not widely accessible and is very costly [Citation58,Citation59]. Therefore, surrogate measures of mitochondrial content are often used, including mitochondrial DNA, specific membrane lipids (cardiolipin), enzyme activities (citrate synthase, succinate dehydrogenase, and cytochrome c oxidase activity), or mitochondrial respiratory protein content. Though no single surrogate measure is likely to predict mitochondrial content under all situations, cardiolipin content and citrate synthase activity have been reported to be among the most accurate predictors in young, healthy individuals [Citation56]. However, there are limitations in using single biomarkers to estimate mitochondrial content, and it is preferable to measure several biomarkers when mitochondrial content is not directly measured via microscopic methods [Citation57].
Though mitochondrial ATP production can be measured directly, oxygen consumption is a common, though indirect, measure of ATP production because the two processes are tightly coupled. Oxygen consumption is commonly measured using a Clark-electrode, with systems such as the Oroboros Oxygraph-2k (Oroboros; Innsbruck, Austria) increasing in popularity worldwide. In this system, the mitochondria are assessed in cultured cells, muscle fiber bundles or isolated mitochondria [Citation60]. The Clark electrode is an excellent tool because it provides the ability to make precise measurements with small tissue samples, while also allowing for a diverse array of substrate titration protocols and a thorough interrogation of oxidative phosphorylation [Citation61]. Another method used to measure oxygen consumption is the Seahorse XF extracellular flux analyzer, which uses optical sensors to measure oxygen content in up to 96 cell culture or tissue samples simultaneously. Other functional measures of mitochondria include enzyme activities of individual protein complexes associated with the electron transport system, or of beta-oxidation and TCA cycle enzymes like 3-hydroxyacyl-CoA dehydrogenase and citrate synthase [Citation7,Citation62,Citation63].
Furthermore, in-vivo methods exist to evaluate mitochondrial function in human subjects, including 31P-magnetic resonance spectroscopy and optical spectroscopy [Citation64]. However, to date there have been no studies conducted to determine in-vivo mitochondrial adaptations which result in response to passive heating. For a more comprehensive discussion current methods for mitochondrial function analyses, the reader is directed to previously published reviews [Citation61,Citation65,Citation66].
3. Mechanistic basis for heat-induced improvements in mitochondrial function
3.1. Passive heat improves mitochondrial function in cell culture, animals, and humans
Exercise generates heat in skeletal muscle, which can reach temperatures above 40 °C in humans [Citation17,Citation18]. As exercise is a powerful driver of mitochondrial biogenesis, it remains unknown how much this increase in muscle temperature contributes to biogenesis, if at all. Of course, it is difficult to remove heat from exercising muscle to properly assess the role of heat stress in exercise-induced adaptations. Therefore, investigations have primarily focused on either applying additional heat to exercising muscle or applying passive heat to resting muscle to investigate the potential of heat stress alone to improve mitochondrial function. The first study to investigate the effects of passive heating on skeletal muscle mitochondrial content was conducted by Liu and Brooks, who heated C2C12 myotubes at 40 °C for 1 h per day for 5 consecutive days. Following the 5 days of heating, mitochondrial complex proteins were all increased compared to cells cultured at a constant 37 °C. Citrate synthase activity was also increased compared to control [Citation20]. Similar work by Patton et al. confirmed this finding in C2C12 myotubes, showing that 6 days of consecutive heating (2 h/day @ 40 °C) also results in increased mitochondrial protein expression and mitochondrial respiratory capacity [Citation21]. Using an in-vivo model, Tamura et al. applied whole-body heat to mice (40 °C) for 30 min per day for 3 weeks. Whole-body heating increased mitochondrial citrate synthase activity compared to control animals (), as well as mitochondrial respiratory protein content. Furthermore, heat applied immediately following exercise training caused greater mitochondrial adaptations than exercise training or heat alone [Citation22].
Figure 1. Maximal citrate synthase activity in plantaris muscle of mice following 3 weeks of exercise training (ET), heat stress (HS), or both (ET + HS) compared to control (CON). Adapted from figure by Tamura et al. [Citation22]. Copyright © 2014 The American Physiological Society. Used with permission.
![Figure 1. Maximal citrate synthase activity in plantaris muscle of mice following 3 weeks of exercise training (ET), heat stress (HS), or both (ET + HS) compared to control (CON). Adapted from figure by Tamura et al. [Citation22]. Copyright © 2014 The American Physiological Society. Used with permission.](/cms/asset/6116ad9c-3c9e-45ee-8329-d7452a234646/ihyt_a_2205066_f0001_b.jpg)
Until 2018, no studies had directly investigated the role of passive heating on muscle mitochondrial function in humans. The first study of this kind was conducted by Hafen et al. which implemented shortwave diathermy to raise the internal muscle temperature of the vastus lateralis to approximately 39.5 °C. This mode of heat was applied for 2 h per day for 6 consecutive days on a single leg. Twenty-four hours after the final heating session, protein content of mitochondrial complexes I and V were increased compared to baseline, and mitochondrial respiration was increased by nearly 30% compared to the untreated control leg [Citation23]. Subsequent studies in human subjects using diathermy and other heating modalities have supported the finding that passive heat can induce mitochondrial adaptations in skeletal muscle [Citation25,Citation67,Citation68]. Importantly, the effects of applying passive heat are not limited to improved mitochondrial function or increased mitochondrial content. For example, in a study by Hafen et al. subjects had a single leg immobilized for 10 consecutive days, resulting in about a 7.6% reduction in the cross-sectional area of the vastus lateralis. However, when heat was applied daily via shortwave diathermy (2 h/day), the reduction in muscle size was limited to 4.5%. This heat-induced preservation of muscle size was associated with preserved mitochondrial respiration and respiratory protein content () [Citation67]. Furthermore, building evidence suggests that passive heat may improve insulin sensitivity and glucose control in individuals with diabetes [Citation69].
