Abstract
Purpose
Cisplatin is commonly prescribed in hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal malignancy. Acute kidney injury (AKI) is regarded as a common complication after HIPEC combined with cytoreductive surgery (CRS). However, post-HIPEC chronic kidney disease (CKD) is scarce and less investigated. This study aims to investigate the incidence of CKD following cisplatin-based HIPEC and to analyse the associated risk factors.
Materials and Methods
From January 2016 to August 2021, a total of 55 patients treated with CRS and cisplatin-based HIPEC for peritoneal carcinomatosis were categorized retrospectively into groups, with and without CKD. Demographics, comorbidity, surgery, postoperative management, and complications were collected to evaluate risk factors for cisplatin-based HIPEC-related CKD. Univariate and multivariate analyses were conducted to confirm the correlation between different variables and CKD occurrence.
Results
Of the 55 patients, 24 (43.6%) patients developed AKI and 17 (70.8%) patients of these AKI patients progressed to CKD. Multivariate regression analysis identified intraoperative use of parecoxib (Odds Ratio (OR) = 4.39) and intraoperative maximum temperature > 38.5°C (OR = 6.40) as major risk factors for cisplatin-based HIPEC-related CKD occurrence. Though type II diabetes mellitus and intraoperative complications were the independent risk factors of AKI following cisplatin-based HIPEC, but they were not shown in CKD analysis.
Conclusion
Intraoperative use of parecoxib during cisplatin-based HIPEC emerged as a significant risk factor for postoperative CKD. Clinicians should exercise caution in prescribing parecoxib during HIPEC procedures. Additionally, maintaining intraoperative body temperature below 38.5°C might be crucial to mitigate the risk of CKD development. This study underscores the importance of identifying and preventing specific risk factors to improve long-term renal outcomes in patients undergoing cisplatin-based HIPEC.
Introduction
Acute kidney injury (AKI) had been regarded as unsolved postoperative complication [Citation1]. In patients receiving non-cardiac surgery with low-grade American Society of Anesthesiologist (ASA) physical status, 6% of patients could develop post-operative AKI [Citation2]. AKI has long been thought of a reversible syndrome. But it did increase risk of chronic kidney disease (CKD) development, which followed end-stage renal disease (ESRD), and mortality [Citation3]. However, current studies about postoperative CKD progression remain poor.
Hyperthermic intraperitoneal chemotherapy (HIPEC) following cytoreductive surgery (CRS) treats peritoneal carcinomatosis in various kinds of cancer. It was first used for pseudomyxoma peritonei (PMP) management in 1980 [Citation4]. Compared to systemic chemotherapy, HIPEC owns the advantage of direct intraperitoneal delivery of chemotherapy and enhanced cytotoxic effect to peritoneal surface malignancy [Citation5]. On the contrary, CRS with HIPEC is related to certain complications such as AKI, intra-abdominal abscess, pulmonary complications, anastomotic leakage, intestinal fistula, and hematological toxicity [Citation6]. Rates of major morbidity range from 22-34% and mortality from 0.8-4.1% after CRS and HIPEC [Citation7–9].
Considering hyperthermia-induced splanchnic vasodilation, arterial hypotension, fluid distribution and the direct effects of nephrotoxic chemotherapeutic drugs, HIPEC was recognized as a high-risk factor to postoperative AKI 10. AKI after HIPEC accounts from 1% to 48% and is\ associated with major complications up to 50% [Citation10,Citation11]. Cisplatin is a well-known nephrotoxicity chemotherapeutic drug and has been widely applied as the HIPEC regimen for various cancers. Although many literatures reported cisplatin-based HIPEC as the independent risk factor of postoperative AKI, with incidence of 26.1–48% [Citation10–12], the incidence of post-operative CKD is rarely mentioned.
Parecoxib, a parenteral-specific cyclooxygenase-2 (COX-2) inhibitor, is effective in treating postoperative pain through inhibiting prostaglandin and thromboxane production [Citation13]. COX-2 primarily limit in site of inflammation, while COX-1 mainly found in most body tissue [Citation14]. Therefore, Parecoxib results minor effect on renal function and has been wildly used during surgery to reduce opioid dose by anesthesiologist. Several clinical trials also emphasized the safety of Parecoxib, even in patients with eGFR <90 ml/min·1.73/m2 [Citation15,Citation16]. However, the combination use of cisplatin and parecoxib had never been documented.
