Abstract
Objectives
To evaluate the timing and safety of hysteroscopic myomectomy for large submucosal fibroids pretreated with high intensity focused ultrasound (HIFU).
Materials and methods
From June 2011 to December 2020, 74 patients with solitary submucousal fibroid with size larger than 4 cm who received HIFU treatment followed by hysteroscopic myomectomy were enrolled.
Results
The average age of patients was 40.2 ± 6.7 years. Among them, 1 had type 0, 18 had type I and 55 patients had type II submucosal fibroids. The mean diameter of fibroids was 5.7 ± 1.2 cm. All patients completed HIFU in one session, and the median non-perfused volume (NPV) ratio achieved in fibroids was 90.5%. Hysteroscopic myomectomy was performed in 0–1, 1–3, 3–6, and 6–12 months after HIFU. The mean shrinkage rate of fibroids post-HIFU was 68.19 ± 19.86%, 61.10 ± 16.89%, and 63.76 ± 26.68% in 1–3 months, 3–6 months and 6–12 months, respectively. All patients completed hysteroscopic myomectomy successfully, and no intrauterine adhesion after HIFU was observed. The complete resection of fibroids achieved in 69 patients in one session of the procedure. The mean operation time was 66.66 ± 31.61 min, the median blood loss was 20 ml, and the median distention medium deficit was 275 ml. No significant difference was observed in the operation time, blood loss and distention medium deficit among patients who received hysteroscopic myomectomy at different time points (p > 0.05).
Conclusions
HIFU can be used as a pretreatment for large submucosal fibroids before hysteroscopic myomectomy. Based on our results, hysteroscopic myomectomy could be performed at any time point, even within 1 month after HIFU.
Introduction
Uterine myomas or fibroids are the most common benign tumors of the reproductive system in reproductive age women. The prevalence of uterine fibroids varies in different races with different ages. The highest prevalence was reported as 70% in African American reproductive age women [Citation1]. Submucosal fibroids account for 5–10% in all types of uterine fibroids and are more likely to cause prolonged menstruation and increased menstrual volume as the fibroids protrude into the uterine cavity [Citation2]. In addition, submucosal fibroids can lead to infertility or miscarriage because they affect the endometrial receptivity and the intrauterine environment. Currently, the standard treatment of submucosal fibroids is hysteroscopic myomectomy/transcervical resection of myoma (TCRM) [Citation3–5]. However, it is difficult to completely resect the submucosal fibroids with size larger than 4 cm by hysteroscopic myomectomy in one session [Citation6]. Pretreatment is usually performed before hysteroscopic myomectomy to reduce the size of the large submucosal fibroids. Gonadotropin-releasing hormone analogue (GnRH-a) is the most commonly used medication of pretreatment for hysteroscopic myomectomy. Recently, a study showed that high intensity focused ultrasound (HIFU) seemed to be superior to GnRH-a in the pretreatment for large submucosal fibroids before hysteroscopic myomectomy [Citation7]. However, there was no study on the timing of hysteroscopic myomectomy after HIFU for submucosal fibroids. Therefore, this study intended to evaluate the timing and safety of HIFU followed by hysteroscopic myomectomy in patients with large submucosal fibroids through a retrospective analysis.
Materials and methods
The protocol for this retrospective study was approved by the ethics committee at our institute (No. CQHF-2022-0011) and the requirement for informed consent was waived.
Patients
From June 2011 to December 2020, 83 patients with a solitary type 0–II submucousal fibroid larger than 4 cm who received HIFU followed by hysteroscopic myomectomy in Chongqing Haifu Hospital were enrolled in this study ().
Inclusion criteria were as follows: 1) patients with a solitary symptomatic submucosal fibroid and the maximum diameter of the submucosal fibroid was larger than 4 cm; 2) patients who voluntarily received HIFU as a pretreatment before hysteroscopic myomectomy.
Exclusion criteria were as follows: 1) patients with suspected or confirmed uterine malignancy during follow-up; 2) patients who were unwilling to accept hysteroscopic myomectomy because of a significant symptom relief after HIFU; 3) patients who conceived after HIFU.
