Abstract
Background and Purpose
Microwave ablation (MWA) is a promising modality that needs to be further investigated for cystic lesions. The present study aimed to determine the effects of MWA on cysts and cystic neoplasms with a tissue-mimicking model.
Methods
Twenty New Zealand White rabbits were randomly divided into Group A (cyst mimic models, n = 10, φ = 5 cm) and Group B (cystic neoplasm mimicking models, n = 10, φ = 5 cm). For each group, ex vivo rabbit healthy bladder and VX2-implanted tumor bladder were fixed and embedded in agarose gel to mimic cyst and cystic neoplasm. In the MWA experimental subgroups, microwave antennas guided by computed tomography (CT) were introduced into these models. A system thermometer was placed at the outer edge of the bladder wall to monitor temperature changes. Immediately after MWA, ex vivo rabbit healthy bladders and VX2-implanted tumor bladders were harvested for gross anatomy and prepared for pathological evaluation.
Results
A total of twenty cyst and cystic neoplasm mimicking models were successfully developed. Ninety percent of the MWA procedures were successful, and no peri-procedural complications were encountered. The temperature of the cystic wall increased with duration in both MWA experimental subgroups and an effective ablation temperature (>60 °C) was achieved. Pathological examination of the cyst and cystic neoplasm mimic models revealed degenerative necrosis of the bladder wall mucosal epithelial cells, loss of bladder wall tissue structure and coagulative necrosis of VX2 tumor cells.
Conclusion
Our data indicate that MWA could cause thermal damage to the tissue structure of cyst and cystic neoplasm, and it is an effective technique for treating cystic diseases.
ex vivo rabbit healthy bladder and VX2-implanted tumor bladder were fixed and embedded in agarose gel to mimic cyst and cystic neoplasm.
The temperature of the cystic wall increased with MWA duration and an effective ablation temperature (> 60 °C) was achieved.
MWA could cause thermal damage to the tissue structure of the cyst and cystic neoplasm and it is effective in treating cystic diseases, as assessed by histopathology.
HIGHLIGHTS
Introduction
In recent years, cysts and cystic neoplasms have become clinically common, complex, and insidious, with a high incidence [Citation1–4]. Open surgical and laparoscopic resection are the conventional approaches to cystic lesions, indicated for cysts in the marginal areas or protruding into the peritoneal cavity, whereas partial excision of organs is feasible for those located in the parenchyma [Citation5]. Percutaneous aspiration with sclerotherapy can be achieved by injecting ethanol, bleomycin, and antitumor agents (paclitaxel and gemcitabine) to destroy the neoplastic epithelial lining of the cyst. It is a minimally invasive, safe, and effective treatment for patients who refuse or are not candidates for surgery [Citation6–11]. A prospective study of 164 patients who underwent cyst ablation with alcohol and paclitaxel reported a treatment response of complete resolution (72.2%), partial resolution (19.6%), and persistent cyst (8.2%) [Citation12].
Image-guided thermal ablation techniques, including radiofrequency ablation (RFA) and microwave ablation (MWA), have been widely used for the treatment of solid tumors in recent years. Based on the principle of local thermal ablation of solid tumors in multiple organ sites, Rhim et al. [Citation13], Song et al. [Citation14], and Park et al. [Citation15] explored and introduced image-guided RFA for the treatment of liver cysts and cystic renal tumors and achieved satisfactory clinical efficacy. A prospective multicenter study using endoscopic ultrasound-guided RFA for the treatment of pancreatic cystic neoplasms reported complete resolution at 6 and 12 months in 47% and 64.7% of cases, and the significant response rate was 65% and 71%, respectively [Citation16]. Compared to RFA, MWA is a more effective technique for cystic lesions and has a higher overall energy utilization [Citation17–19]. In addition, MWA enables energy transfer through electromagnetic radiation with fewer restrictions on the surrounding tissue. Although MWA may be a promising modality for treating cysts and cystic neoplasms, few studies have shown sufficient outcomes.