Therefore, the purpose of this study was to investigate the incidence and predicted risk factors of CKD following cisplatin-based HIPEC and then evaluate the correlation between parecoxib and HIPEC related renal impairment.
Methods
Study population
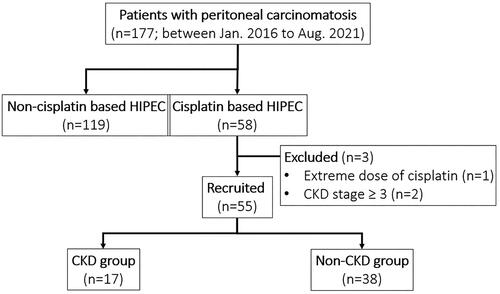
This retrospective study was approved by the local ethics committee of the China Medical University Hospital with the assigned number of CMUH110-REC2-033. Between Jan 2016 and Aug 2021, we included patients who older than 18 years old underwent CRS and HIPEC for curative intent to treat peritoneal carcinomatosis at our institution. Patients with baseline eGFR less than 60 ml/min/1.73m2, extreme dose of cisplatin or undergoing renal replacement therapy were excluded (). The recruited patients were divided into two groups, with or without CKD development after HIPEC, for further analysis and then were evaluated the independent risk factors for CKD, which was our primary observed outcome in this study.
Figure 1. Patient allocation flow diagram. Fifty-five patients completed the study. One patient was excluded for extreme dose of cisplatin due to primary tumor of mesothelioma, and another 2 patients were excluded for advanced CKD stage.
We collected data from our prospectively registered database, including patient’s demographic, tumor-related, intraoperative information, and post-operative variables. Nephrotoxicity related factors, such as history of hypertension, diabetes mellitus (DM), hyperlipidemia or use of NSAIDs, ACE inhibitor and contrast media, were also recorded.
Surgical procedure and perioperative care
All patients received CRS procedures for macroscopically detectable peritoneal tumors with complete cytoreduction (CC) score 0 or 1. We also adapted and published laparoscopic CRS for selected cases [Citation17,Citation18]. At the end of surgery, HIPEC (open or close methods) was started by double inflows and outflows drains after intraperitoneal perfused saline (2 L/m2) reached a temperature of 41 to 44 Celsius degrees. The Cisplatin dose and combined regimen were determined by primary neoplasm [Citation19]. In patients with colorectal cancer (CRC) or appendiceal cancer, we used a HIPEC combination of Cisplatin (25 mg/m2/L) plus MMC (3.3 mg/m2/L) for 60–90 min [Citation20]. For ovarian cancer or primary peritoneal serous carcinoma (PPSC) or Mullerian cancer, single Cisplatin (100 mg/m2) [Citation21], or combination of Cisplatin (100 mg/m2) and PTX (175 mg/m2) [Citation22] were administrated for 60 min. We used Cisplatin (75 mg/m2) for 60 min in endometrial cancer [Citation23]. Intraoperative intravenous parecoxib was given according to anesthesiologist’s clinical judgment. During HIPEC, intravenous fluid resuscitation was done to maintain urine output over 1.5 cc/kg/hr. After operation, patients were sent to intensive care unit (ICU) for continuous advanced hemodynamics monitoring. We performed early post-operative extubation and early nutritional support. Postoperative analgesia consisted of patient-controlled epidural or intravenous analgesia and prophylactic antibiotics with cefmetazole was routinely given.
Definition of renal impairment
Renal impairment was analyzed by blood parameters adapting Kidney Disease Improving Global Outcomes (KDIGO) guideline [Citation24]. Acute kidney injury is defined as increase serum creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 h, or higher than 1.5 times baseline levels in prior 7 days, or urine volume <0.5 ml/kg/h for 6 h; chronic kidney disease is defined as persistent abnormality of kidney function for a period of over 90 days. Creatinine and urea were evaluated on postoperative days (POD) 0,1,2,4,7,30,60,90, and every 6 months till last outpatient department visit. Once if serum creatinine decreased to within the baseline level +0.3 mg/dl or is less than 1.5 times baseline levels, it would not be considered as chronic kidney disease. And postoperative urine output and fluid balance were recorded at least for 5 days. No renal protective medication was regularly administered in patients.