Magnetic resonance imaging examination
MRI examination was performed with a 1.5 T MRI system produced by Shanghai United Imaging Medical Technology Co., Ltd., China. A pelvic contrast-enhanced MRI examination was performed in every patient before HIFU. The sequence included T2-weighted imaging (T2WI), T1-weighted imaging (T1WI) and enhanced T1-weighted imaging, including transverse, sagittal and coronal sequences. The following parameters were used for T2WI sequence: TR, 4692 ms; TE, 75 ms; layer thickness 5 mm; layer spacing, 1 mm. Parameters used for T1WI sequence were as follows: TR, 196 ms; TE, 8.18 ms; layer thickness, 6 mm; layer spacing, 1.2 mm. Parameters used for contrast enhanced MRI were: TR: 4 ms; TE: 2 ms; layer thickness: 5 mm; layer spacing: 1.2 mm.
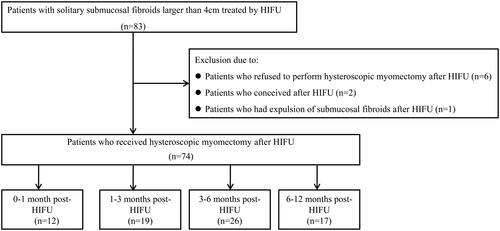
MR images obtained from patients were evaluated by two radiologists with more than 10 years of experience. The fibroids were classified as hypointense (the signal intensity of fibroids was equal to or lower than that of skeletal muscle), isointense (the signal intensity of fibroids was higher than that of skeletal muscle but lower than that of the myometrium), and hyperintense fibroids (the signal intensity of fibroids was equal to or higher than that of the myometrium) on the basis of signal intensity on T2WI of pre-HIFU MRI. Post-HIFU contrast enhanced MR images were used to evaluate the non-perfused volume (NPV) and the shrinkage of fibroids (). NPV indicates the volume of coagulative necrosis. The volume of fibroids and NPV were obtained using the software program, which was programed by the engineers from Chongqing Haifu Medical Technology Co., Ltd., to contour the fibroids and the non-perfused region in every slice of contrast enhanced MR images, then calculated using the same program. The NPV ratio = NPV/fibroid volume × 100%.
Figure 2. Pre- and post-HIFU MRI obtained from a 39-year-old patient with a large submucosal fibroid treated by HIFU. (A) Pre-HIFU contrast-enhanced MRI showed significant enhancement in the fibroid and the size of the fibroid was 5.4 cm × 5.0 cm × 5.2 cm (arrow). (B) One day post-HIFU MRI showed the NPV ratio was 92.5% (arrow). C. MRI obtained before hysteroscopic myomectomy showed the fibroid shrank significantly at 3 months after HIFU. The size of the fibroid was 2.4 cm × 2.6 cm × 2.3 cm (arrow).
HIFU procedure
Bowel preparation and skin preparation were performed before HIFU treatment. Bowel preparation included ingesting semi-liquid food or liquid food 2 days prior, and fasting for 12 h before HIFU. Skin preparation included shaving the hair between the lower edge of belly button and the upper edge of the pubic symphysis, then degreasing and degassing the skin with degassed water. Before HIFU treatment, a urinary catheter was inserted into the bladder, and the bladder volume was adjusted by infusing saline during the treatment to obtain a safe acoustic pathway.
HIFU was performed with Focused Ultrasound Tumor Therapeutic System (Model-JC or JC200) produced by Chongqing Haifu Medical Technology Co., LTD., China. Every patient was positioned prone on the HIFU table, with the anterior abdominal wall in contact with degassed water. The treatment was performed under conscious sedation (using fentanyl at 0.8–1 μg/kg administered at 30–40 min intervals; midazolam hydrochloride, at 0.02–0.03 mg/kg, administered at 30–40 min intervals). Respiratory rate, heart rate, blood pressure, and oxygen saturation were monitored during the procedure. The sagittal ultrasound scanning mode was chosen for both pretreatment planning and sonication. The locations of the fibroids and surrounding tissues were identified on ultrasound imaging and the targeted fibroid was divided into a number of sections by real-time ultrasound, with a distance of 5 mm between any two sections. Point scan was used, and power was set between 300 and 400 watts. The distance from the focal point to the endometrium was at least 1.5 cm. The treatment was terminated when the fibroid showed increased grayscale change or there was an absence of blood supply as evaluated by contrast-enhanced ultrasound immediately after HIFU ablation. Additional treatment was performed if contrast-enhanced ultrasound showed unsatisfactory NPV ratio. Treatment time, treatment power, sonication time and adverse events were recorded.
