Abstract
Thermal ablation (TA) has harvested favorable outcomes in treating low-risk papillary thyroid microcarcinoma (PTMC). Preoperative assessment, intraoperative procedures and postoperative follow-up are all closely linked with the success and safety of TA on PTMC. However, many details in these aspects have not been systematically reviewed. This review firstly described the influence of preoperative assessment, especially for the risk of lymph node metastasis (LNM), as well as the molecular testing on the selection of TA for PTMC. Besides, we also summarized the experiences in treating special PTMC cases by TA, like multifocal lesions, PTMC located in the isthmus or adjacent to the dorsal capsule. At last, we discussed the follow-up strategies, the influence of the thyroid-stimulating hormone (TSH) level on the prognosis of PTMCs, and the management for recurrent cases. In conclusion, the procedures during the entire perioperative period should be standardized to improve the outcomes of TA in treating PTMC patients.
Introduction
Papillary thyroid microcarcinoma (PTMC), with a maximum diameter of 1 cm, has become the most common neoplasm often with a good prognosis [Citation1]. Although no longer considered as a subtype of papillary thyroid cancer in the 2022 World Health Organization Classification of Thyroid Tumors [Citation2], PTMC is still managed based on its size in clinical practice. Generally, low-risk PTMC is defined as that without evidence of clinical lymph node metastasis (LNM) or distant metastasis, invasion to the adjacent trachea or the recurrent laryngeal nerve, and aggressive subtypes of papillary thyroid carcinoma on cytology, etc [Citation3, Citation4]. Surgery is recommended for low-risk PTMC, although the affected patients often need a lifelong medication after surgery. At present, active surveillance (AS) is recommended as a reliable alternative to surgery for low-risk PTMC, which, however, may cause the risk of tumor progression and LNM [Citation4]. Thermal ablation (TA), represented by laser ablation (LA), radiofrequency ablation (RFA) and microwave ablation (MWA), has been used as an effective and safe technique to treat low-risk PTMCs [Citation5, Citation6].
Currently, some issues in the management of low-risk PTMCs by TA remain to be resolved. For example, it is controversial about whether preoperative assessment via cytologic evaluation, molecular testing, contrast-enhanced computed tomography (CT) and other examinations can precisely diagnose PTMCs and exclude LNM. The efficacy of TA in special PTMC cases with multiple foci or those located in the isthmus requires a thorough exploration. Post-TA follow-up by thyroid ultrasound and TSH suppression therapy for PTMC patients have not been standardized. In this review, we detailed the preoperative diagnosis, perioperative procedures and postoperative follow-up of PTMC patients managed by TA.
Preoperative assessment
Preoperative diagnosis
Ultrasonography is the most sensitive method for detecting thyroid nodules. Based on several ultrasound features, the Thyroid Imaging Reporting and Data System (TI-RADS) has been widely adopted to predict the risk of malignancy. Generally, thyroid nodules of TI-RADS 5 have the highest risk of malignancy [Citation7], and this risk may vary slightly across different versions of TI-RADS [Citation8–10]. However, TI-RADS is unable to precisely diagnose thyroid cancer. The fine needle aspiration cytology (FNAC) is often introduced for preoperative pathological diagnosis of thyroid nodules. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) is a useful tool to standardize the cytological reporting of FNAC. The malignant risks of class VI and V thyroid nodules in the latest TBSRTC are 97% (97%-100%) and 74% (67%-83%), respectively [Citation11]. Therefore, the inclusion of TBSRTC V thyroid nodules may underestimate the post-TA recurrence rate of PTMCs in the real world.
BRAFV600E mutation is often used as a diagnostic marker of PTC, with a specificity of 100% and a sensitivity of 69% [Citation12]. Therefore, the BRAFV600E mutation seems to be a diagnostic marker of PTMC as well, although it can also be detected in 30% of poorly differentiated thyroid carcinomas (PDTCs) and 40% of anaplastic thyroid cancer (ATC) [Citation13]. The prognostic potential of BRAFV600E mutation in PTMCs remains controversial. Lin et al. [Citation14] have suggested that the BRAFV600E mutation is correlated with an increased risk of LNM in patients with PTC or even PTMCs. On the contrary, Zheng et al. [Citation15] did not identify a significant correlation between BRAFV600E mutation and the relapse of PTMCs. TA is considered as an effective option for PTMC. The prognosis is acceptable in low-risk PTMC patients carrying BRAFV600E mutation and managed by TA [Citation16]. Compared with wild-type BRAF PTMC, PTMC carrying BRAFV600E mutation is more aggressive and requires an early treatment, like minimally invasive ablation [Citation17].
The application of core needle biopsy (CNB) prior to TA for PTMC has not been reported yet. CNB is featured by rapid diagnosis based on a plenty of tumor tissues [Citation18]. Unlike thyroidectomy, TA is unable to provide postoperative pathological reporting. Therefore, preoperative CNB can be applied to PTMC patients willing to obtain a diagnosis report.