Statistical analysis
Analyses were calculated using SPSS software (IBM SPSS Statistics for Windows, version 26.0, IBM Corp.). Continuous data were reported as the mean ± standard deviation or medians with interquartile ranges and the two groups were compared with Mann-Whitney U test for independent values, respectively. Categorical data were presented as percentages and compared with χ2 test or Fisher’s exact test between groups, as appropriate. Binary logistic regression analysis in a univariable setting was performed to screen possible predictors at first. Parameters with significant correlations were evaluated with multivariate analysis to determinate risk factors of renal impairment. For all results, p-value <0.05 was considered statistically significant.
Results
During the period, 55 patients were recruited with a mean age of 55.4 years old Asians, female-dominated (67.3%), and BMI of 23.84 kg/m2 (). The primary neoplasm type was shown in , including 28 colon cancer (51.0%), 12 ovarian cancer (21.8%), 3 endometrial cancer (5.5%), 3 primary peritoneal serous carcinoma (5.5%), 2 appendiceal cancer (3.6%), 2 Mullerian cell carcinoma (3.6%) and 5 others (9%). The overall comorbidities were listed in .
Figure 2. Distribution of primary neoplasms liocation in 55 patients received cisplatin-based HIPEC.
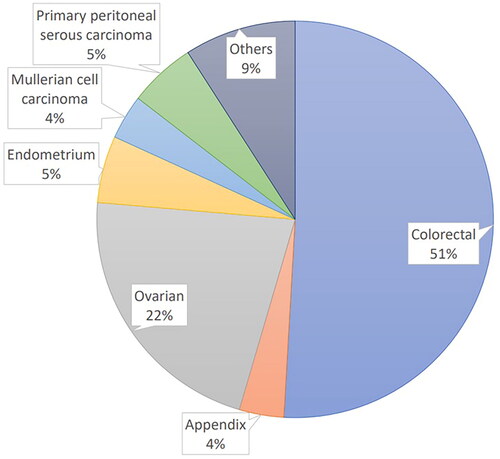
Table 1. Characteristics of patients with cisplatin-based HIPEC.
In the perioperative hospital course, four patients (7.3%) received iodinated radiological contrast for complete tumor staging or clinical evaluation. The concomitant drugs were prescribed postoperatively with metformin in one patient (1.8%), angiotensin converting enzyme inhibitor in 2 (3.6%), diuretic agents in 24 (43.6%) and NSAID in 31 (56.4%). None of patients received aminoglycosides antibiotics.
Renal impairment
Among all the patients, baseline mean eGFR was 90.5 ± 20.4 ml/min/1.73m2 before surgery. There was no patient suffered renal impairment before cisplatin-based HIPEC. Mean eGFR was reduced to 63.9 ± 37.7 ml/min/1.73m2 in the short-term after HIPEC therapy and recovered to a mean of 85.8 ± 38.6 ml/min/1.73m2 in the long-term period. One patient needed temporary hemodialysis for renal insufficiency. Of the 55 patients, most patients experienced ameliorated eGFR in POD1 and deteriorated in POD2. 24 (43.6%) patients developed AKI in first week and 7 patients recovered in POD7. Rest of 17 (70.8%) patients’ renal function got worsen in 1–2 week after surgery and stabilized in 1 month. Once they meet criteria for CKD in 3 months, no patients recovered in the following days (Figure S1).