Figure 2. Passive heating mitigates muscle atrophy and reduced mitochondrial respiration in human subjects following 10 days of single-leg immobilization. A) Percent change in cross-sectional area of the vastus lateralis measured via magnetic resonance imaging with immobilization alone or immobilization + heat. B) Maximal coupled respiration measured in permeabilized muscle fibers before (white bars) and after (gray bars) 10 days of immobilization or immobilization + heat. Figure adapted from work by Hafen et al. [Citation67]. Copyright © 2019 The American Physiological Society. Used with permission.
![Figure 2. Passive heating mitigates muscle atrophy and reduced mitochondrial respiration in human subjects following 10 days of single-leg immobilization. A) Percent change in cross-sectional area of the vastus lateralis measured via magnetic resonance imaging with immobilization alone or immobilization + heat. B) Maximal coupled respiration measured in permeabilized muscle fibers before (white bars) and after (gray bars) 10 days of immobilization or immobilization + heat. Figure adapted from work by Hafen et al. [Citation67]. Copyright © 2019 The American Physiological Society. Used with permission.](/cms/asset/30152dfc-4e2b-4747-b83b-94c0becd7c7b/ihyt_a_2205066_f0002_b.jpg)
3.2. Heat shock proteins
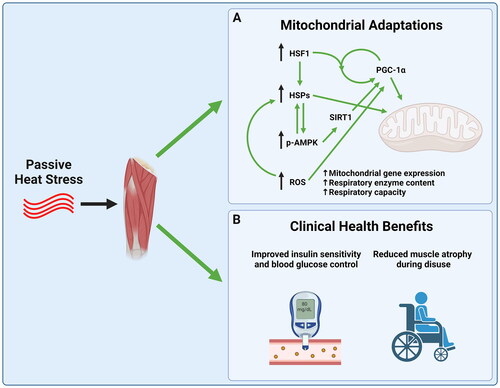
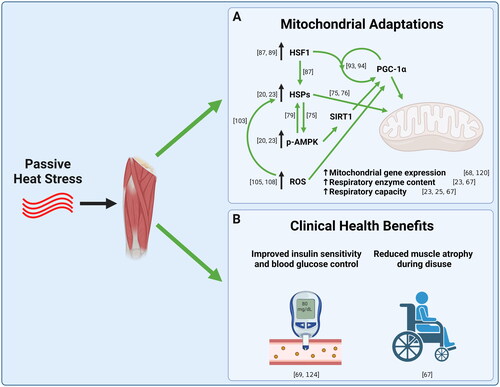
The molecular mechanisms by which heat stress causes improvements in mitochondrial content and function is an active area of research. Heat shock proteins (HSPs) are a family of molecular chaperones (chaperone proteins assist in protein folding and are critically important for normal protein function) which are highly conserved across tissues and organisms [Citation70,Citation71]. Heat shock proteins appear to be central to mitochondrial adaptations that occur following heat stress, with HSP72 being especially important. HSP72 protein expression has been shown to be consistently upregulated in skeletal muscle following heat exposure in cell culture [Citation20], animals [Citation22,Citation24,Citation72,Citation73], and humans [Citation23,Citation67,Citation68], and is associated with increases in mitochondrial content and function [Citation20,Citation22,Citation23,Citation74]. Furthermore, the transgenic overexpression of HSP72 in mouse skeletal muscle results in mitochondrial biogenesis [Citation75,Citation76].
The precise mechanism by which an increase in heat shock proteins results in mitochondrial adaptations is not well understood in human skeletal muscle. However, both heat stress and transgenic overexpression of HSP72 result in an increase in activation of energy stress signaling. Specifically, HSP72 overexpression increases the activity of 5′ AMP-activated protein kinase (AMPK) and the protein expression of sirtuin 1 (SIRT1) [Citation20,Citation23,Citation75]. AMPK and SIRT1 work together to activate a key regulator of mitochondrial biogenesis, peroxisome-proliferator receptor-gamma coactivator 1 (PGC-1α) [Citation77,Citation78]. Interestingly, while overexpression of HSP72 activates AMPK, heat stress activates AMPK prior to an increase in HSP expression, suggesting there exists bidirectional signaling between AMPK and HSP72 [Citation79].
