Abstract
Objective
Long-term re-intervention after ultrasound-guided high intensity focused ultrasound (USgHIFU) ablation was reported, and the prediction of non-perfusion volume ratio (NPVR) in differently aged patients with uterine fibroids (UFs) was explored.
Materials and Methods
Patients with UFs who underwent USgHIFU ablation from January 2012 to December 2019 were enrolled and divided into < 40-year-old and ≥ 40-year-old groups. Cox regression was used to analyze the influencing factors of re-intervention rate, and receiver operating characteristic (ROC) curve was used to analyze the correlation between NPVR and re-intervention rate.
Results
A total of 2141 patients were enrolled, and 1558 patients were successfully followed up. The 10-year cumulative re-intervention rate was 21.9%, and the < 40-year-old group had a significantly higher rate than the ≥ 40-year-old group (30.8% vs. 19.1%, p < 0.001). NPVR was an independent risk factor in both two groups. When the NPVR reached 80.5% in the < 40-year-old group and 75.5% in the ≥ 40-year-old group, the risk of long-term re-intervention was satisfactory.
Conclusion
The long-term outcome of USgHIFU is promising. The re-intervention rate is related to NPVR in differently aged patients. Young patients need a high NPVR to reduce re-intervention risk.
1. Introduction
Uterine fibroids (UFs), also known as uterine leiomyomas, are the most common benign reproductive system tumors in females of childbearing age, with prevalence rates ranging from 4.5% to 68.6% due to population characteristics such as race, region, health status, etc. [Citation1]. The morbidity rates of UFs is clearly related to age. Foth et al. [Citation2] reported that the incidence of UFs is approximately 22.4% in women under 35 years, more than 50% in women aged 36–40 and 65.2% in women aged 46–50. The treatment option for UFs requires individualized clinical management, including symptom relief and desire to experience pregnancy. Hysterectomy is a radical cure for fibroids but is unsuitable for patients who need fertility preservation. In recent years, laparoscopic myomectomy has become a mainstream technology, but it requires high suture techniques. Although uterine artery embolization (UAE) can relieve clinical symptoms and improve the quality of life of 75% patients, the risk of long-term re-intervention is high [Citation3], and the 5-year re-intervention rate is 32% [Citation4]. High intensity focused ultrasound (HIFU) ablation, as a noninvasive technique, has gradually attracted public attention which has a lower rate of complications than myomectomy but a similar effect to quality of life [Citation5]. It can cause instant coagulative necrosis (1-3 s) within a precisely defined area of 1.5 mm × 1.5 mm × 10 mm, including ultrasound-guided HIFU (USgHIFU) ablation and magnetic resonance-guided HIFU (MRgHIFU) ablation.
The short-term efficacy and safety of USgHIFU have been confirmed with the widespread application in clinic [Citation6]. Non-perfusion volume ratio (NPVR), as an important indicator for evaluating the success, is the proportion of non-perfusion areas after HIFU ablation [Citation7,Citation8]. Numerous studies have confirmed that long-term re-intervention is correlated with age both on HIFU, myomectomy and UAE [Citation9–11]. Age may be another important factor influencing long-term re-intervention after USgHIFU. However, it has not been reported that the prediction of NPVR based on different ages. Therefore, this study reported the long-term re-intervention rate and the individualized treatment goal in differently aged patients.
2. Materials and methods
All patients signed informed consent forms before USgHIFU. Verbal consent was obtained for the follow-up. The Chinese Clinical Trial Registry provided full approval for the study protocol (Registration No. CHiCTR2300074797). All procedures were in accordance with ethical standards and the Declaration of Helsinki.
2.1. Patients
The patients with UFs who underwent USgHIFU from January 2012 to December 2019 were enrolled in the minimally invasive and noninvasive treatment center of our hospital.
The inclusion criteria were as follows: (1) premenopausal women aged 18–50 years; (2) patients diagnosed with UFs of International Federation of Obstetrics and Gynecology (FIGO) types I-Ⅵ by magnetic resonance images (MRI); (3) the maximum diameter of fibroid was at least 1 cm; (4) patients underwent USgHIFU firstly.
The exclusion criteria were as follows: (1) patients with special type of fibroids, such as FIGO type 0, Ⅶ or Ⅷ; (2) patients with other gynaecological diseases, such as adenomyosis and ovarian tumors; (3) patients with serious organic lesions; (4) patients diagnosed or suspected malignant disease; (5) patients unwilling to undergo the follow-up.