Hysteroscopic myomectomy
The hysteroscopic myomectomy was performed at different time points after HIFU according to the symptom relief and patient willingness. A disposable cervical dilation mold was placed to dilate the cervical the night before hysteroscopic myomectomy. The patient was placed in lithotomy position and the procedure was carried out under deep sedation. Respiratory rate, heart rate, blood pressure, and oxygen saturation level were monitored during the procedure. Electrolyte monitoring was also performed with the dynamical blood gas analysis to prevent acute water intoxication and hyponatremia. For non-diabetic patients, 5% dextrose solution was used as uterine distention fluid. For diabetic patients, mannitol solution was used. The hysteroscopic dilatation and perfusion system was used, and the intrauterine pressure was set to 80–100 mmHg (1 mmHg = 0.133 kPa) or less than or equal to mean arterial pressure according to the patient’s monitoring. Myomectomy was performed with a monopolar resection ring under ultrasound guidance. The necrotic fibroids were removed with oval clamp and sucked out with large suction tube under negative pressure. Electrocoagulation was used to stop bleeding. The operation was terminated when no abnormality was detected in the uterine cavity. The resected tissues were sent for pathological evaluation. Blood examination was performed within 24 h after hysteroscopic myomectomy.
Statistical analysis
SPSS 27.0 software was used for statistical analysis. Normally distributed data was presented as mean ± standard deviation, and data with skewed distribution was presented as median (with interquartile range). Analysis of variance was used for statistical comparison of operation time in hysteroscopic myomectomy, and Kruskal-Wallis H test for bleeding volume and absorption of uterine distension fluid in hysteroscopic myomectomy. A p-value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics of patients
As shown in , nine patients were excluded since six patients refused the hysteroscopic resection as they felt symptom significantly relieved, two patients conceived, and one patient had spontaneous passage of fibroid after HIFU treatment. The size of fibroids in the six patients who refused the hysteroscopic resection was ranged from 4.5 cm to 6.2 cm in diameter, five fibroids were classified as type II submucosal fibroids, and one was classified as type I. The NPV ratio achieved in these six fibroids was from 84.2% to 94.5%. The shrinkage rate was from 60.3% to 74.0% at 3 months after HIFU. The two patients who conceived after HIFU both had type II submucosal fibroids, with size of 4.2 cm and 5.4 cm in diameter. The NPV ratio achieved in these two fibroids was 97.3% and 92.2%, respectively. The shrinkage rate was 73.6% and 71.4% at 3 months after HIFU. One patient conceived 5 months after HIFU, and another one conceived at 8 months after HIFU. Both patients had a normal pregnancy and delivery. After HIFU treatment, one patient with a type 0 submucosal fibroid had spontaneous passage of the treated fibroid. The size of the submucosal fibroid was 4.4 cm in its largest diameter before HIFU.
Overall, a total of 74 patients who met the inclusion criteria were finally enrolled in this study. Among them, 1 patient had type 0, 18 patients had type I and 55 patients had type II submucosal fibroids. The average age of patients was 40.2 ± 6.7 years. The average body mass index (BMI) was 22.4 ± 3.1 kg/m2. Based on the signal intensity of fibroids on T2WI of MRI, 37 patients had hypointense, 22 patients had isointense, and 15 patients had hyperintense fibroids. The mean maximum diameter of fibroids was 5.7 ± 1.2 cm, the median volume of fibroids was 67.2 cm3 and the median volume of uterine was 258.7 cm3 before HIFU treatment ().
Table 1. Baseline characteristics of patients before HIFU.
Therapeutic results of HIFU
All patients successfully completed HIFU procedure without serious adverse effects or complications. The median sonication time was 710.5 s and the median treatment time was 72.5 min. The median NPV ratio achieved in fibroids was 90.5% ().
Table 2. Therapeutic parameters of HIFU.