Multifocal PTMC
Multifocality is considered as a risk factor for the LNM of PTMC [Citation19]. Compared with that of bilateral multifocal PTMC (a single focus in each lobe), the risk of cervical LNM is much higher in patients with unilateral multifocal PTMC [Citation20]. A meta-analysis has reported that the multifocal thyroid cancer is highly likely to recur [Citation21]. Hence, TA may not be suitable for patients with multifocal PTMCs.
Nagaoka et al. have reported that the 10-year rates of tumor enlargement (11.4% vs. 14.8%) and LNM development (1.1% vs. 2.4%) are comparable between unifocal and multifocal low-risk PTMC patients during AS [Citation22]. However, the main, usually biggest, tumor (index tumor) and others (64.6% of non-index tumors) are confirmed by FNAC and high-suspicious sonographic features, respectively, which may overestimate the feasibility of AS for multifocal PTMCs. In a cohort involving 44 RFA-treated and 53 surgically treated patients with unilateral multifocal PTMCs who had been followed up for five years or longer, no significant differences in disease progression rate, LNM rate, persistent lesion rate and recurrence-free survival (RFS) are found between groups [Citation23]. Yu et al. have reported that the progression-free survivals (PFSs) at 6, 12, 24, 36 and 48 months of MWA in patients with multifocal PTMCs are 100.0%, 96.4%, 96.4%, 70.3% and 52.7%, respectively, and the disease progression rate is 11.4% [Citation24]. Therefore, MWA is an optional treatment for multifocal PTMCs with less than three foci.
PTMCs in the isthmus
Thyroid tumors located in the isthmus are more aggressive and prone to capsular invasion and LNM [Citation25]. The incidences of LNM and capsular invasion in patients with PTMCs located in the isthmus may rise to 50% and 64.7%, respectively [Citation26]. Notably, a wider-than-tall shape is a typical ultrasound feature of PTC with LNM and capsular invasion, due to the narrow space for the isthmus to grow [Citation27]. Zheng et al. have revealed that the efficacy of TA on PTMC in the isthmus reaches 100%, and cases of recurrence, LNM and severe complications are not reported [Citation28]. During the process of TA, hydrodissection and moving-shot technique can be carefully performed to ensure safety.
PTMCs adjacent to the dorsal capsule
AS is not recommended to patients with PTMCs adjacent to the dorsal capsule, due to the risk of capsular invasion, and they can be managed by TA. A multicenter cohort study has compared the feasibility, efficacy, and safety of MWA in the treatment of PTMCs with either capsular invasion determined by ultrasound or not [Citation29]. It is shown that the success rate (99% vs. 100%), incidence of complications (1% vs. 3%) and disease progression rate (2% vs. 1%) are comparable between groups, suggesting the acceptable short-term outcomes of TA for PTMCs adjacent to the dorsal capsule.
Assessment of LNM
High-resolution ultrasonography is the first choice for preoperative noninvasive assessment of cervical lymph nodes. Wang et al. have demonstrated that the sensitivity and specificity of ultrasound in diagnosing central LNM of PTMCs are 73.3% and 64.2%, respectively [Citation30]. Xing et al. have consistently found the high specificity of ultrasound and high sensitivity of CT in diagnosing the central, lateral and cervical LNM [Citation31]. Collectively, ultrasound helps to prevent the misdiagnosis, and CT reduces the rate of missed diagnosis. Through early enhancement in metastatic lymph nodes, preoperative ultrasound combined with contrast-enhanced CT can visualize lymph nodes that do not show up on ultrasound scans, including those in the retropharyngeal, retrosternal region, and mediastinal region [Citation32].
However, contrast-enhanced CT may increase the risk of thyroid dysfunction by 2-3 times [Citation33]. We found that the incidences of thyroid dysfunction at 3, 6, 12, and 24 months of MWA for PTMC, when preoperatively examined by contrast-enhanced CT, are 12.84%, 15.19%, 18%, and 4.5%, respectively, all significantly higher than those of TA for benign thyroid nodules [Citation34, Citation35]. As a result, the European Thyroid Association (ETA) has recommended a regular follow-up for thyroid function after the use of iodine-based contrast [Citation36].
Examination of the vocalization and throat
TA may cause transient or permanent damages to the recurrent laryngeal nerve and superior laryngeal nerve. A 5-year, single-institution cohort study showed that four PTMC patients developed hoarseness after MWA and during the follow-up period. Among them, three patients recovered within one week, and the remaining one recovered six months later [Citation35]. In a cohort involving 715 PTMC patients managed by TA, voice change, as a major complication, was reported in 11 patients, including 10 with transient voice change for a minimum of 1 month, and 1 with permanent voice change. In addition, immediate hoarseness occurred in 7 patients, and all resolved within 10 min to 1 week postoperatively [Citation37]. Therefore, we recommended the examination of vocalization prior to TA, at least by inquiring patients about their voice abnormalities or changes. This may help to exclude recurrent laryngeal neuropathy. Fiberoptic laryngoscopy through the nose or mouth is the gold standard for assessing the vocal cord motility in people with voice abnormalities [Citation38].