Operative features
The operative information of all patients was described in . and was classified into 2 subgroups based on CKD development. The average dose of Cisplatin was 94.45 ± 14.46 mg/m2. The median Peritoneal Cancer Index score (PCI) of all patients was 9 (3–15), 76% had achieved CC-0 and 40% were completed by laparoscopic CRS. Most HIPEC (96.4%) were completed by closed method. Compare between CKD and non-CKD group, there was no significant difference in mean Cisplatin dose, median PCI, CC score, duration of surgery, intraoperative blood loss, blood transfusion, intraoperative complications. Analyzed the intraoperative core body temperature during HIPEC, maximum temperature (Tmax) was statistically significantly higher in CKD group than non-CKD group (38.64 ± 0.59 vs. 38.21 ± 0.64, p = 0.027). But temperature change (△T) did not reveal significantly different in both groups (p = 0.356). Analyzed urine output (mL/kg/hr) during HIPEC, CKD group presented a trend of less urine output than non-CKD group (1.33 ± 1.20 vs. 2.19 ± 1.84, p = 0.056). However, urine output during CRS period did not show any trend. Subgroup analysis of intraoperative complications revealed iatrogenic vessel injury (p = 0.036) with statistically significant difference between CKD and non-CKD groups. In addition, there was no statistically significant difference in post-operative ICU stay (1.59 ± 1.54 vs. 1.42 ± 0.89, p = 0.613) and hospital stay (12.41 ± 2.55 vs. 14.32 ± 9.7, p = 0.431).
Table 2. Operative profile.
Risk factors of HIPEC related AKI
As shown in , univariate and multivariate analysis model confirmed 4 independent factors, diabetes mellitus (OR = 18.72, p = 0.030), Tmax ≥ 38.5 C during HIPEC (OR = 11.76, p = 0.004), intraoperative complications (OR= 5.76, p = 0.041) and use of parecoxib (OR = 5.49, p = 0.030) could be predictors of cisplatin-HIPEC related AKI.
Table 3. Parameter associated to acute kidney injury after cisplatin based HIPEC Obesity, body mass index ≥ 27.
Risk factors of HIPEC related CKD
As shown in , univariate analysis for post-HIPEC CKD disclosed to the neoadjuvant chemotherapy (at least 4 cycles), Tmax >38.5 C during HIPEC, and intraoperative use of parecoxib. In subgroup of neoadjuvant chemotherapy, no significant difference in cisplatin, carboplatin nor oxaliplatin was shown. Other demographic and intraoperative/postoperative variable were not statistically different in CKD development. However, the multivariate analysis only confirmed Tmax >38.5 C during HIPEC (OR = 6.40, p = 0.010) and intraoperative use of parecoxib (OR = 4.39, p = 0.037) as the independent risk factors.
Table 4. Parameter associated to chronic kidney disease after cisplatin-based HIPEC.
Parecoxib subgroup analysis
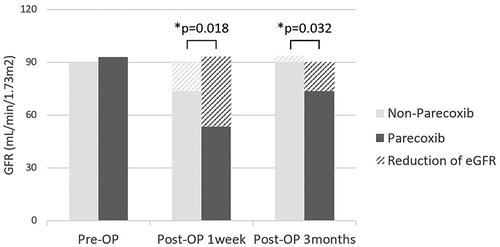
22 (40%) patients used parecoxib intraoperatively. The baseline eGFR average value in patients with and without use of parecoxib was 93.0 ± 18.5 and 89.9 ± 22.3 ml/min/1.73m2 separately. The mean follow-up was 25.4 ± 18.0 months. As shown in , reduction of the average eGFR in parecoxib group was 39.5 ± 34.5 and 18.7 ± 39.5 ml/min/1.73m2 at 1 week and 3 months after cisplatin-based HIPEC, which showed statistically significantly higher than the eGFR reduction in non-parecoxib group (16.3 ± 34.3 and −3.7 ± 31.7 ml/min/1.73m2).