We further compared therapeutic results according to the signal intensity of the fibroids. As shown in , the median NPV ratio was 92.9% in hypointense fibroids, it was 86.0% in isointense fibroids, and 89.0% in hyperintense fibroids. A significant difference was observed in NPV ratio between hypointense fibroids and isointense fibroids, but not between hypointense fibroids and hyperintense fibroids, as well as between isointense and hyperintense fibroids. We also didn’t find any significant difference in fibroid volume, treatment time, sonication time, sonication energy, and NPV among the submucosal fibroids with different signal intensity.
Table 3. Comparison of the Therapeutic results in fibroids with different MR signal intensity.
Comparison of fibroids and hemoglobin of patients who received hysteroscopic myomectomy at different time points after HIFU
These patients were offered HIFU as a pre-surgical. Before HIFU treatment, all patients were suggested to have hysteroscopic myomectomy at 1–3 months after HIFU. However, patients often made their own decisions to undergo hysteroscopic myomectomy at different time points based on their subjective reasons. Among these patients, 12 received hysteroscopic myomectomy at 0–1 month after HIFU, 19 received hysteroscopic myomectomy at 1–3 months after HIFU, 26 received hysteroscopic myomectomy at 3–6 months after HIFU, and 17 patients received hysteroscopic myomectomy at 6–12 months after HIFU. Therefore, these patients were divided into four groups based on the time points they received hysteroscopic myomectomy after HIFU. As shown in , the mean size of fibroids before HIFU was 5.9 ± 1.9 cm, 5.8 ± 1.0 cm, 5.5 ± 1.2 cm and 5.7 ± 0.9 cm in diameter in the groups of patients who received hysteroscopic myomectomy at 0–1 month, 1–3 months, 3–6 months, and 6–12 months after HIFU, respectively. The mean diameter of fibroids decreased to 3.8 ± 1.3 cm, 4.1 ± 1.0 cm, and 4.0 ± 0.9 cm in 1–3 months, 3–6 months, and 6–12 months after HIFU. The average shrinkage rate was 68.19 ± 19.86%, 61.10 ± 16.89%, and 63.76 ± 26.68% in 1–3 months, 3−6 months, and 6–12 months after HIFU, respectively. The average hemoglobin of patients in these four groups was 93.1 ± 19.0 g/L, 90.1 ± 20.3 g/L, 95.2 ± 22.2 g/L, and 91.2 ± 20.7 g/L, respectively. The hemoglobin level of patients before hysteroscopic myomectomy in these four groups increased to 95.3 ± 13.5 g/L, 102.1 ± 14.9 g/L, 101.1 ± 18.3 g/L, and 108.4 ± 12.3 g/L, respectively. The hemoglobin of patients in 1–3 months, 3–6 months and 6–12 months after HIFU was significantly higher than that of patients before HIFU treatment. All patients completed hysteroscopic myomectomy successfully without any serious adverse event. During the procedure, no intrauterine adhesion after HIFU was observed (). The fibroid was completely resected in 69 patients in one session of hysteroscopic myomectomy. Partial removal of the treated submucosal fibroids was achieved in the other 5 patients. The mean operation time was 66.66 ± 31.61 min, the median blood loss was 20 ml, and the median distention fluid deficit was 275 ml. No significant difference was observed in the operation time, blood loss and distention fluid deficit of hysteroscopic myomectomy in patients who received hysteroscopic myomectomy at 0–1 month, 1−3 months, 3–6 months, and 6–12 months after HIFU (p > 0.05) (). There was no significant difference in operation time, blood loss, and distention fluid deficit during the procedure of hysteroscopic myomectomy among the submucosal fibroids with different signal intensity of T2WI of MRI ().
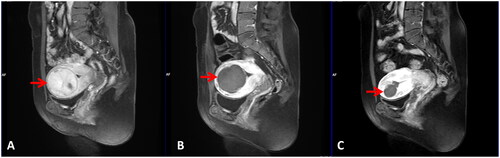
Figure 3. The procedure of hysteroscopic myomectomy for a 39-year-old patient with a large submucosal fibroid pretreated by HIFU. The operation time was 30 min. The distention fluid deficit was 150 ml and the blood loss was 20 ml. (A–C) Hysteroscopic images showed the surface of the treated fibroid before hysteroscopic myomectomy (red asterisks). (D) A hysteroscopic image showed the fibroid was resected completely (green asterisk).