Perioperative procedures of TA
Complete ablation
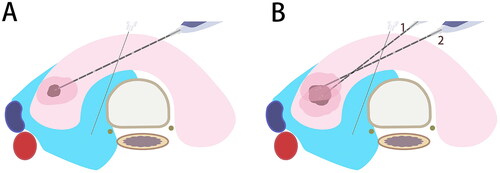
The goal of TA is to reduce the thyroid nodule volume by irreversible coagulation necrosis [Citation38]. The fixed-needle technique combined with the moving-shot technique can achieve layer-by-layer, point-by-point ablation of lesions and surrounding tissues [Citation39]. Hydrodissection prior to TA is a useful technique to expand the ablation area and prevent the potential damage to adjacent tissues simultaneously. Vaporization is observed around the probe or electrode during the ablation, and the replacement of transient hyperechoic area by complete hyperechoic area in the tumor lesions indicates a complete ablation ( and ). The efficacy of TA is usually assessed by ultrasound. A complete ablation is defined as no contrast perfusion into the ablation zone, with a minimum safety margin of 3 mm [Citation40]. Tuttle et al. have proposed a safe margin of at least 2 mm outside the tumor lesion [Citation41]. However, the ablation area has not been determined for a complete destruction of malignant thyroid nodules. The initial ablation ratio (IAR), defined as the ratio of the ablation area measured by contrast-enhanced ultrasonography on the day or the next day of MWA to preoperative nodular volume, is an indicator to predict the curative effect of benign thyroid nodules after TA. Our latest study revealed that PTMC patients with an IAR of 15 or above faced a low risk of recurrence [Citation35]. Generally, the fixed-needle technique is sufficient to completely ablate small-volume PTMC (). For large-volume PTMC, the fixed-needle technique combined with moving-shot technique is recommended to achieve a layer-by-layer, point-by-point complete ablation (). Layers or points of ablated tissues can be appropriately added based on the site and size of lesions, thus ensuring a complete ablation.
Figure 1. Schematic diagram of microwave ablation for papillary thyroid microcarcinomas. A. Anatomical structure of the neck; B. Normal saline is injected as a spacer fluid to separate the normal tissues from the ablation area; C. The needle or antenna is inserted into the tumor lesion; D. An extended ablation area is introduced.
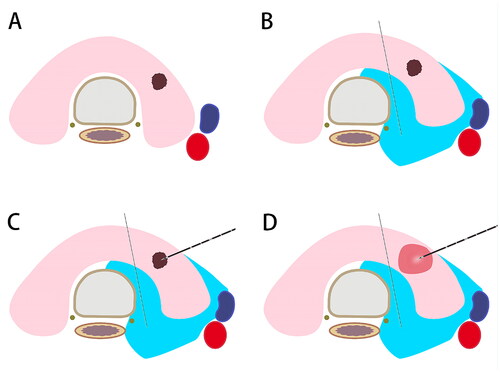
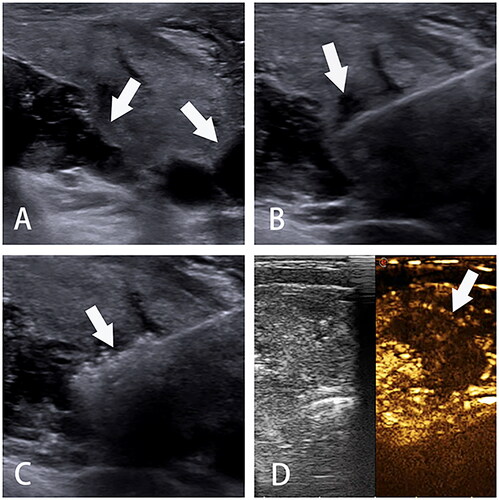
Figure 2. Ultrasonographic imaging of TA. A. Hydrodissection is performed to build up a barrier to protect normal tissues from heat injury; B. The needle or antenna is percutaneously inserted into the PTMC; C. The power turns off with tissue degeneration; D. Contrast-enhanced ultrasonography after ablation with a filling defect. TA, thermal ablation. PTMC, papillary thyroid microcarcinoma.
PTMCs adjacent to dorsal capsule
Some special perioperative procedures of TA are required for PTMCs adjacent to dorsal capsule. To prevent potential thermal injury to normal tissues, it is suggested that normal saline should be continuously injected into the space between isthmus and trachea for hydrodissection during TA [Citation42]. Consistently, the Korean Society of Thyroid Radiology (KSTHR) recommends the formation of a 10-mm isolation or larger via injecting normal saline into the isthmus and peritracheal soft tissues during TA to prevent thermal injury [Citation43]. After the hyperechoic ablation zone has covered the entire tumor lesion and with a 2-mm ring outer the tumor margin (including the adjacent thyroid capsule), TA is terminated.