Discussion
CRS and HIPEC has emerged as a viable alternative for treating peritoneal surface neoplasms. Despite its efficacy, HIPEC is associated with notable morbidity, particularly AKI. Existing literature indicates that major abdominal surgery without HIPEC carries a 13.4% AKI incidence [Citation25]. However, when HIPEC is introduced post cytoreductive surgery, the prevalence of AKI exhibits considerable variability, ranging from 0.9% to 48% [Citation6,Citation8–12,Citation26–32]. Liesenfeld had underscored a heightened risk of AKI (OR = 16.29) with cisplatin-based HIPEC regimens [Citation9]. Their research revealed 61.2% of AKI incidence with cisplatin, compared to 14.6% with non-cisplatin HIPEC. Other investigations also reported increased AKI rate (26.1-48%) with Cisplatin-based HIPEC [Citation11,Citation26,Citation27,Citation31,Citation32]. In our study, the AKI rates after cisplatin-based HIPEC was 43.6%, aligning with previous findings. Notably, of the 24 patients with AKI, 70.8% progressed to CKD, a less documented outcome. Our study identified contributors to this high CKD rate, including uncontrolled high body temperature (>38.5 °C) during HIPEC (OR = 6.40) and intraoperative use of parecoxib (OR = 4.39). To our knowledge, relevant data on CKD following HIPEC therapy are lacking.
Cisplatin-based HIPEC, wildly used for peritoneal neoplasms across malignancies, carries a known risk of kidney injury [Citation10,Citation27,Citation31,Citation32]. The complex pathophysiology of cisplatin-induced AKI involves cellular uptake and efflux, apoptosis, vascular injury, oxidative stress, and inflammation [Citation33]. Systemic cisplatin administration results in an overall nephrotoxicity incidence of 23.7%, with 8.1% after the first dose [Citation34]. While intraperitoneal cisplatin is believed to have limited systemic diffusion [Citation35], several literatures associate cisplatin-based HIPEC with AKI and renal impairments are notably linked to cisplatin use [Citation10,Citation26,Citation27,Citation31]. However, conflicting results exist, such as a study by Heakeam reporting only a 3.7% AKI incidence following cisplatin-based HIPEC [Citation30]. Ceresoli et al. demonstrated the HIPEC-related AKI is associated to volume changes during cytoreduction and hyperthermia-induced vasodilatation than cisplatin [Citation28]. Clinical evidence of progressing to CKD after HIPEC is scarce.
In our efforts to mitigate renal impairment, we implemented a program based on Raspe’s and Cooksley’s study [Citation36,Citation37], including pre-operative nutrition status evaluation, perioperative adequate hydration with strict glycemic control, HIPEC perfusate with normal saline-based solution, closely intraoperative urine output monitoring and routine postoperative fresh frozen plasma (FFP) supplement. Sodium thiosulfate was used for nephroprotection in HPIEC, though not introduced in Taiwan. We also avoid the use of nephrotoxic antibiotics and radiocontrast agents in program.
Despite these efforts, some patients still experienced AKI following CRS-HIPEC, with portion progressing to irreversible damage. In the literatures, age, preexisting renal dysfunction, high body mass index, preoperative hypoalbuminemia, hyperglycemia, hypertension, neoadjuvant chemotherapy, and use of NSAID were relatively high risks of post-operative AKI [Citation1,Citation2,Citation10–12,Citation25–27,Citation32,Citation38]. In our study, diabetes mellitus emerged as a significant comorbidity associated with AKI. Although it seems reasonable that the diabetic patients increase risk of renal dysfunction by arterial thickening and glomerulopathic changes, no relevant data had been published. Systemic administration of cisplatin was known as risk factor of nephrotoxicity; therefore, cisplatin was avoided in patients with renal impairment. In subgroup analysis of neoadjuvant chemotherapy regimen, it did not displayed relationship between neoadjuvant chemotherapy and AKI or CKD. Besides, patients receiving cisplatin chemotherapy are mostly afflicted with ovarian cancer, totaling around 20 individuals. Patients receiving cisplatin exhibited normal renal function, and under rigorous control, no association was found between adjuvant cisplatin therapy and CKD. High cisplatin dose, high PCI score, increased blood loss, low intraoperative and postoperative diuresis were intraoperative factors linked to AKI [Citation27,Citation30].
However, only iatrogenic vascular injury during operation increased risk of CKD. Urine output is a sensitive and early marker of kidney perfusion and influenced by several factors, such as hemodynamic, sympathetic tone, aldosterone and antidiuretic hormone concentration during CRS-HIPEC. Some studies demonstrated the intraoperative low urine output was significantly relative to AKI [Citation39,Citation40]. Instead of AKI, our study showed low urine output during HIPEC had a trend to CKD (p = 0.055).