Aside from the apparent role of HSP72 in activating PGC-1α through AMPK and SIRT1, heat shock proteins play a vital role in the import of nuclear-encoded mitochondrial proteins into the mitochondrial matrix, as well as in helping fold and assemble them into complexes [Citation52]. The mitochondrial proteome is composed of over 1,000 proteins, 99% of which are nuclear encoded, with only 13 being coded for by mitochondrial DNA [Citation80]. Because most mitochondrial proteins are translated outside the mitochondria, specialized import machinery is required to introduce newly synthesized proteins into the mitochondrial matrix. Two primary players in this process are the translocase of the outer membrane (TOM), and the translocase of the inner membrane (TIM) [Citation81]. Interestingly, both cytosolic and mitochondrial heat shock proteins are vital for this translocation process, partially due to their interactions with TIM and TOM [Citation81–83]. Furthermore, once introduced into the mitochondria, heat shock protein 60 is necessary for the proper folding and assembly of the respiratory complexes of the electron transport system [Citation84]. To date, it is unknown if passive heating in humans or animals improves protein import and folding due to increased HSP content or activation in skeletal muscle. However, the vital role of HSPs in mitochondrial protein import and assembly suggests that this is an important area for future research.
Mitochondria are constantly undergoing a dynamic process of renewal/remodeling. This includes membrane fusion and membrane splitting or fission as well as mitophagy (degradation of damaged or unhealthy mitochondria). HSP72 is an apparent regulator of mitochondrial remodeling through mitophagy, fission, and fusion. Mitofusin and parkin are two essential proteins involved in mitochondrial fusion and mitophagy and can only perform their roles if HSP72 is present [Citation85]. This may be part of the reason why heat stress improves mitochondrial health. Furthermore, heat stress increases markers of mitophagy following denervation in mouse skeletal muscle, resulting in improved muscle function [Citation86]. Overall, the effects of heat stress on mitophagy and fission/fusion have not been well studied in skeletal muscle but may be an important area for future research.
3.3. Heat shock factor 1
The expression of HSPs are regulated by a transcription factor called Heat Shock Factor 1 (HSF1), which is vital for the heat shock response in skeletal muscle [Citation87], and is highly conserved across tissues and species [Citation88]. HSF1 regulates HSP gene transcription by forming a homotrimer which binds the promoter region of DNA. Purified HSF1 has been shown to trimerize in response to heat, indicating that it is a literal thermo-sensor [Citation89,Citation90]. However, it should be noted that heat is not the only method for activating HSF1, as other types of stress, including oxidative stress, can lead to HSF1 trimerization and binding to DNA [Citation90]. HSF1 increases the transcription of HSPs like HSP72 and HSP90, as well as many other genes in the cell [Citation91,Citation92]. Interestingly, PGC-1α has been shown to interact with HSF1 protein as HSF1 binds to the promoter region of genes encoding HSPs and genes associated with oxidative phosphorylation [Citation93,Citation94]. Therefore, aside from regulating HSP content in muscle, HSF1 may directly regulate mitochondrial biogenesis, though there is relatively little research in this area, particularly in humans.
3.4. Reactive oxygen species
Reactive oxygen species (ROS) have long been known to be generated by contracting skeletal muscle [Citation95,Citation96]. While initially thought to be merely a harmful by-product of exercise, it was suggested in 1982 that ROS production in muscle may provide a stimulus for mitochondrial biogenesis [Citation97]. ROS are now considered to be key regulators of muscle force production and mediators of exercise-induced adaptations, including mitochondrial biogenesis [Citation98,Citation99]. For example, ROS production induced by electrical stimulation of cultured primary rat cells upregulates PGC-1α [Citation100]. In human subjects, antioxidant supplementation inhibits some of the beneficial adaptations of exercise training, including increased signaling for mitochondrial biogenesis and improvements in insulin sensitivity [Citation101]. Furthermore, exercise is known to cause an increase in heat shock protein expression, and antioxidant supplementation may limit this response [Citation102,Citation103]. These findings suggest that the oxidative stress generated by contracting skeletal muscle may be necessary for the mitochondrial and heat shock adaptations which follow.
Elevated muscle temperature during exercise is an important stimulus for ROS production [Citation104]. Furthermore, passive heat is known to increase the production of ROS in skeletal muscle fibers [Citation105,Citation106] and to increase oxidative stress in cardiac tissue, which leads to mitochondrial adaptations and cardio-protection [Citation107]. Therefore, it is likely that ROS are part of the mechanism by which heat induces mitochondrial adaptations in skeletal muscle. To date, the mechanism by which ROS are generated during passive heating is not entirely clear. However, it is possible that ROS production increases during heating due to both mitochondrial and nonmitochondrial sources [Citation108,Citation109]. Given that ROS have been shown to be important for exercise-induced mitochondrial adaptations, an area for future research may be to determine to what extent heat-induced improvements in mitochondrial content are mediated by increased ROS production.
4. Models of passive heating and applications
4.1. Animal models of passive heating
While some inconsistencies exist in the literature regarding the adaptive effects of passive heat stress on muscle mitochondria, this may be partially attributed to the variety of heating methods, and lengths/temperatures of the interventions used (). For example, several studies have implemented heating chambers to for passive whole-body heating of mice and rats. Tamura et al. used a heat chamber set to 40 °C, and heated animals for 7 days [Citation86] or 3 weeks [Citation22], both of which were sufficient to induce mitochondrial adaptations. Lower temperatures in heating chambers have also been used, such as 37 ± 3 °C for 1 h/day, 5 days/week for 6 weeks. This lower temperature resulted in much more mild benefits, suggesting there may be a temperature threshold, or a dose of heat required to induce mitochondrial adaptations [Citation74]. However, there is also likely a temperature limit that, if exceeded, will cause mitochondrial impairments [Citation110,Citation111]. A limitation of current research in this field is that most animal studies do not report internal muscle temperatures associated with different heating modalities, though core temperatures are often reported.