Multifocal PTMCs
A thorough preoperative assessment for multifocal PTMCs prior to TA helps to identify the number and site of tumor lesions, but also determine the optimal approach for ablation [Citation44]. Hyperechoic areas during ablation can mask inferior structures, and therefore, deep lesions should be initially ablated. Both the trans-isthmic approach and hydrodissection contribute to preventing heat injury during TA for multifocal PTMCs. In addition, mastering a perfect ultrasonic understanding about the neck anatomy is critically important to prevent nerve damages.
Hydrodissection
Continuous hydrodissection is the key for effectively and safely extending the ablation for PTMCs, especially those adjacent to the trachea, esophagus, internal carotid artery, and thyroid capsule [Citation16]. Generally, the first injection is performed between the thyroid capsule and trachea, with the following one between the thyroid capsule and the anterior strap muscles [Citation28]. The KSTHR recommends the administration of lidocaine and normal saline into the isthmus and peritracheal soft tissues, to control pain and prevent heat injury [Citation43]. The administration of 5% dextrose dissolved in cold water (4 °C), which is non-conductive, is effective to provide a thermal barrier isolating the target organ [Citation45]. Because of its large density, optimal elasticity and long absorption time (>2 h), sodium hyaluronate is also used as a type of fluid to isolate tissues during TA and prevent postoperative adhesions.
Differences in TA techniques
A meta-analysis has shown that the mean volume reduction ratio (VRR) of PTMCs managed by RFA is significantly higher than those of MWA and LA (99.3% vs. 95.3% vs. 88.6%, p < 0.001) [Citation37]. Meanwhile, the pooled incidence of major complications is higher in PTMC patients managed by MWA than in those managed by RFA and LA, although a difference is non-detectable. Tong et al. have reported that RFA, MWA and LA all reduce the volume of PTMC, achieving comparable proportions of complete disappearance (76.2% vs. 62.9% vs. 57.3%, p > 0.05), postoperative recurrence (0.01% vs. 0.85% vs. 1.87%, p > 0.05) and complications (1.7% vs. 6.0% vs. 0.92%, p > 0.05) [Citation46]. Collectively, RFA, MWA and LA are all effective and safe for ablating PTMCs.
Postoperative follow-up
Follow-up examinations
After TA, PTMC patients can be followed up through regular clinical, radiographic, and biochemical monitoring of treatment-related events and thyroid function. The therapeutic efficacy of TA on PTMCs is assessed according to VRR, complete resolution of malignant lesions, and long-term tumor progression [Citation38]. However, follow-up strategies for PTMCs after TA are controversial. Generally speaking, follow-up visits are arranged at 1, 3, 6 and 12 months postoperatively, and then every 6 months [Citation43, Citation47, Citation48]. Ultrasonography is the most recommended follow-up tool to assess the efficacy, newly developed thyroid cancer and abnormal lymph nodes. The value of reexamination CT in follow-up remains unclear.
Guidelines are currently scant for carrying out post-TA pathological analysis after the complete ablation by CNB. Our previous data have revealed that fibroblastic proliferation and chronic inflammation are the most common pathological characteristics detected by CNB at 3 and 6 months after MWA in 42 PTMC patients [Citation49]. In addition, local residual and recurrent tumors are not identified by CNB. Postoperative CNB seems ineffective to manage PTMC after TA. On the contrary, in a cohort of 202 patients with 211 low-risk PTMCs, three cases of tumor cell residual are identified by CNB at 3 or 6 months of RFA, all managed by reoperation of TA [Citation50].
TSH suppression therapy
TSH level is correlated with the malignant risk of thyroid nodules and postoperative recurrence of differentiated thyroid cancer. So far, the influence of TSH level on the progression of thyroid cancer during AS remains debated [Citation51–53]. The outcomes of TSH suppression therapy to low-risk PTMC patients after TA have not been comprehensively analyzed. Our previous data have reported that RFS is comparable between MWA-treated PTMC patients with preoperative TSH ≥2 mU/L and <2 mU/L [Citation35]. Li et al. have recruited PTC patients managed by RFA and divided them into low-level TSH group (<2 mU/L) and high-level TSH group (≥2 mU/L) by propensity score matching [Citation54]. There are no significant differences in the local tumor progression (6.6% vs. 9.4%), 1-year disease-free survival (DFS, 97.2% vs. 100%), 3-year DFS (95.3% vs. 92.5%) and 5-year DFS (93.3% vs. 90.6%) between groups.
Management of recurrence: AS, re-ablation or surgery
Due to the potential risk of occult carcinoma and LNM in PTMC patients, TA may cause an incomplete ablation of primary thyroid cancer that requires a re-operation. Zhu et al. have reported that at 18 and 30 months after RFA, ipsilateral cervical LNM develops in 2/102 (1.9%) of PTMC patients, one in the region IV and the other in VI [Citation55]. Both are surgically treated, and recurrence is not reported during the follow-up period. Our previous study has shown that 6/7 of recurrent PTMC patients after TA are managed by delayed surgery, and adhesions or inflammatory changes caused by the initial ablation are not found [Citation35]. Recurrent cases and deaths are not reported after delayed surgery. The remaining patient is managed by AS. Collectively, delayed surgery in recurrent PTMC patients after TA may not influence the overall survival.