Our study identified 30.9% of patients with cisplatin-based HIPEC developed CKD, with parecoxib use being a significant risk factor for AKI and CKD in . In the univariate analysis, we overviewed all the medications during admission, especially in the HIPEC course. There was no overall difference in use of metformin, angiotensin converting enzyme inhibitor, angiotensin II receptor blockers, loop diuretics, nor NSAID. Surprisingly, in 22 patients prescribed parecoxib, 14 patients (63.6%) developed AKI (OR = 5.49, p = 0.003). Later, 11 patients of them (50.0%) progressed to CKD (OR = 4.39, p = 0.037). Meanwhile, in patients without parecoxib, 10 patients (30.3%) developed AKI and 6 patients (18.2%) progressed to CKD. 3-month GFR reduction in both groups was 39.5 and 17.6 respectively. Both NSAIDs and COX-2 inhibitors have the potential to elicit renal injury, but most clinical studies disclosed no significant renal injury after COX-2 inhibitors [Citation16,Citation41].
Parecoxib is approved in over 80 countries as perioperative analgesia to prevent or reduce postoperative pain. In our practice, parecoxib was administered intravenously 40 mg per 12 h only during the operation and was not prescribed in ICU or in the general ward. In a pooled analysis study from 9287 patients in 28 comparative trials, the author demonstrated the rate of renal failure was not different between parecoxib (1.0%) and placebo (0.9%) [Citation15]. Currently, no drug-drug interaction between cisplatin and parecoxib has been proven. It is speculated that COX-2 accumulates in kidney after CRS to recovery renal function, but administration of parecoxib hampers the program. The following Cisplatin-based HIPEC deteriorates the situation by renal hypo-perfusion secondary to the hyperthermia-induced vasodilatation and Cisplatin absorption into plasma.
Notable, we found intraoperative maximum core body temperature more than 38.5°C was one of independent risk factors to AKI and CKD. Hendrix et al. also showed the strong correlation (OR = 3.77) between body temperature ≥ 39.5°C and postoperative complications in patients underwent CRS/HIPEC [Citation42]. Ceresoli et al. demonstrated the hyperthermia induced musculocutaneous vasodilatation, stimulated the splanchnic nerve and released massive cytokine into plasma. These situations implicate severe depletion of intravascular volume and result organ hypoperfusion during HIPEC 28. In our study, a total of 25 patients with Tmax ≥ 38.5 °C during HIPEC, 64% had AKI (OR = 11.76) and 52% developed CKD (OR = 6.40). Active cooling of patients has been recommended to prevent intraoperative high body temperature during CRS/HIPEC. However, a significant proportion of patients still develop episodes of intraoperative hyperthermia. In Carlos’ publishment [Citation43], he pointed out longer HIPEC perfusion time and blood transfusions were associated with hyperthermia ≥39 °C.
The aim of this study disclosed the risk factor of nephrotoxicity post cisplatin-based HIPEC therapy. Our data suggested that prescribing parecoxib during cisplatin-based HIPEC therapy causes irreversible renal damage. Prohibit of simultaneous use of parecoxib and cisplatin-based HIPEC should be considered. And presence of intraoperative hyperthermia ≥38.5 °C might be a predictor of post-HIPEC renal dysfunction, which could help to improve patients’ selection to prevent kidney injury.
The limitations conditioned by the retrospective nature of the study can be pointed out [Citation44]. First, inherent bias of patient selection could not be avoided. Second, this was single-center research, and the results need to be verified in multicenter trials. Third, the conclusion from our database is predominately suitable for Asian population. Furthermore, a limited sample size of 55 with low event rate and wide confidence interval was documented.
Data available statement
The data set for this work will be made available on EASY by Data Archiving and Networked Services (DANS): https://doi.org/10.17026/dans-2c7-5v8y
Supplemental Material
Download PDF (27.7 KB)Acknowledgements
We are grateful to Sheng-Chi Chang for leading the program and Hung-Chieh Yeh for alerting increasing CKD prevalence after HIPEC therapy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67–75. doi: 10.1053/j.ackd.2012.10.003.
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379.