In this study, we aimed to determine the effects of MWA on cystic lesions using tissue-mimicking models of cysts and cystic neoplasms.
Materials and methods
Animals
This study was approved by the Institutional Animal Care and Use Committee and all experiments were performed in accordance with animal care guidelines. Twenty New Zealand White rabbits (weight: 2.5–3.5 kg; age: 3–4 months), provided by the Beijing Keyu Animal Breeding Center (Beijing, China; license number: SCXK 2018-0010), were used in this study. A tumor-bearing rabbit was used to develop the tumor masses for implantation. The VX2 tumor strain (National Infrastructure of Cell Line Resources, Beijing, China) was implanted into the lateral hind thigh muscles of tumor-bearing rabbit. After two to three weeks, the implanted tumor mass grew to an average size of 2.0 cm3. The VX2 tumor mass was then excised and the necrotic tissue was discarded. The tissue at the edge of the tumor was selected and trimmed into small fragments of approximately 1.0 mm3. Ten recipient New Zealand White rabbits were anesthetized with 30–35 mg/kg intravenous sodium pentobarbital. The rabbit VX2 bladder tumor model was established as described by Bin et al. [Citation20]. Briefly, 2–3 VX2 tumor fragments were implanted into the submucosa of rabbit bladders using an 18-gauge coaxial needle. The presence of the rabbit bladder tumor was confirmed by computed tomography (CT; SOMATOM Perspective, Siemens, Germany). Two weeks later, an implanted tumor mass with a minimum of 1.0 cm in diameter was eligible to establish the tumor model, and both plain and contrast-enhanced CT scans were performed on the ten rabbits. Ten rabbits served as healthy bladder providers.
Preparation of cyst and cystic neoplasm-mimicking models
The rabbits were fasted for 6 h prior to surgery and anesthetized by injecting 3 ml/kg of 3% pentobarbital sodium at the edge of the ears. A 3-cm incision was made longitudinally in the lower abdomen, fully exposing the bladder in the field of view. The bladder neck and bilateral ureteral connections to the bladder were ligated, and the healthy bladder and VX2-implanted tumor bladder were excised ().
Figure 1. Preparation of a cystic neoplasm mimic model. (A) The VX2-implanted tumor bladder showed that the bladder mucosa was smooth, the blood vessels were clearly visible on the surface, and the VX2 tumor (yellow arrow) was located in the anterior wall of the bladder. (B) The VX2-implanted tumor bladder was immersed in agarose solution that had been cooled to 40–45 °C and kept intact in the solution. The gel fixation matrix was formed by leaving it at room temperature (23 °C) for approximately 40 min to complete the development of cystic neoplasm mimic models.
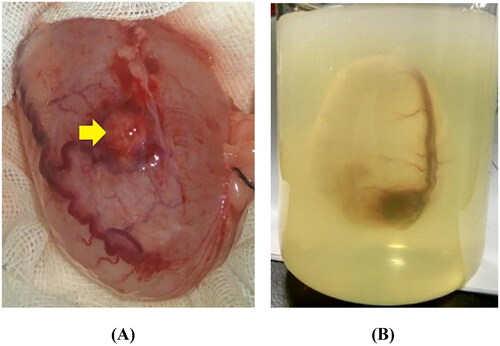
Agarose (Agarose G-10, Biowest, Spain) at a concentration of 4 g/200 ml was selected as the gelling agent for the preparation of a visualization and fixation matrix. The temperature of the agarose solution was maintained at 40–45 °C. A 1-ml syringe needle was used to puncture the fixed end of the bladder cavity, the initial urine was aspirated, and 0.9% sodium chloride was injected into the bladder. The liquid was maintained at a temperature of 37 °C. The volume of injected fluid was determined according to the maximum transverse diameter of the bladder (5.0 cm/60 ml). Then, ex vivo rabbit healthy bladder and VX2-implanted tumor bladder were immersed in agarose solution that had been cooled to 40–45 °C and kept intact in the solution. The gel fixation matrix was formed by leaving it at room temperature (23 °C) for approximately 40 min, which completed the development of cyst and cystic neoplasm mimic models ().