Table 1. Animal studies investigating effects of passive heating or HSP72 overexpression on mitochondria.
Other methods of whole-body heating in animals include the use of heated pads/blankets or warm water immersion. While these methods allow for well controlled heating sessions and accurate measurement of core body temperature, the use of anesthesia during every heating session introduces an extra layer of difficulty in carrying out these studies. However, both methods have been shown to raise animal core temperature to ≥ 40 °C [Citation24,Citation73,Citation76,Citation112,Citation113] and to improve metabolic parameters which may be associated with improved mitochondrial respiration or content [Citation24,Citation73,Citation76,Citation114,Citation115]. Further research using these passive heating methods may help to further elucidate the role of heat stress on mitochondrial adaptations.
Animal models are also useful because they can be genetically manipulated to study heat shock. For example, transgenic HSP72 overexpression or HSP72 knockout models have been used to determine whether HSP72 directly affects mitochondrial content and function, and by what mechanisms this may occur [Citation75,Citation85,Citation116]. Upstream of HSP72, HSF1 has also been silenced to determine the mechanistic basis for the heat shock response [Citation87]. While these animal models have not been used extensively to study the effects of passive heating on mitochondrial function, they may be particularly valuable for determining the mechanism for heat-induced benefits on muscle mitochondria. Pharmacological induction or inhibition of heat shock proteins is another potential way to investigate the mechanisms involved in the heat stress response, and to evaluate the potential therapeutic benefits of heat stress on mitochondria and other metabolic parameters [Citation75,Citation88,Citation117].
4.2. Human models of passive heating
In humans, various models of passive heating have been utilized. Some models have focused on raising whole-body temperature [Citation68,Citation118], while others have localized the heat to only one limb [Citation23,Citation67,Citation68,Citation119,Citation120]. Models of whole-body heating include saunas/heat chambers, water immersion and water-perfused suits, with the temperature and duration of these various heating modalities varying greatly. For example, sauna usage in Finland typically entails short bouts of heating (10–20 min) at very high temperatures (60–100 °C) [Citation121,Citation122], while other studies have used lower temperatures for slightly longer heating bouts [Citation68,Citation118]. When using extremely high temperatures, some studies use multiple short bouts of heating with a cold shower separating each bout [Citation123]. Currently, only a small number of human studies have directly investigated the effects of whole-body heating on muscle mitochondria (). Furthermore, the focus of most whole-body heating studies has been on raising core temperature, and not necessarily internal muscle temperature, which is likely a key factor for inducing mitochondrial adaptations. An exception to this was a study performed by Ihsan et al. which involved whole-body heating using a water-perfused suit and raised internal muscle temperature to approximately 39 °C. Following a single session of heating, signaling for mitochondrial biogenesis increased, while a localized heating approach from the same study failed to induce mitochondrial biogenesis possibly due to a more mild change in muscle temperature to about 38 °C () [Citation68]. Warm water or hot tub immersion is another form of whole-body heat which has been shown to improve insulin sensitivity and blood glucose control in diabetic individuals [Citation124]. While improved insulin sensitivity is often associated with improvements in mitochondrial health [Citation47], we are not aware of any water immersion studies which have directly investigated the adaptive effects of passive heating on mitochondria. However, given the apparent clinical utility of hot water immersion on blood glucose control, this may be an interesting avenue for future research.
Table 2. Human studies investigating effects of passive heating on mitochondria.
Localized heating methods include heat applied directly to the skin or deep-heating methods such as shortwave diathermy. Modalities which apply heat to the skin include single-leg water-perfused suits and steam-heated sheets/wraps; these methods cause changes in skin temperature, but the heat may not effectively penetrate deep into the muscle [Citation68,Citation119,Citation120]. Deep heating methods include ultrasound and shortwave diathermy. Ultrasound can induce mild increases in muscle temperature [Citation125–127], but to our knowledge, has not been studied for its effects on mitochondrial function in humans. One of the most effective methods for inducing changes in muscle temperature, and for improving mitochondrial function, is shortwave diathermy [Citation23,Citation25,Citation67]. Diathermy has been reported to raise muscle temperature to 40–41 °C without a concomitant change in core temperature, which makes it a valuable tool for investigating the molecular pathways activated by heat stress in skeletal muscle [Citation23,Citation67]. When applied to the vastus lateralis of human subjects either short- or long-term, shortwave diathermy has been shown to increase mitochondrial respiratory capacity [Citation23,Citation25]. It has also been shown to reduce muscle atrophy during limb disuse [Citation67]. However, a clear clinical limitation of diathermy and other localized heating modalities is that they only heat individual muscle groups. Furthermore, shortwave diathermy may have contraindications for a variety of clinical conditions including pregnancy, artificial implants, and pacemakers [Citation128]. Therefore, shortwave diathermy and other localized heating modalities may not be suitable for many of the patients who could benefit most from passive heating. further summarizes the human studies which have investigated the effects of passive heating on skeletal muscle mitochondria.