2.2. MRI evaluation and classification
All patients received MRI scan 1 week before and within 3 days after the treatment. A series of T1WI, T2WI and enhanced T1WI were performed with a 3.0-T MRI system (GE Medical system, Milwaukee, WI, USA). The MRI were analyzed by a radiologist who had completed 5 years of specialization in abdominal MR imaging (Reader 1) and they were validated by another radiologist (Reader 2) who had 15 years of experience in abdominal MR imaging; in case of disagreement, Reader 2 retraced the image and this was considered as the final decision.
The MR images were evaluated and measured as follows: (1) Type of fibroid: types I-Ⅵ. The fibroids were categorized according to the FIGO [Citation12]. (2) Location of fibroid: anterior or posterior. The fibroid that the acoustic pathway did not pass through the uterine cavity during USgHIFU ablation was anterior fibroid. Otherwise, it was posterior fibroid. (3) Signal intensity on T2WI: hypointense, isointense or hyperintense. (4) Enhancement type on T1WI: mild, moderate or significant. The degree of enhancement was compared with normal myometrium according to the dynamic contrast-enhanced MR image within 60 s of the contrast medium injection. (5) Maximum diameter of fibroid: three dimensions were measured on T2WI: longitudinal diameter (D1), anteroposterior diameter (D2) and transverse diameter (D3). The maximum of three dimensions was selected as the maximum diameter of fibroids. (6) Calculation of fibroid volume and NPVR. The fibroid volume and NPVR were calculated by using the following equation: V = 0.5233 × D1 × D2 × D3 [Citation13]. The fibroid volume was measured by preoperative MRI while the non-perfused volume (NPV) was measured by post-operative MRI. The NPVR was defined as NPV/fibroid volume × 100%. The largest fibroid was selected as the primary fibroid for the above measurements when it comes to patients with multiple fibroids.
2.3. USgHIFU ablation
One-session USgHIFU ablation was performed by HIFU-licensed physicians with at least 3 years of HIFU clinical experience. A focused ultrasound tumor therapeutic system (Model-JC, Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China) was used. The ultrasound transducer worked with a frequency of 0.5–1.5 MHz, and energy was adjusted in within a range of 0–400 W. The guided ultrasound frequency used for real-time monitoring was 3.5 MHz (Esaote, MyLab70, Italy).
All patients received diet preparation, enema cleansing and skin preparation (shaving, degreasing and degassing) before treatment. Fentanyl (0.8–1 μg/kg) and midazolam hydrochloride (0.015–0.03 mg/kg) were administered to maintain conscious sedation while reducing patient discomfort. Changes in grey scale on real-time ultrasonographic imaging served as ablation indication. Sonication was terminated when the increased grey scale covered the planned ablation area. Sonication time was controlled within 3000s. Patients were instructed in prone position for 2 h after ablation.
2.4. Follow-up
A gynecologist who had worked for more than 3 years followed up the patients through telephone according to the follow-up items and criteria set by our research team. When patients had undergone re-intervention because of UFs, the reason, time and method were recorded. The follow-up end point was set as any re-intervention due to fibroids or hysterectomy due to other reasons. Whether patients had menopause more than 12 months and whether diagnosed with adenomyosis were used as parts of follow-up items. Moreover, we had asked patients whether underwent intervention repeatedly. If patients failed to be contacted multiple times within 3 days, it was confirmed as lost to follow-up.
2.5. Statistical analysis
SPSS version 26.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Normally distributed data were reported as mean ± standard deviation and non-normally distributed data were reported as medians and interquartile ranges (IQRs). Categorical data were expressed as numerals and percentages (%). Comparisons among groups were made using Mann-Whitney U test, t-test and chi-square test. p < 0.05 was considered statistically significant. Survival analysis was used to calculate cumulative re-intervention rates, and Cox regression was used to analyze the influencing factors. Receiver operating characteristic (ROC) curve was used to find out the correlation between NPVR and re-intervention rates. The cutoff value, area under curve (AUC), sensitivity and specificity were used to estimate the prediction of NPVR.
3. Results
3.1. Patients and USgHIFU ablation results
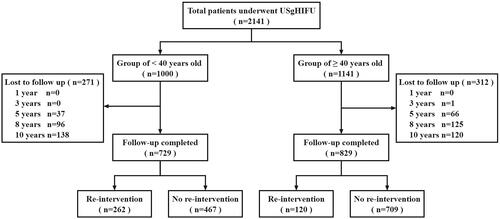
A total of 2141 patients were enrolled: 1558 (72.8%) patients were successfully followed up, and 583 patients were lost (). The age was 40.0 (35.0, 44.0) years, and the BMI was 22.1 (20.4, 23.9) kg/m2 of the 2141 patients. The maximum diameter was 56.0 (47.0, 67.0) mm, and the volume of the fibroid was 68.1 (40.7, 118.8) cm3. The ultrasonic power was 400.0 (IQR 395.0, 400.0) W, the reatment time was 83.0 (IQR 57.0, 119.0) min, the sonication time was 900.0 (IQR 564.0, 1410.0) s, the dose was 348.6 (IQR 210.3, 554.2) kJ and the NPVR was 87.0% (IQR 75.0%, 94.0%) (). No serious complications occurred during and after USgHIFU ablation, such as skin rupture and intestinal injury. No long-term complications were related to USgHIFU.