Re-ablation is also an option for recurrent PTMC. In a cohort involving 414 unifocal low-risk PTMC patients having been followed up for 42.15 ± 11.88 months, the proportions of complete disappearance of tumors and local tumor progression are 88.41% and 3.62%, respectively [Citation56]. Among them, 1 (0.24%) case of residual carcinoma, 4 (0.97%) cases of LNM and 10 (2.42%) cases of recurrence are detected by CNB. A total of 13 PTMC patients are managed by another round of RFA, and the complete ablation is achieved in 11 patients during the follow-up period. A relevant meta-analysis has demonstrated that recurrent PTMC patients after TA are all treated with re-ablation or delayed surgery, and deaths or distant metastases are not reported [Citation46].
Choice for low-risk PTMC: AS or TA
Overdiagnosis and overtreatment of PTMC have been concerned in recent years. Usually, low-risk PTMC has an acceptable prognosis and requires no surgical procedures. AS is an effective method recommended to patients with very low-risk PTMCs in guidelines [Citation57]. In a study of 5646 patients performed by Miyauchi et al. 57.1% chose AS with a median follow-up 7.3 (1.0, 29.3) years, and 42.9% chose immediate surgery with a median follow-up 11.9 (1.0, 29.3) years. In the AS group, 124 patients (3.8%) had tumor enlargement, and the 10- and 20-year enlargement rates were 4.7% and 6.6%, respectively. The rate of lymph node metastasis was significantly higher in the AS group than in the IS group (1.1% vs. 0.4% and 1.7% vs. 0.7% at 10 and 20 years, respectively; p = 0.009), but the differences were small [Citation58].
However, in the application of AS, the effects of repeated ultrasounds, biopsies, and blood sampling, as well as psychological distress of living with a known cancer should be assessed [Citation59]. A prospective study has found that psychological distress and sleep disturbance worsen in patients with suspicious malignant tumors after treatment with AS [Citation60]. It is worth noting that PTMC patients managed by AS experience fewer health-related problems than those treated with surgery [Citation61]. Several studies have confirmed the efficacy and safety of TA in the treatment of low-risk PTMC (). Compared with AS, TA has similar follow-up intervals and contents, but inactivated the original tumor. TA is suitable for low-risk thyroid cancer, or those patients who are suitable for AS but willing to actively treat their cancer [Citation59]. Hence, TA may serve as an alternative to AS and surgery for low-risk PTMC (). However, whether TA can improve the quality of life of low-risk PTMC patients needs further evidence.
Figure 4. Flow chart for the multidisciplinary management of PTMCs. Surgery is preferred to high-risk PTMCs if feasible; otherwise, they can be managed by as or TA based on patients’ desire, tumor features and medical resources, etc. Surgery is still preferred to PTMC patients with tumor progression or recurrence detected during as after TA. PTMC, papillary thyroid microcarcinoma; as, active surveillance; TA, thermal ablation; MWA, microwave ablation; LA, laser ablation; RFA, radiofrequency ablation.
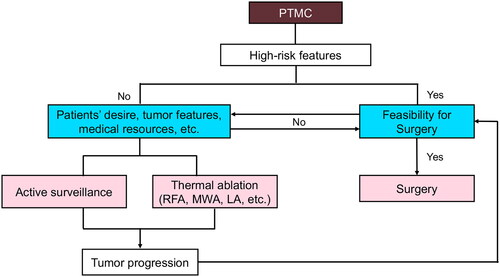
Table 1. Characteristics of selected studies (2018–2023).
Conclusions
The efficacy and safety of TA in the treatment of low-risk PTMCs have been well validated, and TA is now a popular management option for thyroid cancer. Preoperative assessment, perioperative procedures and postoperative follow-up are all closely linked with the efficacy and safety of TA for PTMC. Rigorous prospective studies are needed to analyze their details in the future, thus standardizing the clinical application of TA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33(1):27–63. doi:10.1007/s12022-022-09707-3.
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. doi:10.1089/thy.2013.0367.
- Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult Low-Risk papillary thyroid microcarcinoma: consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31(2):183–192. doi:10.1089/thy.2020.0330.
- Xu D, Ge M, Yang A, et al. Expert consensus workshop report: Guidelines for thermal ablation of thyroid tumors (2019 edition). J Can Res Ther. 2020;16(5):960–966. doi:10.4103/jcrt.JCRT_558_19.
- Mauri G, Hegedus L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197. doi:10.1159/000516469.
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892–899. doi:10.1148/radiol.11110206.
- Zhou J, Yin L, Wei X, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020;70(2):256–279. doi:10.1007/s12020-020-02441-y.