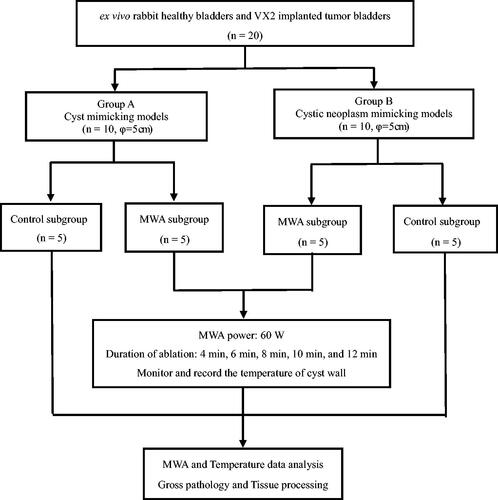
The prepared models were assigned to the cyst mimic model group (Group A, n = 10, φ = 5 cm) and the cystic neoplasm mimic model group (Group B, n = 10, φ = 5 cm). Each group was randomly divided into a control subgroup (n = 5) and a MWA experimental subgroup (n = 5). Except for the interventional radiologist who performed the procedures, none of the participants knew which rabbits and models belonged to each group.
MWA
MWA was performed using a microwave generator (Microwave Ablation/Resection System, SurblateTM, Vison Medical, Nanjing, China). The microwave generator had a microwave frequency of 2,450 ± 50 MHz, a continuous wave output power of 5–120 W adjustable in steps, and a temperature range of 40–112 °C with a measurement error of ±3 °C. The system was equipped with a thermistor temperature measuring device and a digital thermometer (multi-channel thermometer with an accuracy of 1 °C). The microwave antenna (Vison Medical, Nanjing, China) was 15 cm in effective length and 17 G in outside diameter, with a 15 mm active tip.
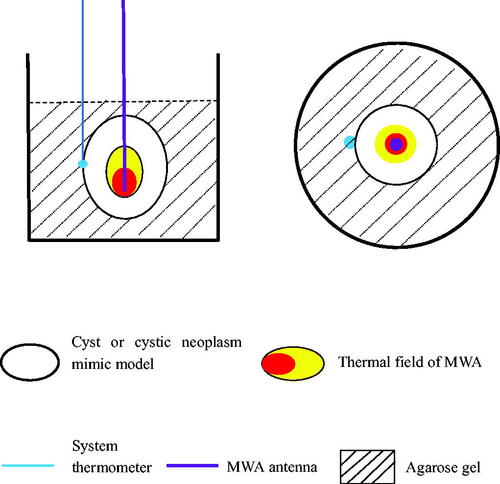
During the MWA procedure, a system thermometer was placed at the outer edge of the bladder wall to monitor temperature changes. shows the locations of the microwave antenna, system thermometer, and cyst and cystic neoplasm mimic models.
MWA procedure
shows the flowchart of the experimental procedure used in this study.
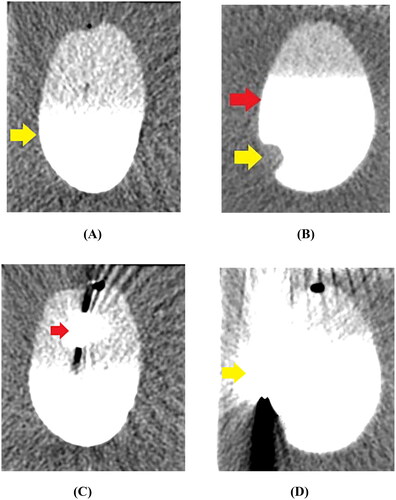
First, a cyst mimic model and a cystic neoplasm mimic model were placed in front of the bed section of the CT, the scan parameters were set (tube voltage, 80 kV; tube current, 120 mA; slice thickness, 5 mm; reconstruction interval, 1 mm), and the scanning program was initiated. CT was used to determine the maximum cross-sectional slice of ex vivo healthy bladder and VX2-implanted tumor bladder in agarose gel, and the puncture points were marked (). Then, a 17 G microwave antenna (15 cm) was inserted along the length of the cyst and cystic neoplasm mimic models, perpendicular to the level of the agarose gel fixation base, and repeated CT scans were performed to assess if the antenna tip had reached the base of the cystic space (). Finally, the microwave energy was set at 60 W and 2450 MHz, the duration of MWA was 12 min, and the position of the microwave antenna was fixed during the ablation process. Moreover, the system thermometer was placed on one side of the cyst wall, and the changes in the wall temperature were monitored and recorded at 4, 6, 8, 10, and 12 min (). The correlations between temperature and microwave duration were illustrated, which recorded the data obtained from the experimental groups. All the procedures were performed by the same interventional radiologist with >20 years of experience.