4.3. Internal muscle temperature is key
Whether whole-body or localized heating methods are employed, it appears that a key factor for eliciting changes in mitochondrial function is the degree to which internal muscle temperature rises during the intervention. Passive heating methods that cause muscle temperature to rise to or above 39–40 °C typically elicit mitochondrial adaptations [Citation23,Citation67,Citation68], while it is less clear for more mild heating methods [Citation68,Citation118,Citation119] (). Interestingly, diathermy is one method which induces large increases in muscle temperature and causes significant improvements in mitochondrial respiration. Therefore, we believe that diathermy is an excellent heating modality for investigating the molecular pathways involved in heat-induced mitochondrial adaptations. However, whole-body heating methods may be more practical in a clinical setting because they are not focused on a single muscle group, allowing for more clinically relevant benefits. The potential limitation of whole-body heat is simply whether the muscle temperature can rise enough to activate the same pathways that are affected by interventions like shortwave diathermy. Recent work has shown that hot-water immersion at 42 °C can elevate internal muscle temperature to approximately 39 °C [Citation129]. Thus, it is possible that hot-water immersion can raise muscle temperature sufficiently to improve muscle mitochondrial function. Furthermore, hot-water immersion at slightly lower temperatures (∼37–41 °C) can improve blood glucose control, suggesting that this heating method has clinical utility [Citation124].
4.4. Clinical applications for passive heating
A future area of research may be to compare different forms of whole-body heating (i.e., sauna, hot-tub) to determine which methods (and temperatures) cause mitochondrial adaptations, and if these adaptations are required for improvements in blood glucose control and other metabolic benefits. Current evidence suggests that passive heating may be especially therapeutic for patients with insulin resistance and type II diabetes mellitus [Citation69,Citation124]. Patients with sarcopenia and cancer cachexia may also benefit from passive heat exposure. Pre-clinical evidence suggests that heat may improve muscle regeneration, muscular dystrophy, and other muscle-related pathologies [Citation114,Citation130–132]. However, only one study in humans has investigated the potential for passive heating to reduce muscle atrophy associated with the preservation of mitochondrial function [Citation67]. Furthermore, most of the research investigating heat-induced mitochondrial adaptations has been performed in young populations. Older populations are certainly more susceptible to insulin resistance and atrophy. Therefore, future work in older populations is warranted.
5. Conclusions
Though work is still very preliminary in this area, passive heat stress has been shown to have therapeutic potential through improving mitochondrial function in skeletal muscle. Future areas of research may include determining what heating modalities improve mitochondrial function, and the duration and temperature required to do so. Functional measures of mitochondria, including in-vivo and ex-vivo methods, may be valuable tools for better understanding the clinical utility of passive heating. Furthermore, pre-clinical models aiming to elucidate the molecular mechanisms at play in heat-induced benefits could open doors for pharmacological investigations to activate these same pathways.
Acknowledgements
The graphical abstract and Figure 3 were created using BioRender.com
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482.
- Romanello V, Sandri M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol Life Sci. 2021;78(4):1305–1328.
- Brown JL, Rosa-Caldwell ME, Lee DE, et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017;8(6):926–938.
- Sangwung P, Petersen KF, Shulman GI, et al. Mitochondrial dysfunction, insulin resistance, and potential genetic implications. Endocrinology. 2020;161(4):bqaa017.
- Hesselink MK, Schrauwen-Hinderling V, Schrauwen P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(11):633–645.
- Memme JM, et al. Mitochondrial bioenergetics and turnover during chronic muscle disuse. Int J Mol Sci. 2021;22(10):5179.
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242(9):2278–2282.
- Oliveira AN, Richards BJ, Slavin M, et al. Exercise is muscle mitochondrial medicine. Exerc Sport Sci Rev. 2021;49(2):67–76.
- Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2018;52(8):505–513.
- Lobelo F, Stoutenberg M, Hutber A. The exercise is medicine global health initiative: a 2014 update. Br J Sports Med. 2014;48(22):1627–1633.
- Ramirez-Velez R, Lobelo F, Izquierdo M. Exercise for disease prevention and management: a precision medicine approach. J Am Med Dir Assoc. 2017;18(7):633–634.
- Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. 2016;101(1):17–22.
- Powers SK, Deminice R, Ozdemir M, et al. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. 2020;9(5):415–425.
- Valenzuela PL, Morales JS, Pareja-Galeano H, et al. Physical strategies to prevent disuse-induced functional decline in the elderly. Ageing Res Rev. 2018;47:80–88.
- Nguyen TTN, Choi H, Jun HS. Preventive effects of dulaglutide on disuse muscle atrophy through inhibition of inflammation and apoptosis by induction of Hsp72 expression. Front Pharmacol. 2020;11:90.
- Owen PJ, Armbrecht G, Bansmann M, et al. Whey protein supplementation with vibration exercise ameliorates lumbar paraspinal muscle atrophy in prolonged bed rest. J Appl Physiol (1985). 2020;128(6):1568–1578.
- Saltin B, Gagge AP, Stolwijk JA. Muscle temperature during submaximal exercise in man. J Appl Physiol. 1968;25(6):679–688.
- Parkin JM, Carey MF, Zhao S, et al. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol (1985). 1999;86(3):902–908.