- Russ G, Bonnema SJ, Erdogan MF, et al. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237. doi:10.1159/000478927.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and Imaging-Based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370–395. doi:10.3348/kjr.2016.17.3.370.
- Ali SZ, Baloch ZW, Cochand-Priollet B, et al. The 2023 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2023;12(5):319–325. doi:10.1016/j.jasc.2023.05.005.
- Fnais N, Soobiah C, Al-Qahtani K, et al. Diagnostic value of fine needle aspiration BRAF(V600E) mutation analysis in papillary thyroid cancer: a systematic review and meta-analysis. Hum Pathol. 2015;46(10):1443–1454. doi:10.1016/j.humpath.2015.06.001.
- Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci. 2021;22(4):1726. doi:10.3390/ijms22041726.
- Lin KL, Wang OC, Zhang XH, et al. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. 2010;17(12):3294–3300. doi:10.1245/s10434-010-1129-6.
- Zheng X, Wei S, Han Y, et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013;20(7):2266–2273. doi:10.1245/s10434-012-2851-z.
- Lin Y, Wu ZR, Shi YP, et al. Radiofrequency ablation of unifocal papillary thyroid microcarcinoma with BRAF V600E mutation. J Clin Endocrinol Metab. 2023;108(11):e1298–e1305. doi:10.1210/clinem/dgad269.
- Attia AS, Hussein M, Issa PP, et al. Association of BRAF(V600E) mutation with the aggressive behavior of papillary thyroid microcarcinoma: a Meta-Analysis of 33 studies. Int J Mol Sci. 2022;23(24):15626. doi:10.3390/ijms232415626.
- Lan L, Luo Y, Zhou M, et al. Comparison of diagnostic accuracy of thyroid cancer with ultrasound-guided fine-needle aspiration and core-needle biopsy: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:44. doi:10.3389/fendo.2020.00044.
- Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. 2012;97(4):1250–1257. doi:10.1210/jc.2011-1546.
- Cai J, Fang F, Chen J, et al. Unilateral multifocality and bilaterality could be two different multifocal entities in patients with papillary thyroid microcarcinoma. Biomed Res Int. 2020;2020:9854964.
- Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta-analysis. Int J Surg. 2018;50:121–125. doi:10.1016/j.ijsu.2017.12.035.
- Nagaoka R, Ebina A, Toda K, et al. Multifocality and progression of papillary thyroid microcarcinoma during active surveillance. World J Surg. 2021;45(9):2769–2776. doi:10.1007/s00268-021-06185-2.
- Yan L, Zhen Y, Li Y, et al. Five-year outcome between radiofrequency ablation vs. Surgery for unilateral multifocal papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2023;15:dgad360. doi:10.1210/clinem/dgad360.
- Yu XY, Zhou HD, Wei Y, et al. Preliminary study of microwave ablation for multifocal papillary thyroid microcarcinoma in nonoperative candidates. J Vasc Interv Radiol. 2023;34(6):999–1006. doi:10.1016/j.jvir.2023.01.035.
- Chai YJ, Kim SJ, Choi JY, et al. Papillary thyroid carcinoma located in the isthmus or upper third is associated with Delphian lymph node metastasis. World J Surg. 2014;38(6):1306–1311. doi:10.1007/s00268-013-2406-x.
- Luo H, Yan F, Lan L, et al. Ultrasonographic features, nodule size, capsular invasion, and lymph node metastasis of solitary papillary carcinoma of thyroid isthmus. Front Oncol. 2020;10:558363. doi:10.3389/fonc.2020.558363.
- Karatzas T, Charitoudis G, Vasileiadis D, et al. Surgical treatment for dominant malignant nodules of the isthmus of the thyroid gland: a case control study. Int J Surg. 2015;18:64–68. doi:10.1016/j.ijsu.2015.04.039.
- Zheng L, Liu FY, Yu J, et al. Thermal ablation for papillary thyroid microcarcinoma located in the isthmus: a study with 3 years of follow-up. Future Oncol. 2022;18(4):471–480. doi:10.2217/fon-2021-0463.
- Zheng L, Dou JP, Han ZY, et al. Microwave ablation for papillary thyroid microcarcinoma with and without US-detected capsule invasion: a multicenter prospective cohort study. Radiology. 2023;307(3):e220661. doi:10.1148/radiol.220661.
- Wang Y, Nie F, Wang G, et al. Value of combining clinical factors, conventional ultrasound, and Contrast-Enhanced ultrasound features in preoperative prediction of Central lymph node metastases of different sized papillary thyroid carcinomas. Cancer Manag Res. 2021;13:3403–3415. doi:10.2147/CMAR.S299157.
- Xing Z, Qiu Y, Yang Q, et al. Thyroid cancer neck lymph nodes metastasis: meta-analysis of US and CT diagnosis. Eur J Radiol. 2020;129:109103. doi:10.1016/j.ejrad.2020.109103.