Figure 4. CT imaging of the MWA procedure. (A) The cyst mimic model showed a regular morphology with clear borders after contrast injection into the cystic cavity (yellow arrow). (B) The cystic neoplasm mimic model showed a regular morphology with clear borders (red arrow). The filling defect of the VX2-implanted tumor (yellow arrow) was visible in the cystic cavity after contrast injection. (C) A 17 G microwave antenna (red arrow) was inserted along the length of the cystic neoplasm mimic model, perpendicular to the level of the agarose gel fixation base. (D) The system thermometer (yellow arrow) was placed at the outer edge of the bladder wall to monitor temperature changes.
Histology
Immediately after MWA, ex vivo rabbit healthy bladders and VX2-implanted tumor bladders were harvested from the agarose gel fixation base, respectively. The gross anatomical morphology of the bladder and VX2 tumor was observed. Tissue samples were fixed with 4% paraformaldehyde for 24 h, paraffin-embedded, and cut into 4-μm-thick sections. Pathological changes were observed by hematoxylin-eosin (H&E) staining, and histological features and necrosis were observed under an optical microscopy (Nikon TE2000, Japan). A senior pathologist performed the histopathological evaluation using microscopy.
Statistical analysis
Statistical analyses were performed with SPSS 24.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as mean ± standard deviation. T-test was used to analyze the enumeration data. Statistical significance was set at p < 0.05.
Results
VX2 tumor and cyst and cystic neoplasm mimic model
The VX2 tumor was successfully implanted in the bladders of ten New Zealand White rabbits. The diameter of the tumors was approximately 1.5 cm (range, 1.0–2.0 cm) two weeks after implantation. A total of twenty ex vivo rabbit healthy bladders and VX2-implanted tumor bladders were used to establish ten cyst mimic models and ten cystic neoplasm mimic models.
MWA procedure Results
Except one, all MWA procedures were successful, and no peri-procedural complications were encountered. In the MWA experimental subgroup of Group A, one cyst mimic model was excluded due to puncture failure, in which the bladder wall was damaged by the microwave antenna tip resulting in fluid leakage from the lumen.
shows the temperature variations of the cyst wall during the MWA procedure at each time point in the experimental subgroup of Group A. The initial temperature of the cyst wall was 36.1 ± 0.8 °C, rising slowly over a period of 4 min and then rapidly after 6 min. shows the temperature changes in the experimental subgroup of Group B. The initial temperature of cystic neoplasm mimic model wall was 36.2 ± 2.1 °C, 56.3 ± 2.4 °C, and 73.1 ± 3.4 °C at 4 and 6 min, and peaked at 95.5 ± 0.9 °C at 12 min. The temperature of the cyst wall in both groups increased with the duration of MWA, reaching 60 °C within 6 min. The final temperature reached nearly 100 °C after the procedure.