Table 1. Baseline characteristics of patients and results of USgHIFU ablation.
3.2. Long-term efficacy of USgHIFU
The follow-up time after USgHIFU was 87 (IQR 67,108) months, ranging from 45 to 129 months. The cumulative re-intervention rates for 1, 3, 5, 8 and 10 years after USgHIFU were 3.1%, 9.9%, 14.7%, 20.3% and 21.9% through survival analysis. The time of re-intervention was 36 (IQR 24,60) months after treatment. A total of 382 patients underwent re-intervention, of which 19 (4.9%) underwent re-interventions twice and 3 (0.8%) underwent re-interventions thrice. Reenlargement of original fibroid after a period of shrinkage (44.2%, 169/382), followed by symptom recurrence (18.1%, 69/382), new fibroid (12.3%, 47/382), fibroid removal through other surgery, such as cesarean section, oophorocystectomy, etc. (9.4%, 36/382), psychological factors due to the follow-up image of ablated residual fibroid which was even asymptomatic and did not grow (4.5%, 21/382), poor ablation (5.0%, 19/382), pre-fertility preparation (3.4%, 13/382) and hysteroscopic surgery because of the expulsion of submucosal fibroids (1.0%, 4/382). Re-intervention methods included myomectomy (62.3%, 238/382), USgHIFU (19.1%,73/382), hysterectomy (17.8%, 68/382) and UAE (0.8%, 3/382). The median time of re-intervention is 36.0 months. In addition, 11 (0.7%) patients were diagnosed with adenomyosis after USgHIFU. 399 patients were at the menopause and the median menopause age was 50 years.
3.3. Relationship between age and re-intervention
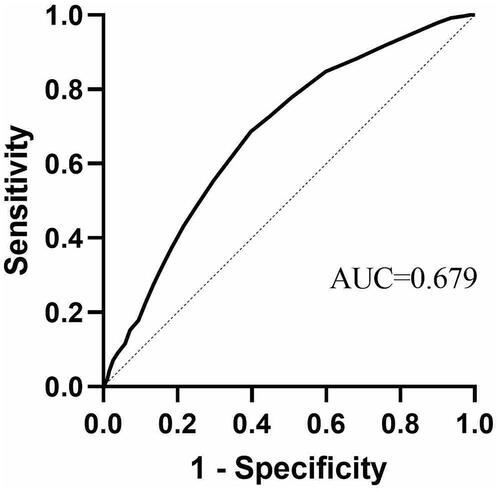
To clarify the relationship between age and re-intervention, 1558 patients who were successfully followed up were enrolled for the ROC curve analysis (). The AUC was 0.679 (95% CI, 0.649–0.709), the cutoff value was 39.5 years, sensitivity was 0.603 and specificity was 0.686.
3.4. Comparison of patients in different age groups
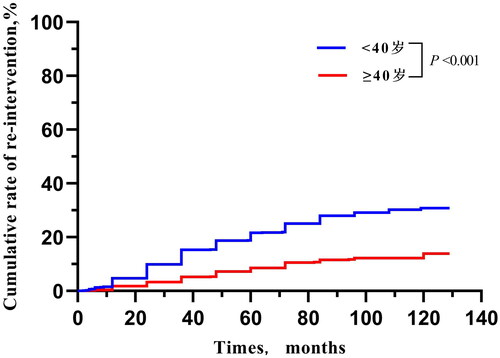
According to the above results, 2141 patients were divided into < 40-year-old group and ≥ 40-year-old group. There were no significant differences in history of lower abdominal surgery, type, location, signal intensity on T2WI and enhancement type on T1WI of fibroid between the two groups. In addition, the difference in NPVR between the groups and among different types of T2WI were non-significant. However, the median BMI, ratio of childbirth history, ratio of multiple fibroids, maximum diameter and volume of fibroid in the < 40-year-old group were significantly lower than those in the ≥ 40-year-old group (21.4 kg/m2 vs. 22.6 kg/m2, 53.2% vs. 91.6%, 32.9% vs. 48.6%, 53.0 mm vs. 57.0 mm, 60.1 cm3 vs. 75.0 cm3, p < 0.001; ). The < 40-year-old group had a higher re-intervention rate than the ≥ 40-year-old group in different types of T2WI (). Through survival analysis, the cumulative re-intervention rate of the < 40-year-old group was significantly higher than that in the ≥ 40-year-old group (4.7% vs. 2.4% within 1 year; 15.3% vs. 7.1% for 3 years; 16.8% vs. 11.7% for 5 years; 21.6% vs. 16.9% for 8 years; 30.8% vs.19.1% for 10 years, p < 0.001; ).