- Baek JH, Cho SJ. Thermal ablation for small papillary thyroid cancer: a potential game changer. Radiology. 2021;300(1):217–218. doi:10.1148/radiol.2021210424.
- Rhee CM, Bhan I, Alexander EK, et al. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172(2):153–159. doi:10.1001/archinternmed.2011.677.
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: a systematic review and meta-analysis. Endocrine. 2020;67(1):35–43. doi:10.1007/s12020-019-02019-3.
- Ren Y, Han X, Li Y, et al. Initial ablation ratio predicting the recurrence of low-risk papillary thyroid microcarcinomas treated with microwave ablation: a 5-year, single-institution cohort study. Endocr Connect. 2023;12(9):e230128. doi:10.1530/EC-23-0128.
- Bednarczuk T, Brix TH, Schima W, et al. 2021 European thyroid association guidelines for the management of iodine-based contrast media-induced thyroid dysfunction. Eur Thyroid J. 2021;10(4):269–284. doi:10.1159/000517175.
- Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and Meta-Analysis. Thyroid. 2020;30(5):720–731. doi:10.1089/thy.2019.0707.
- Orloff LA, Noel JE, Stack BC, Jr., et al. Radiofrequency ablation and related ultrasound‐guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. 2022;44(3):633–660. doi:10.1002/hed.26960.
- Teng DK, Li WH, Du JR, et al. Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid. 2020;30(12):1752–1758. doi:10.1089/thy.2020.0049.
- Song Q, Gao H, Ren L, et al. Radiofrequency ablation versus total thyroidectomy in patients with papillary thyroid microcarcinoma located in the isthmus: a retrospective cohort study. Int J Hyperthermia. 2021;38(1):708–714. doi:10.1080/02656736.2021.1916625.
- Tuttle RM, Li D, Ridouani F. Percutaneous ablation of low-risk papillary thyroid cancer. Endocr Relat Cancer. 2023;30(3):e220244. doi:10.1530/ERC-22-0244.
- Wu J, Zhao ZL, Cao XJ, et al. A feasibility study of microwave ablation for papillary thyroid cancer close to the thyroid capsule. Int J Hyperthermia. 2021;38(1):1217–1224. doi:10.1080/02656736.2021.1962549.
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid radiofrequency ablation guideline: Korean society of thyroid radiology. Korean J Radiol. 2018;19(4):632–655. doi:10.3348/kjr.2018.19.4.632.
- Yan L, Zhang M, Song Q, et al. Clinical outcomes of radiofrequency ablation for multifocal papillary thyroid microcarcinoma versus unifocal papillary thyroid microcarcinoma: a propensity-matched cohort study. Eur Radiol. 2022;32(2):1216–1226. doi:10.1007/s00330-021-08133-z.
- Chung SR, Baek JH, Choi YJ, et al. Thermal ablation for the management of papillary thyroid microcarcinoma in the era of active surveillance and hemithyroidectomy. Curr Oncol Rep. 2022;24(8):1045–1052. doi:10.1007/s11912-022-01268-2.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Yan L, Li Y, Li XY, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for solitary T1N0M0 papillary thyroid carcinoma: a retrospective study with more than 5 years of follow-up. Cancer. 2023;129(16):2469–2478. doi:10.1002/cncr.34802.
- Yan L, Zhang M, Song Q, et al. Ultrasound-guided radiofrequency ablation versus thyroid lobectomy for low-risk papillary thyroid microcarcinoma: a propensity-matched cohort study of 884 patients. Thyroid. 2021;31(11):1662–1672. doi:10.1089/thy.2021.0100.
- Lu C, Li X, Chu X, et al. Clinical effects of microwave ablation in the treatment of low-risk papillary thyroid microcarcinomas and related histopathological changes. Front Endocrinol (Lausanne). 2021;12:751213. doi:10.3389/fendo.2021.751213.
- Yan L, Luo Y, Zhang Y, et al. The clinical application of core-needle biopsy after radiofrequency ablation for low-risk papillary thyroid microcarcinoma: a large cohort of 202 patients study. J Cancer. 2020;11(18):5257–5263. doi:10.7150/jca.42673.
- Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014;38(3):673–678. doi:10.1007/s00268-013-2335-8.
- Kim HI, Jin M, Ko NG, et al. Effect of TSH levels during active surveillance of PTMC according to age. Endocr Relat Cancer. 2022;29(4):191–200. doi:10.1530/ERC-21-0403.
- Yamamoto M, Miyauchi A, Ito Y, et al. Active surveillance outcomes of patients with low-risk papillary thyroid microcarcinoma according to levothyroxine treatment status. Thyroid. 2023;33(10):1182–1189. doi:10.1089/thy.2023.0046.
- Li X, Yan L, Xiao J, et al. Optimal thyrotropin level for low-risk papillary thyroid carcinoma after ultrasound-guided radiofrequency ablation. Int J Hyperthermia. 2023;40(1):2160880.