Table 1. MWA experimental group with cyst mimicking models (Power/Transverse diameter: 60 W/5 cm) (Mean ± SD, °C)
Table 2. MWA experimental group with cystic neoplasm mimic models (Power/Transverse diameter: 60 W/5 cm) (Mean ± SD, °C)
Pathologic evaluation Results
Gross pathology
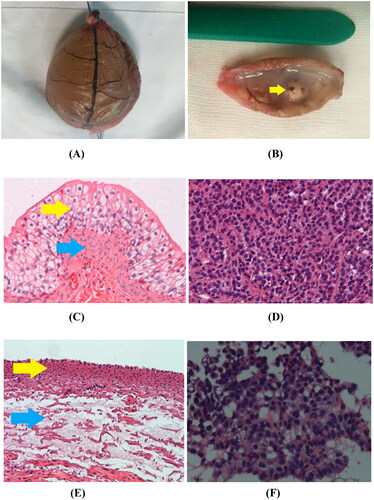
The ex vivo rabbit healthy bladders and VX2-implanted tumor bladders were grossly intact. With increasing MWA duration, the bladder wall gradually dehydrated, the mucosal surface became wrinkled, the color changed from light red to withered yellow, and the blood vessels in the bladder wall appeared withered and resembled dendritic branches, the color changed from dark red to dark brown; the bladder wall and blood vessels within the bladder were finally charred (). The VX2-implanted tumor on the bladder wall (yellow arrow) appeared grayish white and soft after MWA ().
Figure 5. Gross specimen and pathological examination imaging. (A) Gross morphology image of ex vivo rabbit healthy bladder treated with MWA after 12 min duration showed that the blood vessels in the bladder wall appeared withered and looked like dendritic branches, and the bladder surface appeared dark brown. (B) Gross morphology image of VX2 implanted tumor (yellow arrow) treated with MWA after 12 min duration appeared grayish white and soft. (C) In the control group, 100× H&E staining showed that the integrity of the mucosal structure and the epithelial cells (yellow arrow) were maintained, and the lamina propria and muscularis (blue arrow) were intact. (D) In the control group, H&E staining of the VX2 tumor showed irregular tumor cell morphology, large deep-stained nuclei, obvious atypia, and high mitotic rates. (E) Immediately after MWA, 100× H&E histology showed that complete shedding of mucosal epithelial cells of the bladder wall, necrosis of the muscularis mucosa (yellow arrow), and the loss of histological patterns and structures in all layers of the bladder wall (blue arrow). (F) Immediately after MWA, 100× H&E histology showed coagulative necrosis in most of the VX2 tumor cells, along with karyopyknosis and deeply stained cytoplasm.
Histopathological evaluation
show the histopathological anatomy of ex vivo healthy bladder and VX2-implanted tumor in the control subgroups of Group A and Group B. Histopathological examination suggested that the morphology and intracellular structure of the epithelial cells of the inner wall of the normal bladder lumen were intact, as were the morphology and structure of the cells of the lamina propria and muscularis. The VX2 tumor demonstrated infiltrative growth with irregular tumor cell morphology, large nuclei with dark staining, evident atypia, and karyokinesis.
shows the pathological changes in the MWA experimental subgroups in Group A after 12 min of MWA duration. Histopathological examination revealed complete shedding of mucosal epithelial cells in the bladder wall, necrosis of the muscularis mucosa, and loss of histological patterns and structures in all layers of the bladder wall. reveals the changes in the VX2-implanted tumor structure in Group B after 12 min of MWA duration. Histopathological examination showed coagulative necrosis in most of the VX2 tumor cells, along with karyopyknosis and deeply stained cytoplasm. The histopathological results of the bladder wall were the same as the corresponding changes for Group A.
Discussion
Microwaves transfer energy to the surrounding medium (solid tissue or fluid) in the form of emitted electromagnetic waves with an inherent thermal field that is positively correlated with the output power and duration [Citation21]. The higher the effective thermal conductivity, the more microwave energy is absorbed and more heat is generated. Temperatures in excess of 60 °C are considered to cause instantaneous cell death [Citation22]. Cystic lesions are water-rich tissues and hypoxic, resulting in a lower potential of hydrogen (pH) and are more sensitive to heat than solid tissues. The MWA technique, utilizing the microwave-induced heat effect causes direct lethality via coagulative necrosis, which theoretically allows cyst and cystic neoplasm to achieve the therapeutic effect of thermal destruction without any chemicals, and is safer and more efficient than sclerotherapy.