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–260.
- Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27(6):365–374. doi: 10.1053/ctrv.2001.0232.
- Wajekar AS, Solanki SL, Patil VP. Postoperative complications and critical care management after cytoreduction surgery and hyperthermic intraperitoneal chemotherapy: a systematic review of the literature. World J Crit Care Med. 2022;11(6):375–386. doi: 10.5492/wjccm.v11.i6.375.
- Foster JM, Sleightholm R, Patel A, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw Open. 2019;2(1):e186847. doi: 10.1001/jamanetworkopen.2018.6847.
- Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol. 2016;7(1):99–111. doi: 10.3978/j.issn.2078-6891.2015.100.
- Naffouje SA, Tulla KA, Chorley R, et al. Acute kidney injury increases the rate of major morbidities in cytoreductive surgery and HIPEC. Ann Med Surg . 2018;35:163–168. doi: 10.1016/j.amsu.2018.09.036.
- Liesenfeld LF, Wagner B, Hillebrecht HC, et al. HIPEC-Induced acute kidney injury: a retrospective clinical study and preclinical model. Ann Surg Oncol. 2022;29(1):139–151. doi: 10.1245/s10434-021-10376-5.
- Sin EIL, Chia CS, Tan GHC, et al. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690–695. doi: 10.1080/02656736.2017.1293304.
- Tang Y-Z, Zeng P, Liao Y, et al. Correlation between perioperative parecoxib use and postoperative acute kidney injury in patients undergoing non-cardiac surgery: a retrospective cohort analysis. BMJ Open. 2021;11(8):e047840. doi: 10.1136/bmjopen-2020-047840.
- Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50 (Suppl):S29–S34. doi: 10.1194/jlr.R800042-JLR200.
- Xu N, Pang K, Qi S, et al. Correlation between perioperative parecoxib use and postoperative acute kidney injury in patients undergoing radical mastectomy: a retrospective cohort analysis. BMC Anesthesiol. 2022;22(1):155. doi: 10.1186/s12871-022-01688-4.
- Chang SC, Fingerhut A, Chen WT. Short and long-term outcomes of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for colorectal and appendiceal cancer peritoneal metastasis: propensity score-matched comparison between laparoscopy vs. open approaches. Surg Oncol. 2022;43:101766. doi: 10.1016/j.suronc.2022.101766.
- Chang S-C, Seow-En I, Ke T-W, et al. Laparoscopic total pelvic peritonectomy for colorectal cancer pelvic carcinomatosis: a retrospective case series and photographic/videographic step-by-step guide. Surg Endosc. 2022;36(3):2178–2191. doi: 10.1007/s00464-021-08719-0.
- Koole S, van Stein R, Sikorska K, et al. Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int J Gynecol Cancer. 2020;30(6):888–892. doi: 10.1136/ijgc-2020-001231.
- Pinto A, Pocard M. Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: a systematic review of the literature. Pleura Peritoneum. 2019;4(2):20190006. doi: 10.1515/pp-2019-0006.
- Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618.
- Coccolini F, Campanati L, Catena F, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61. doi: 10.3802/jgo.2015.26.1.54.
- Cornali T, Sammartino P, Kopanakis N, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for patients with peritoneal metastases from endometrial cancer. Ann Surg Oncol. 2018;25(3):679–687. doi: 10.1245/s10434-017-6307-3.
- Fliser D, Laville M, Covic A, et al. A European renal best practice (ERBP) position statement on the kidney disease improving global outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375.
- O'Connor ME, Kirwan CJ, Pearse RM, et al. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016;42(4):521–530. doi: 10.1007/s00134-015-4157-7.
- Cata JP, Zavala AM, Van Meter A, et al. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34(5):538–544. doi: 10.1080/02656736.2017.1368096.
- Chen KL, Shamavonian R, Karpes JB, et al. Acute kidney injury following hyperthermic intraperitoneal chemotherapy with cisplatin. Anticancer Res. 2021;41(3):1641–1646. doi: 10.21873/anticanres.14926.
- Ceresoli M, Coccolini F, Ansaloni L. HIPEC and nephrotoxicity: a cisplatin induced effect? Eur J Surg Oncol. 2016;42(6):909–910. doi: 10.1016/j.ejso.2015.08.174.