- Hawley JA, Lundby C, Cotter JD, et al. Maximizing cellular adaptation to endurance exercise in skeletal muscle. Cell Metab. 2018;27(5):962–976.
- Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol (1985). 2012;112(3):354–361.
- Patton MG, Gillum TL, Szymanski MC, et al. Heat acclimation increases mitochondrial respiration capacity of C2C12 myotubes and protects against LPS-mediated energy deficit. Cell Stress Chaperones. 2018;23(5):871–883.
- Tamura Y, Matsunaga Y, Masuda H, et al. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R931–43.
- Hafen PS, Preece CN, Sorensen JR, et al. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol (1985). 2018;125(5):1447–1455.
- Gupte AA, Bomhoff GL, Touchberry CD, et al. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol (1985). 2011;110(2):451–457.
- Marchant ED, et al. Localized heat therapy improves mitochondrial respiratory capacity but not fatty acid oxidation. Int J Mol Sci. 2022;23(15):8500.
- Mao X, Gu Y, Sui X, et al. Phosphorylation of Dynamin-Related protein 1 (DRP1) regulates mitochondrial dynamics and skeletal muscle wasting in cancer cachexia. Front Cell Dev Biol. 2021;9:673618.
- Kitaoka Y, Miyazaki M, Kikuchi S. Voluntary exercise prevents abnormal muscle mitochondrial morphology in cancer cachexia mice. Physiol Rep. 2021;9(16):e15016.
- Ferri E, et al. Role of Age-Related mitochondrial dysfunction in sarcopenia. Int J Mol Sci. 2020;21(15):5236.
- Kim KW, Baek M-O, Yoon M-S, et al. Deterioration of mitochondrial function in the human intercostal muscles differs among individuals with sarcopenia, obesity, and sarcopenic obesity. Clin Nutr. 2021;40(5):2697–2706.
- Leduc-Gaudet JP, et al. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci. 2021;22(15):8179.
- Fealy CE, et al. Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging. Trends Mol Med. 2021;27(11):1033–1044.
- Gilliam LAA, Fisher-Wellman KH, Lin C-T, et al. The anticancer agent doxorubicin disrupts mitochondrial energy metabolism and redox balance in skeletal muscle. Free Radic Biol Med. 2013;65:988–996.
- Morton AB, Mor Huertas A, Hinkley JM, et al. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion. 2019;45:52–62.
- Min K, Kwon O-S, Smuder AJ, et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J Physiol. 2015;593(8):2017–2036.
- Buso A, Comelli M, Picco R, et al. Mitochondrial adaptations in elderly and young men skeletal muscle following 2 weeks of bed rest and rehabilitation. Front Physiol. 2019;10:474.
- Kenny HC, Rudwill F, Breen L, et al. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia. 2017;60(8):1491–1501.
- Dirks ML, Miotto PM, Goossens GH, et al. Short-term bed rest-induced insulin resistance cannot be explained by increased mitochondrial H2 O2 emission. J Physiol. 2020;598(1):123–137.
- Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces Whole-Body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875.
- Standley RA, Distefano G, Trevino MB, et al. Skeletal muscle energetics and mitochondrial function are impaired following 10 days of bed rest in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(9):1744–1753.
- Genders AJ, Holloway GP, Bishop DJ. Are alterations in skeletal muscle mitochondria a cause or consequence of insulin resistance? Int J Mol Sci. 2020;21(18):6948.
- Goodpaster BH. Mitochondrial deficiency is associated with insulin resistance. Diabetes. 2013;62(4):1032–1035.
- Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19(9):324–330.
- Sergi D, Naumovski N, Heilbronn LK, et al. Mitochondrial (dys)function and insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol. 2019;10:532.
- Holloszy JO. Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes. 2013;62(4):1036–1040.
- Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: a case of fat overconsumption, not mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2008;105(22):7627–7628.
- Miova B, Dimitrovska M, Dinevska-Kjovkarovska S, et al. The heat stress response and diabetes: more room for mitochondrial implication. Curr Pharm Des. 2016;22(18):2619–2639.
- Krako Jakovljevic N, et al. Targeting mitochondria in diabetes. Int J Mol Sci. 2021;22(12):6642.
- Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–581.
- Min K, Smuder AJ, Kwon O-S, et al. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985). 2011;111(5):1459–1466.
- Smuder AJ, Roberts BM, Wiggs MP, et al. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents Colon 26 cancer-induced cardiorespiratory muscle weakness. Oncotarget. 2020;11(38):3502–3514.
- Willingham TB, Ajayi PT, Glancy B. Subcellular specialization of mitochondrial form and function in skeletal muscle cells. Front Cell Dev Biol. 2021;9:757305.
- Hood DA, Tryon LD, Carter HN, et al. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473(15):2295–2314.
- Pan X, Liu J, Nguyen T, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15(12):1464–1472.
- Ji LL, Yeo D, Kang C, et al. The role of mitochondria in redox signaling of muscle homeostasis. J Sport Health Sci. 2020;9(5):386–393.
- Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50(3):222–233.
- Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590(14):3349–3360.
- Groennebaek T, Nielsen J, Jespersen NR, et al. Utilization of biomarkers as predictors of skeletal muscle mitochondrial content after physiological intervention and in clinical settings. Am J Physiol Endocrinol Metab. 2020;318(6):E886–E889.