In the present study, we demonstrate that MWA causes thermal damage to the epithelial lining of the bladder wall and VX2-implanted tumor at gradually increasing temperatures, resulting in tissue structure destruction. When the microwave antenna was inserted into the cystic cavity and the microwave system was activated, an ellipsoidal thermal field was generated in the local space near the center of the microwave antenna tip. The fluid in the cyst transferred the heat accumulated at the center to the cyst wall via convective heat transfer. When the temperature of the cyst wall exceeded 60 °C, tissue cells underwent dehydration and degeneration, resulting in coagulative necrosis of the epithelial tissue of the cyst wall. Previous studies have explored the therapeutic mechanism of thermal ablation for cystic lesions. Carrafiello et al. [Citation23] and Floridi et al. [Citation24] reported the clinical application of image-guided percutaneous MWA in cystic renal lesions and preliminarily validated the safety and feasibility of MWA in treating cysts or cystic neoplasms. These observations support our findings. Histopathological examination revealed that MWA caused complete shedding of mucosal epithelial cells of the bladder wall, necrosis of the muscularis mucosa, and coagulative necrosis of VX2 tumor cells after the procedure. Thus, the current study confirms that MWA is effective in treating cystic lesions.
During the MWA procedure, the transverse diameter of the cyst and cystic neoplasm mimic models was set at 5 cm. Considering that cystic lesions with a transverse diameter of 5 cm are common, the current results provide a theoretical basis for clinical practice. Based on our experience with solid tumor ablation, MWA in treating the tumors (φ<3 cm or >5 cm) was leading to excessive or insufficient thermal ablation time and ablation range. Therefore, cyst and cystic neoplasm mimic models (φ = 5 cm) were included, and a fixed power of 60 W was used in the MWA experimental groups. Although MWA used in cyst and cystic neoplasm mimic studies may not be directly used in human clinical trials, the findings may provide histopathological evidence and MWA parameters for clinical applications. Thus, our experiments predict that thermal ablation has promising applications in the treatment of cystic diseases.
Nevertheless, the present study had several limitations. First, ex vivo rabbit healthy bladders and VX2-implanted tumor bladders did not show complete tissue metabolism after isolation. Although a normal cell structure could be maintained for a specific period of time, there was still variability compared to intra-organic cysts and cystic neoplasms. Second, the fixed ablation parameters in the experiments and the small sample size caused bias in the assessment of technical parameters, such as operating power, temperature, and MWA duration for cysts and cystic neoplasms. This phenomenon should be avoided in future studies. Finally, the present study only determined the temperature at a single point on the cyst wall, while the temperature changes at the top, bottom, and side walls and the cystic fluid were not measured, which does not reflect the overall temperature changes. Therefore, multipoint real-time temperature measurements and morphological distribution of the ablation thermal field should be the focus of future studies.
In conclusion, the results of this present study show that MWA is feasible and effective for cystic diseases, providing critical information for clinical application. However, it is necessary to confirm the safety and efficacy of MWA in the treatment of cysts and cystic neoplasms in large prospective patient samples.
Author contributions
Conceived and designed the experiments: Xiao-guang Li. Performed the experiments: Bin Li. Analyzed the data: Xiao-guang Li. Contributed reagents/materials/analysis tools: Bin Li. Wrote the manuscript: Bin Li.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
No data was used for the research described in the article.
Additional information
Funding
References
- van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, et al. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16(11):676–689.
- Aziz H, Acher AW, Krishna SG, et al. Comparison of society guidelines for the management and surveillance of pancreatic cysts: a review. JAMA Surg. 2022;157(8):723–730.
- Babu BI, Shapiro AMJ. Current evidence in the management of premalignant cystic lesions of the pancreas in patients undergoing liver transplantation. Pancreas. 2022;51(2):117–120.