- Zhu Y, Che Y, Gao S, et al. Long-term follow-up results of PTMC treated by ultrasound-guided radiofrequency ablation: a retrospective study. Int J Hyperthermia. 2021;38(1):1225–1232. doi:10.1080/02656736.2021.1963850.
- Yan L, Lan Y, Xiao J, et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol. 2021;31(2):685–694. doi:10.1007/s00330-020-07128-6.
- Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan associations of endocrine surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020;67(7):669–717. doi:10.1507/endocrj.EJ20-0025.
- Miyauchi A, Ito Y, Fujishima M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33(7):817–825. doi:10.1089/thy.2023.0076.
- Pace-Asciak P, Russell JO, Tufano RP. Review: improving quality of life in patients with differentiated thyroid cancer. Front Oncol. 2023;13:1032581. doi:10.3389/fonc.2023.1032581.
- Li R, Li G, Wang Y, et al. Psychological distress and sleep disturbance throughout thyroid nodule screening, diagnosis, and treatment. J Clin Endocrinol Metab. 2021;106(10):e4221–e4230. doi:10.1210/clinem/dgab224.
- Jeon MJ, Lee YM, Sung TY, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: a cross-sectional study. Thyroid. 2019;29(7):956–962. doi:10.1089/thy.2018.0711.
- Teng D, Sui G, Liu C, et al. Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol. 2018;144(4):771–779. doi:10.1007/s00432-018-2607-7.
- Ji L, Wu Q, Gu J, et al. Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging. 2019;19(1):16. doi:10.1186/s40644-019-0204-x.
- Teng DK, Li HQ, Sui GQ, et al. Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine. 2019;64(1):109–117. doi:10.1007/s12020-019-01868-2.
- Lim HK, Cho SJ, Baek JH, et al. US-guided radiofrequency ablation for Low-Risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol. 2019;20(12):1653–1661. doi:10.3348/kjr.2019.0192.
- Yue WW, Qi L, Wang DD, et al. US-guided microwave ablation of Low-Risk papillary thyroid microcarcinoma: longer-Term results of a prospective study. J Clin Endocrinol Metab. 2020;105(6):dgaa128. doi:10.1210/clinem/dgaa128.
- Cho SJ, Baek SM, Lim HK, et al. Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid. 2020;30(12):1745–1751. doi:10.1089/thy.2020.0106.
- Wu R, Luo Y, Tang J, et al. Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: a retrospective analysis of 198 patients. Int J Hyperthermia. 2020;37(1):168–174. doi:10.1080/02656736.2019.1708480.
- Kim HJ, Chung SM, Kim H, et al. Long-term efficacy of ultrasound-guided laser ablation for papillary thyroid microcarcinoma: results of a 10-year retrospective study. Thyroid. 2021;31(11):1723–1729. doi:10.1089/thy.2021.0151.
- Cao XJ, Yu MA, Zhu YL, et al. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: a multicenter retrospective study. Int J Hyperthermia. 2021;38(1):916–922. doi:10.1080/02656736.2021.1936218.
- Yan L, Zhang M, Song Q, et al. The efficacy and safety of radiofrequency ablation for bilateral papillary thyroid microcarcinoma. Front Endocrinol (Lausanne). 2021;12:663636. doi:10.3389/fendo.2021.663636.
- Mauri G, Orsi F, Carriero S, et al. Image-Guided thermal ablation as an alternative to surgery for papillary thyroid microcarcinoma: preliminary results of an Italian experience. Front Endocrinol (Lausanne). 2020;11:575152. doi:10.3389/fendo.2020.575152.
- Song Q, Gao H, Tian X, et al. Evaluation of ultrasound-guided radiofrequency ablation as a treatment option for papillary thyroid microcarcinoma in the isthmus: a retrospective study. Front Endocrinol (Lausanne). 2020;11:599471. doi:10.3389/fendo.2020.599471.
- Juan Z, Yongping L, Han X, et al. A 5-year follow-up study on the efficacy and safety of ultrasound-guided laser ablation in elderly patients with papillary thyroid microcarcinoma: a retrospective, single-center study from China. Front Endocrinol (Lausanne). 2022;13:972589. doi:10.3389/fendo.2022.972589.
- Zhang L, Zhang GP, Zhan WW, et al. The feasibility and efficacy of ultrasound-guided percutaneous laser ablation for multifocal papillary thyroid microcarcinoma. Front Endocrinol (Lausanne). 2022;13:921812. doi:10.3389/fendo.2022.921812.
- Yan L, Liu Y, Li W, et al. Long-term outcomes of ultrasound-guided thermal ablation for the treatment of solitary low-risk papillary thyroid microcarcinoma: a multicenter retrospective study. Ann Surg. 2023;277(5):846–853. doi:10.1097/SLA.0000000000005800.
- Han ZY, Dou JP, Zheng L, et al. Safety and efficacy of microwave ablation for the treatment of low-risk papillary thyroid microcarcinoma: a prospective multicenter study. Eur Radiol. 2023;33(11):7942–7951. doi:10.1007/s00330-023-09802-x.