- Dahl R, Larsen S, Dohlmann TL, et al. Three-dimensional reconstruction of the human skeletal muscle mitochondrial network as a tool to assess mitochondrial content and structural organization. Acta Physiol (Oxf). 2015;213(1):145–155.
- Glancy B, Hartnell LM, Malide D, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523(7562):617–620.
- Doerrier C, Garcia-Souza LF, Krumschnabel G, et al. High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol. 2018;1782:31–70.
- Schmidt CA, Fisher-Wellman KH, Neufer PD. From OCR and ECAR to energy: perspectives on the design and interpretation of bioenergetics studies. J Biol Chem. 2021;297(4):101140.
- Chi MM, Hintz CS, Coyle EF, et al. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244(3):C276–87.
- Lowry CV, Kimmey JS, Felder S, et al. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978;253(22):8269–8277.
- Campbell MD, Marcinek DJ. Evaluation of in vivo mitochondrial bioenergetics in skeletal muscle using NMR and optical methods. Biochim Biophys Acta. 2016;1862(4):716–724.
- Acin-Perez R, Benincá C, Shabane B, et al. Utilization of human samples for assessment of mitochondrial bioenergetics: gold standards, limitations, and future perspectives. Life Basel. 2021;11(9):949.
- Perry CGR, Kane DA, Lanza IR, et al. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62(4):1041–1053.
- Hafen PS, Abbott K, Bowden J, et al. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J Appl Physiol (1985). 2019;127(1):47–57.
- Ihsan M, Deldicque L, Molphy J, et al. Skeletal muscle signaling following Whole-Body and localized heat exposure in humans. Front Physiol. 2020;11:839.
- Sebok J, et al. Heat therapy shows benefit in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Int J Hyperthermia. 2021;38(1):1650–1659.
- Madrigal-Matute J, et al. Heat-shock proteins in cardiovascular disease. Adv Clin Chem. 2011;54:1–43.
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796.
- Gupte AA, Bomhoff GL, Swerdlow RH, et al. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–578.
- Kavanagh K, Davis AT, Jenkins KA, et al. Effects of heated hydrotherapy on muscle HSP70 and glucose metabolism in old and young vervet monkeys. Cell Stress Chaperones. 2016;21(4):717–725.
- Tardo-Dino P-E, Taverny C, Siracusa J, et al. Effect of heat acclimation on metabolic adaptations induced by endurance training in soleus rat muscle. Physiol Rep. 2021;9(16):e14686.
- Henstridge DC, Bruce CR, Drew BG, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63(6):1881–1894.
- Chung J, Nguyen A-K, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744.
- Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060.
- Lee WJ, Kim M, Park H-S, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340(1):291–295.
- Goto A, Egawa T, Sakon I, et al. Heat stress acutely activates insulin-independent glucose transport and 5’-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep. 2015;3(11):e12601.
- Rath S, Sharma R, Gupta R, et al. MitoCarta3.0: an updated mitochondrial proteome now with Sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021;49(D1):D1541–D1547.
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749.
- Bauer MF, Hofmann S, Neupert W, et al. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10(1):25–31.
- Jores T, Lawatscheck J, Beke V, et al. Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial beta-barrel proteins. J Cell Biol. 2018;217(9):3091–3108.
- Fan F, Duan Y, Yang F, et al. Deletion of heat shock protein 60 in adult mouse cardiomyocytes perturbs mitochondrial protein homeostasis and causes heart failure. Cell Death Differ. 2020;27(2):587–600.
- Drew BG, Ribas V, Le JA, et al. HSP72 is a mitochondrial stress sensor critical for parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63(5):1488–1505.
- Tamura Y, Kitaoka Y, Matsunaga Y, et al. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J Physiol. 2015;593(12):2707–2720.
- Neueder A, Gipson TA, Batterton S, et al. HSF1-dependent and -independent regulation of the mammalian in vivo heat shock response and its impairment in huntington’s disease mouse models. Sci Rep. 2017;7(1):12556.
- Kurop MK, Huyen CM, Kelly JH, et al. The heat shock response and small molecule regulators. Eur J Med Chem. 2021;226:113846.
- Mosser DD, Duchaine J, Massie B. The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol Cell Biol. 1993;13(9):5427–5438.
- Zhong M, Orosz A, Wu C. Direct sensing of heat and oxidation by drosophila heat shock transcription factor. Mol Cell. 1998;2(1):101–108.
- Kmiecik SW, Mayer MP. Molecular mechanisms of heat shock factor 1 regulation. Trends Biochem Sci. 2021;47(3):218–234.
- Kovacs D, et al. HSF1Base: a comprehensive database of HSF1 (heat shock factor 1) target genes. Int J Mol Sci. 2019;20(22):5815.
- Ma X, Xu L, Alberobello AT, et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1alpha transcriptional axis. Cell Metab. 2015;22(4):695–708.
- Xu L, Ma X, Bagattin A, et al. The transcriptional coactivator PGC1alpha protects against hyperthermic stress via cooperation with the heat shock factor HSF1. Cell Death Dis. 2016;7(2):e2102.
- Dillard CJ, Litov RE, Savin WM, et al. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. 1978;45(6):927–932.
- Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847(2):185–190.
- Davies KJ, Quintanilha AT, Brooks GA, et al. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107(4):1198–1205.
- Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016;594(18):5135–5147.
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276.
- Silveira LR, Pilegaard H, Kusuhara K, et al. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta. 2006;1763(9):969–976.
- Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670.
- Dimauro I, Mercatelli N, Caporossi D. Exercise-induced ROS in heat shock proteins response. Free Radic Biol Med. 2016;98:46–55.
- Fischer CP, Hiscock NJ, Basu S, et al. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol (1985). 2006;100(5):1679–1687.
- King MA, Clanton TL, Laitano O. Hyperthermia, dehydration, and osmotic stress: unconventional sources of exercise-induced reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2016;310(2):R105–14.
- Zuo L, Christofi FL, Wright VP, et al. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279(4):C1058–66.
- van der Poel C, Stephenson DG. Effects of elevated physiological temperatures on sarcoplasmic reticulum function in mechanically skinned muscle fibers of the rat. Am J Physiol Cell Physiol. 2007;293(1):C133–41.
- Mreisat A, Kanaani H, Saada A, et al. Heat acclimation mediated cardioprotection is controlled by mitochondrial metabolic remodeling involving HIF-1alpha. J Therm Biol. 2020;93:102691.
- Kikusato M, Toyomizu M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS One. 2013;8(5):e64412.
- Zuo L, Christofi FL, Wright VP, et al. Lipoxygenase-dependent superoxide release in skeletal muscle. J Appl Physiol (1985). 2004;97(2):661–668.
- Yu T, Dohl J, Chen Y, et al. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J Cell Physiol. 2019;234(8):13292–13302.
- Yu T, Dohl J, Wang L, et al. Curcumin ameliorates Heat-Induced injury through NADPH Oxidase-Dependent redox signaling and mitochondrial preservation in C2C12 myoblasts and mouse skeletal muscle. J Nutr. 2020;150(9):2257–2267.
- Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R134–9.
- Yoshihara T, Naito H, Kakigi R, et al. Heat stress activates the akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol (Oxf). 2013;207(2):416–426.
- Ohira T, Higashibata A, Seki M, et al. The effects of heat stress on morphological properties and intracellular signaling of denervated and intact soleus muscles in rats. Physiol Rep. 2017;5(15):e13350.
- Chen HW, et al. Previous hyperthermic treatment increases mitochondria oxidative enzyme activity and exercise capacity in rats. Kaohsiung J Med Sci. 1999;15(10):572–580.
- Marshall JPS, Estevez E, Kammoun HL, et al. Skeletal muscle-specific overexpression of heat shock protein 72 improves skeletal muscle insulin-stimulated glucose uptake but does not alter whole body metabolism. Diabetes Obes Metab. 2018;20(8):1928–1936.
- Kavanagh K, Flynn DM, Jenkins KA, et al. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300(5):E894–901.
- Hesketh K, Shepherd SO, Strauss JA, et al. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am J Physiol Heart Circ Physiol. 2019;317(1):H114–H123.
- Kim K, Reid BA, Casey CA, et al. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J Appl Physiol (1985). 2020;128(3):483–492.
- Goto K, Oda H, Kondo H, et al. Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol. 2011;111(1):17–27.
- Kunutsor SK, Häkkinen A, Zaccardi F, et al. Short-term effects of finnish sauna bathing on blood-based markers of cardiovascular function in non-naive sauna users. Heart Vessels. 2018;33(12):1515–1524.
- Laukkanen JA, Laukkanen T, Kunutsor SK. Cardiovascular and other health benefits of sauna bathing: a review of the evidence. Mayo Clin Proc. 2018;93(8):1111–1121.
- Behzadi P, Gravel H, Neagoe P-E, et al. Impact of finnish sauna bathing on circulating markers of inflammation in healthy Middle-aged and older adults: a crossover study. Complement Ther Med. 2020;52:102486.
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925.
- Garrett CL, Draper DO, Knight KL. Heat distribution in the lower leg from pulsed short-wave diathermy and ultrasound treatments. J Athl Train. 2000;35(1):50–55.
- Draper DO, Hawkes AR, Johnson AW, et al. Muscle heating with megapulse II shortwave diathermy and ReBound diathermy. J Athl Train. 2013;48(4):477–482.
- Draper DO, Knight K, Fujiwara T, et al. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther. 1999;29(1):13–18. discussion 19-22.
- Shields N, Gormley J, O'Hare N. Contraindications to continuous and pulsed short-wave diathermy. Physical Therapy Reviews. 2002;7(2):133–143.
- Rodrigues P, Trajano GS, Wharton L, et al. Muscle temperature kinetics and thermoregulatory responses to 42 degrees C hot-water immersion in healthy males and females. Eur J Appl Physiol. 2020;120(12):2611–2624.
- Apostolopoulos A, Nakamura A, Yokoyama S, et al. Nuclear accumulation of HSP70 in mouse skeletal muscles in response to heat stress, aging, and unloading with or without reloading. Front Genet. 2018;9:617.
- Gehrig SM, van der Poel C, Sayer TA, et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484(7394):394–398.
- Kami K, Ohira T, Oishi Y, et al. Role of 72-kDa heat shock protein in heat-stimulated regeneration of injured muscle in rat. J Histochem Cytochem. 2019;67(11):791–799.