- European study group on cystic tumours of the pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018 May;67(5):789–804.
- Gomez A, Wisneski AD, Luu HY, et al. Contemporary management of hepatic cyst disease: techniques and outcomes at a tertiary hepatobiliary center. J Gastrointest Surg. 2021;25(1):77–84.
- Yonguc T, Sen V, Aydogdu O, et al. The comparison of percutaneous ethanol and polidocanol sclerotherapy in the management of simple renal cysts. Int Urol Nephrol. 2015;47(4):603–607.
- Du C, Chai N, Linghu E, et al. Long-term outcomes of EUS-guided lauromacrogol ablation for the treatment of pancreatic cystic neoplasms: 5 years of experience. Endosc Ultrasound. 2022;11(1):44–52.
- Chen C, Zhang R, Wan R. Novel technique for treating simple hepatic cysts: endoscopic transgastric hepatic cyst deroofing. Endoscopy. 2022;54(S 02):E1045–E1046.
- Tartaglia N, Lascia D, Cianci A, et al. A. Surgical management of non-parasitic hepatic cysts a single center experience and a review of the literature. Ann Ital Chir. 2019;90:514–519.
- Levy MJ, Bendel EC, Bjarnason H, et al. Endoscopic Ultrasound-Guided dual ultrasound hepatic cyst aspiration and sclerotherapy to ameliorate portal hypertension. Am J Gastroenterol. 2022;117(5):715–716.
- Neijenhuis MK, Wijnands TFM, Kievit W, et al. Symptom relief and not cyst reduction determines treatment success in aspiration sclerotherapy of hepatic cysts. Eur Radiol. 2019;29(6):3062–3068.
- Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy. 2017;49(9):866–873.
- Rhim H, Kim YS, Heo JN, et al. Radiofrequency thermal ablation of hepatic cyst. J Vasc Interv Radiol. 2004;15(1 Pt 1):95–96.
- Song H, Rhim H, Choi JB, et al. Radiofrequency thermal ablation of benign cystic lesions: an experimental pilot study in a porcine gallbladder model. J Korean Radiol Soc. 2001;44(5):571–576.
- Park BK, Kim CK, Lee HM. Image-guided radiofrequency ablation of bosniak category III or IV cystic renal tumors: initial clinical experience. Eur Radiol. 2008;18(7):1519–1525.
- Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51(9):836–842.
- Huang XW, Nie F, Wa ZC, et al. Thermal field distributions of ablative experiments using cyst-mimicking phantoms: comparison of microwave and radiofrequency ablation. Acad Radiol. 2018;25(5):636–642.
- Ni Y, Huang G, Yang X, et al. Microwave ablation treatment for medically inoperable stage I non-small cell lung cancers: long-term results[J]. Eur Radiol. 2022;32(8):5616–5622.
- Zheng H, Liu K, Yang Y, et al. Microwave ablation versus radiofrequency ablation for subcapsular hepatocellular carcinoma: a propensity score-matched study. Eur Radiol. 2022;32(7):4657–4666.
- Li B, Zhang Q, Wang ZW, et al. Transplanted VX2 bladder carcinoma model in rabbits: an experimental study. J Intervent Radiol. 2016;25(11):991–995.
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83.
- Moynagh MR, Dowdy SC, Welch B, et al. Image-guided tumor ablation in gynecologic oncology: review of interventional oncology techniques and case examples highlighting a collaborative, multidisciplinary program. Gynecol Oncol. 2021;160(3):835–843.
- Carrafiello G, Dionigi G, Ierardi AM, et al. Efficacy, safety and effectiveness of image-guided percutaneous microwave ablation in cystic renallesions bosniak III or IV after 24 months follow up[J]. Int J Surg. 2013;11(Suppl 1):s 30–5.
- Floridi C, De Bernardi I, Fontana F, et al. Microwave ablation of renal tumors: state of the art and development trends[J]. Radiol Med. 2014;119(7):533–540.