Table 4. Comparison of fibroids and hemoglobin of patients who received hysteroscopic myomectomy at different time points after HIFU.
Table 5. Comparison of parameters of hysteroscopic myomectomy at different time points after HIFU.
Discussion
Currently, hysteroscopic myomectomy has become the treatment of choice for submucosal fibroids, with excellent success rate. However, the safety of this procedure remains the main concern in clinical practice [Citation8]. Lima et al. reported that the complication rate of hysteroscopic myomectomy was about 13%, and the surgical risk was associated with size and myometrial invasion of submucosal fibroids [Citation9]. Mazzon et al. showed that the size of submucosal fibroids was significantly associated with the successful resection rate of hysteroscopic myomectomy [Citation10]. A consensus statement from the global congress on hysteroscopy scientific committee indicated that it was difficult to completely resect the submucosal fibroids in a single hysteroscopic operation when the fibroid tumor was larger than 4 cm, and a second hysteroscopic operation was often performed [Citation6]. Therefore, pretreatment is usually required to reduce the size of large submucosal fibroids before hysteroscopic myomectomy.
GnRH-a is the most commonly used pre-operative medication before hysteroscopic myomectomy. Previous studies showed that GnRH-a could be used to reduce the blood supply and the size of fibroids by inhibiting the secretion of hormones, and it could be also used to reduce the thickness of endometrium to improve the visibility of endometrial cavity [Citation2, Citation11, Citation12]. However, the response of uterine fibroids to GnRH-a varies among patients. Mavrelos et al. reported that there was no significant difference in the complete resection rate of fibroids, operation time, fluid volumes infused, and complications between the GnRH-a and placebo groups [Citation13]. Meanwhile, some patients are unable to complete treatment due to the side effects of GnRH-a. In patients treated with GnRHa, they often reported headaches, hot flushes, mood swings [Citation14, Citation15]. In contrast, pre-operative HIFU has less adverse effects [Citation16].
Recently, Liao et al. compared the efficacy of HIFU and GnRH-a followed by hysteroscopic myomectomy in the treatment of type II submucosal fibroids with size larger than 4 cm in diameter [Citation7]. Their results showed that there was no significant difference in the fibroid volume reduction between patients pretreated with GnRH-a and patients pretreated with HIFU. There were also no significant differences in complications and complete resection rate of hysteroscopic myomectomy between the two groups. However, the mean operation time, blood loss and volume of the hysteroscopic fluid distention media in the HIFU group were significantly less than those in the GnRH-a group. In the present study, a total of 83 patients with submucosal fibroids who were not indicated for hysteroscopic myomectomy were pretreated with HIFU. Before HIFU treatment, the mean maximum diameter of fibroids was 5.7 ± 1.2 cm. All patients completed HIFU treatment without major adverse effects, and an average non-perfused volume ratio of 90.5% was achieved in the fibroids. We further compared the NPV ratio in submucosal fibroids with different MR signal intensity. The median NPV ratio was 92.9% in hypointense fibroids, it was 86.0% in isointense fibroids, and 89.0% in hyperintense fibroids. The median NPV ratio higher than 80% was achieved in every group of fibroids according to MR signal intensity. A significant difference was observed in NPV ratio between hypointense fibroids and isointense fibroids, but not between hypointense fibroids and hyperintense fibroids, as well as between isointense and hyperintense fibroids. Our results indicated that a higher than 80% of NPV ratio could also be achieved in hyperintense submucosal fibroids. Recently, Zhang et al. reported their results of 68 patients with type II submucosal fibroids treated with hysteroscopic myomectomy. The average size of the fibroids was 4.5 ± 0.9 cm, the intraoperative bleeding volume was 17.41 ± 4.49 ml. However, complete resection was not achieved in 6 patients because of the large tumor size and intraoperative liquid absorption [Citation17]. In our study, all the 74 patients completed hysteroscopic myomectomy successfully without any serious adverse event in different time points after HIFU. The mean operation time was 66.66 ± 31.61 min, the median blood loss was 20 ml and the median distention fluid deficit was 275 ml. The fibroid was completely resected in 69 out of 74 patients in one session of hysteroscopic myomectomy. In the other 5 patients, the treated submucosal fibroids were not completely removed. We reviewed these cases and found that the average NPV ratio of 78.1% was achieved, but the average largest diameter was 7.1 cm when the procedure of hysteroscopic myomectomy was performed. Although only achieved a partial removal of the treated submucosal fibroids, no further hysteroscopic myomectomy was required during the 3-year follow-up period.