Table 2. Comparison of characteristics and ultrasound ablation results between different ages.
3.5. Influencing factors of re-intervention in different age groups
To determine the influencing factors of re-intervention in different age groups, the patients’ characteristics were analyzed by Cox regression analysis. In the < 40-year-old group, re-intervention was related to the number, maximum diameter, volume, signal intensity on T2WI, enhancement type on T1WI and the NPVR of fibroid. The NPVR was an independent risk factor with a negative correlation. In the ≥ 40-year-old group, the age, maximum diameter, volume, signal intensity on T2WI, enhancement type on T1WI and the NPVR of fibroid were associated with re-intervention. Age, signal intensity on T2WI and NPVR were the independent risk factors (). Elder patients, high NPVR and low signal intensity on the T2WI of fibroid may have low risk of re-intervention.
Table 3. The influencing factors of re-intervention at different ages with Cox regression analysis.
3.6. Risk assessment of re-intervention based on different ages
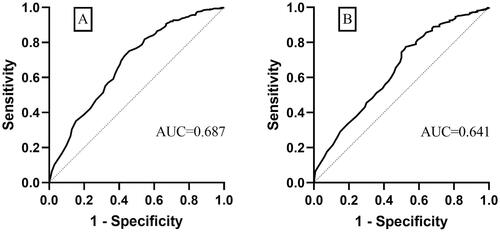
A total of 729 cases in the < 40-year-old group and 829 cases in the ≥ 40-year-old group were successfully followed up. NPVR was set as the test variable and nonintervention as the outcome variable in the ROC curve (). In the < 40-year-old group, when NPVR was > 80.5%, the patients had a low risk of re-intervention (AUC: 0.687, 95% CI: 0.638–0.721, sensitivity: 0.751, specificity: 0.540). In the ≥ 40-year-old group, when NPVR was > 75.5%, the patients had low risk of re-intervention (AUC: 0.641, 95% CI: 0.585–0.696, sensitivity: 0.774, specificity: 0.475).
4. Discussion
Although UFs are benign tumors, they still have a significant negative effect on women’s quality of life and fertility. USgHIFU, as an absolute noninvasive technique, is an alternative treatment for women with UFs [Citation14]. The long-term efficacy of USgHIFU has attracted considerable interest, but over 8 years of follow-up is rarely reported. On the basis of real-world data, we evaluated its long-term efficacy by the Kaplan-Meier method. Although the rate of lost to follow-up was 27.2% in our study, the characteristics between patients of lost to follow-up and patients who completed the response were no significant differences. Thus, the results can still be considered as representative of the whole population of the study.
In this study, the cumulative re-intervention rates 1, 3, 5, 8 and 10 years after USgHIFU were 3.1%, 9.9%, 14.7%, 20.3% and 21.9%. Previous researches reported that the re-intervention rate was 19.0% for 50 months [Citation15] and 20.7% for 70 months after USgHIFU [Citation11]. Sandberg et al. [Citation16] found that the highest re-intervention rates of myomectomy were 15% within 1 year, 34.7% for 3 years and 21.2% for 5 years. The re-intervention rate of 5 years after USgHIFU in this study was slightly lower than that in previous studies due to a higher median NPVR. The proportion of patients with NPVR ≥ 80% was 68%. The main reason of re-intervention was reenlargement of original fibroids (44.2%), of which 10 patients was related to pregnancy. The fibroids should be completely ablated as far as possible to prevent the regrowth of residual part. Moreover, the median time of re-intervention was 36 months after USgHIFU. Therefore, the important follow-up management period is 36 months after USgHIFU. However, long-term management still should not be ignored.