- Chen C-Y, Chang H-Y, Lu C-H, et al. Risk factors of acute renal impairment after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2020;37(1):1279–1286. doi: 10.1080/02656736.2020.1846793.
- Canda AE, Sokmen S, Terzi C, et al. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20(4):1082–1087. doi: 10.1245/s10434-012-2853-x.
- Hakeam HA, Breakiet M, Azzam A, et al. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail. 2014;36(10):1486–1491. doi: 10.3109/0886022X.2014.949758.
- Ye J, Ren Y, Wei Z, et al. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: a retrospective cohort study. Surg Oncol. 2018;27(3):456–461. doi: 10.1016/j.suronc.2018.05.025.
- Angeles MA, Quenet F, Vieille P, et al. Predictive risk factors of acute kidney injury after cytoreductive surgery and cisplatin-based hyperthermic intra-peritoneal chemotherapy for ovarian peritoneal carcinomatosis. Int J Gynecol Cancer. 2019;29(2):382–391. doi: 10.1136/ijgc-2018-000099.
- McSweeney KR, Gadanec LK, Qaradakhi T, et al. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers (Basel). 2021;13(7):1572. doi: 10.3390/cancers13071572.
- Burns CV, Edwin SB, Szpunar S, et al. Cisplatin-induced nephrotoxicity in an outpatient setting. Pharmacotherapy. 2021;41(2):184–190. doi: 10.1002/phar.2500.
- Kusamura S, Azmi N, Fumagalli L, et al. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur J Surg Oncol. 2021;47(1):82–88. doi: 10.1016/j.ejso.2019.06.022.
- Raspé C, Flöther L, Schneider R, et al. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol. 2017;43(6):1013–1027. doi: 10.1016/j.ejso.2016.09.008.
- Cooksley TJ, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J Surg Oncol. 2011;9(1):169. doi: 10.1186/1477-7819-9-169.
- Iyigun M, Aykut G, Tosun M, et al. Perioperative risk factors of acute kidney injury after non-cardiac surgery: a multicenter, prospective, observational study in patients with low grade American society of anesthesiologists physical status. Am J Surg. 2019;218(3):457–461. doi: 10.1016/j.amjsurg.2019.01.031.
- Arjona-Sánchez A, Cadenas-Febres A, Cabrera-Bermon J, et al. Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances. Eur J Surg Oncol. 2016;42(6):869–876. doi: 10.1016/j.ejso.2015.12.016.
- Mizota T, Yamamoto Y, Hamada M, et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth. 2017;119(6):1127–1134. doi: 10.1093/bja/aex255.
- Myles PS, McIlroy DR, Bellomo R, et al. Importance of intraoperative oliguria during major abdominal surgery: findings of the restrictive versus liberal fluid therapy in major abdominal surgery trial. Br J Anaesth. 2019;122(6):726–733. doi: 10.1016/j.bja.2019.01.010.
- Kirkby NS, Chan MV, Zaiss AK, et al. Systematic study of constitutive cyclooxygenase-2 expression: role of NF-κB and NFAT transcriptional pathways. Proc Natl Acad Sci U S A. 2016;113(2):434–439. doi: 10.1073/pnas.1517642113.
- Schug S, Parsons B, Li C, et al. The safety profile of parecoxib for the treatment of postoperative pain: a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data. J Pain Res. 2017;volume10:2451–2459. doi: 10.2147/JPR.S136052.
- Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281(1):F1–11. doi: 10.1152/ajprenal.2001.281.1.F1.
- Puolakka PAE, Rintala S, Yli-Hankala A, et al. The effect of parecoxib on kidney function at laparoscopic hysterectomy. Ren Fail. 2009;31(4):284–289. doi: 10.1080/08860220902780051.
- Hendrix RJ, Kassira JP, Lambert LA. Elevated maximum core body temperature during hyperthermic intraperitoneal chemoperfusion (HIPEC) is associated with increased postoperative complications. Ann Surg Oncol. 2020;27(1):232–239. doi: 10.1245/s10434-019-07495-5.