In clinical practice, another concern is about intrauterine adhesion after HIFU treatment for submucosal fibroids. In this study, we did not observe intrauterine adhesion after HIFU during the procedure of hysteroscopic myomectomy (). Therefore, as long as the focal point is kept at a distance of more than 1.5 cm from the endometrium during HIFU treatment, the fibroid tissue near the capsule can be ablated through thermal diffusion to avoid overtreatment, and complete ablation of the fibroids can be achieved without damaging the endometrium (). Our results indicated that HIFU followed by hysteroscopic myomectomy is a safe and effective treatment for submucosal fibroids larger than 4 cm.
Previous studies have shown that the size of uterine fibroids can be reduced by 35–65%, using GnRH-a over three cycles. Thus, it is recommended to perform hysteroscopic myomectomy at this time point after administering GnRH-a over three cycles [Citation18–20]. What time is the best to undergo hysteroscopic myomectomy for patients with submucosal fibroids who had preoperative HIFU? A previous study reported that the absorption rate of necrotic fibroids at 3-, 6- and 12-month after HIFU was 54.7%, 62.5%, and 73.8%, respectively [Citation21]. In this study, 12 patients had hysteroscopic myomectomy within 1 month, 19 patients had hysteroscopic myomectomy in 1–3 months, 26 patients in 3–6 months, and 17 patients received hysteroscopic myomectomy in 6–12 months. The mean diameter of fibroids in patients who received hysteroscopic myomectomy at 1–3 months, 3–6 months, and 6–12 months after HIFU decreased to 3.8 ± 1.3 cm, 4.1 ± 1.0 cm, and 4.0 ± 0.9 cm, respectively. The shrinkage rates of fibroids at 1–3 months, 3–6 months, and 6–12 months after HIFU were 68.19 ± 19.86%, 61.10 ± 16.89%, and 63.76 ± 26.68%, respectively. Although the size of fibroids didn’t change much within 1 month after HIFU, the procedure of hysteroscopic myomectomy was completed safely. Also, we did not find any significant difference in the operation time, blood loss and distention fluid deficit among these four groups. This phenomenon may be explained by that coagulation necrosis of the submucosal fibroids makes resection easier because it significantly reduced the risk of intraoperative bleeding. Based on our results, we believed that pre-surgical HIFU treatment for large submucosal fibroids decreased the risk of hysteroscopic myomectomy not only by reducing the size, but also by coagulation necrosis of the submucosal fibroids. Therefore, our results indicated that hysteroscopic myomectomy could be performed safely at any time after HIFU to improve the symptoms of patients.
This study is limited because it is retrospective and has no comparisons with other treatment groups such as hysteroscopic myomectomy alone or pre-operative medication combined with hysteroscopic myomectomy. This study is also limited because of the small sample size at each time points of hysteroscopic myomectomy after HIFU. Future studies with large number of subjects and a comparison with other treatment groups are needed.
Conclusions
HIFU could be used as a pre-operative treatment for patients with large submucosal fibroids who intend to have hysteroscopic myomectomy. Based on our results, hysteroscopic myomectomy could be safely performed at any time point after HIFU to improve the symptoms of patients. Future studies with large sample size are needed to confirm these findings.
Disclosure statement
Lian Zhang is senior consultant to Chongqing Haifu. The other authors have no potential conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
Data availability statement
The data that support the findings of this case report are available upon request from the corresponding author, L.Z. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
Additional information
Funding
References
- Sparic R, Mirkovic L, Malvasi A, et al. Epidemiology of uterine myomas: a review. Int J Fertil Steril. 2016;9:424–435.
- Di Spiezio Sardo A, Mazzon I, Bramante S, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update. 2008;14(2):101–119. doi: 10.1093/humupd/dmm041.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25(2):199–208. doi: 10.1016/j.jmig.2017.08.009.
- Vitale SG, Sapia F, Rapisarda AMC, et al. Hysteroscopic morcellation of submucous myomas: a systematic review. Biomed Res Int. 2017;2017:6848250. doi: 10.1155/2017/6848250.
- Bhave Chittawar P, Franik S, Pouwer AW, et al. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;10:CD004638.
- Lagana AS, Alonso Pacheco L, Tinelli A, et al. Management of asymptomatic submucous myomas in women of reproductive age: a consensus statement from the global congress on hysteroscopy scientific committee. J Minim Invasive Gynecol. 2019;26(3):381–383. doi: 10.1016/j.jmig.2018.06.020.
- Liao P, Jiang J, Zeng Y, et al. Comparison of outcomes of hysteroscopic myomectomy of type 2 submucous fibroids greater than 4 cm in diameter via pretreatment with HIFU or GnRH-a. Int J Hyperthermia. 2021;38(1):183–188. doi: 10.1080/02656736.2021.1874546.
- Camanni M, Bonino L, Delpiano EM, et al. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol. 2010;17(1):59–65. doi: 10.1016/j.jmig.2009.10.013.
- Lima MPJS, Costa-Paiva L, Brito LGO, et al. Factors associated with the complications of hysteroscopic myomectomy. Rev Bras Ginecol Obstet. 2020;42(8):476–485. doi: 10.1055/s-0040-1713915.
- Mazzon I, Favilli A, Grasso M, et al. Risk factors for the completion of the cold loop hysteroscopic myomectomy in a One-Step procedure: a post hoc analysis. Biomed Res Int. 2018;2018:8429047. doi: 10.1155/2018/8429047.
- Muzii L, Boni T, Bellati F, et al. GnRH analogue treatment before hysteroscopic resection of submucous myomas: a prospective, randomized, multicenter study. Fertil Steril. 2010;94(4):1496–1499. doi: 10.1016/j.fertnstert.2009.05.070.
- Lethaby A, Puscasiu L, Vollenhoven B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst Rev. 2017;11(11):CD000547.
- Mavrelos D, Ben-Nagi J, Davies A, et al. The value of pre-operative treatment with GnRH analogues in women with submucous fibroids: a double-blind, placebo-controlled randomized trial. Hum Reprod. 2010;25(9):2264–2269. doi: 10.1093/humrep/deq188.
- Bizzarri N, Ghirardi V, Remorgida V, et al. Three-month treatment with triptorelin, letrozole and ulipristal acetate before hysteroscopic resection of uterine myomas: prospective comparative pilot study. Eur J Obstet Gynecol Reprod Biol. 2015;192:22–26. doi: 10.1016/j.ejogrb.2015.06.018.
- Osuga Y, Enya K, Kudou K, et al. Oral Gonadotropin-Releasing hormone antagonist relugolix compared with leuprorelin injections for uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2019;133(3):423–433.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676. doi: 10.1016/j.ultsonch.2015.05.031.
- Zhang R, Wu W, Zou Q, et al. Comparison of clinical outcomes and postoperative quality of life after surgical treatment of type II submucous myoma via laparoscopy or hysteroscopy. J Int Med Res. 2019;47(9):4126–4133. doi: 10.1177/0300060519858027.
- Di Lieto A, De Falco M, Pollio F, et al. Clinical response, voscular change, and angiogenesis in gonadotropin-releasing hormone analogue-treated women with uterine myomas. J Soc Gynecol Investig. 2005;12(2):123–128. doi: 10.1016/j.jsgi.2004.10.008.
- Di Lieto A, De Falco M, Mansueto G, et al. Preoperative administration of GnRH-a plus tibolone to premenopausal women with uterine fibroids:evaluation of the clinical response, the immunohistochemical expression of PDGF.bFGF and VEGF and the vascular pattern. Steroids. 2005;70(2):95–102. doi: 10.1016/j.steroids.2004.10.008.
- Palomba S, Orio F, Jr, Russo T, et al. Long -term effectiveness and safety of GnRH agonist plus raloxirene administration in women with uterine leiomyomas. Hum Reprod. 2004;19(6):1308–1314. doi: 10.1093/humrep/deh296.
- Lee JS, Hong GY, Lee KH, et al. Safety and efficacy of ultrasound-guided high-intensity focused ultrasound treatment for uterine fibroids and adenomyosis. Ultrasound Med Biol. 2019;45(12):3214–3221. doi: 10.1016/j.ultrasmedbio.2019.08.022.