Among the 1558 patients in this study, patients aged > 39.5 years old had a lower risk of re-intervention lower than patients aged > 39.5 years old. This finding was similar to that in UAE [Citation3]. The number and sizes of UFs are negatively correlated with re-intervention [Citation17,Citation18]. In this study, compared with the ≥ 40-year-old group, the < 40-year-old group had lower rates of fertility history (53.2% vs. 91.6%), fewer patients with multiple fibroids (32.9% vs. 48.6%), smaller fibroid (60.1 cm3 vs. 75.0 cm3; p < 0.001), but there was no statistically significant difference in NPVR between two groups (p = 0.133). Nevertheless, the re-intervention rate in the < 40-year-old group was significantly higher than that in the ≥ 40-year-old group (30.8% vs. 19.1%, p < 0.001). UFs are hormone-dependent tumors [Citation19] so that their growth and recurrence occur easily in young people. As for elder patients, especially for perimenopausal patients, even if fibroid reoccurred, it can be left without intervention and observed until menopause.
NPVR is a predictor of efficacy in short-medium term after USgHIFU [Citation7,Citation8]. In our study, it is equally effective as indicator of long-term re-intervention. When NPVR is over 70%, the 2-year clinical cumulative recurrence rates are lower than those after myomectomy [Citation18,Citation20]. Moreover, when the NPVR is higher, the volume of UFs is reduced more, and the symptoms are relieved more [Citation21,Citation22]. Fibroids are reduced by 43% with NPVR > 80% and 20% with NPVR < 80% after MRgHIFU [Citation23]. Furthermore, satisfactory clinical efficiency can be achieved with NPVR > 80%, and re-intervention rate can be reduced to 5% approximately 12 months after treatment [Citation9,Citation24]. Wang et al. [Citation25] found that both MRgHIFU and USgHIFU were safe and effective for complete ablation. Gong et al. [Citation26] showed that experienced doctors could achieve 80% of NPVR by using USgHIFU and even higher than 90% with their medical experience increasing. In our study, 111 patients (5.2%) got NPVR of 100%, and the re-intervention rate was only 6.3%. However, safety and patient tolerance during USgHIFU ablation should be considered at the same time. Gong [Citation27] showed that intraoperative tolerance reduced and the rate of postoperative complications increased with the NPVR increasing, especially when NPVR exceeded 90%. Therefore, our study suggested that NPVR of 80.5% for young patients and NPVR of 75.5% for patients aged ≥ 40 years were required both considering satisfactory long-term efficacy and surgical safety. In addition, hyperintense on the T2WI of UFs is considered one of the important factors affecting the efficacy of HIFU. And hyperintense fibroids had lower NPVR and higher re-intervention rates than other Signal intensity on T2WI both in different age groups. Comprehensive treatment strategies should be individualized for T2WI hyperintense fibroids to improve clinical outcomes [Citation28]. Thus, how to improve the NPVR of fibroids with hyperintense, especially in young patients, is a hard but hotspot issue at present. Some clinical doctors are exploring some new ways such as combined with GnRH[28] or intratumoral ethanol injection [Citation29].
Uma et al. [Citation30] reported that history of uterine surgery may significantly increase the risk of adenomyosis. In our study, the incidence rate of adenomyosis after USgHIFU was 0.7% (11/1558), which was similar to that in normal population [Citation31]. USgHIFU ablation for UFs may not increase the incidence of adenomyosis. A total of 399 patients were at the menopause when we followed up, and the median menopause age was 50 years old, which was similar to the average menopause age in China (48.5 years). USgHIFU has no significant negative effect on ovarian function and does not increase the risk of premature ovarian failure.
This paper systematically reviewed 8-year data, and the maximum follow-up time was 129 months. The cutoff value of NPVR for differently aged patients was developed. These large number of data and long follow-up time can provide evidence for the efficacy of USgHIFU. Thus, doctors can screen appropriate patients for USgHIFU according to the results of influencing factors, formulate individualized treatment plans based on age and estimate long-term outcome after the treatment. However, there were some limitations in this study. We used the ROC curve to find out the cutoff value. However, the AUC was less than 0.7 because we only enrolled one variable into analysis. Perhaps a more appropriate statistical method should be developed which can find the cutoff value in multiple variables simultaneously. The retrospective research may cause some recall bias due to the long interval. Many patients did not complete the symptomatic evaluation so that score of symptomatic relief was not reported. More prospective studies are needed to validate this conclusion, and symptoms and quality of life should be assessed during follow-ups, and a comparative sy of myomectomy during the same period is recommended.
5. Conclusion
The long-term outcome of USgHIFU is promising. The long-term re-intervention rate is related to NPVR in differently aged patients. Young patients need a high NPVR to reduce the long-term re-intervention risk.
Supplemental Material
Download MS Word (166.7 KB)Disclosure statement
No potential conflict of interest was reported by author(s).
Data availability statement
The data of this study are available on request from the corresponding author. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
Additional information
Funding
References
- Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124(10):1501–1512. doi:10.1111/1471-0528.14640.
- Foth D, Röhl FW, Friedrich C, et al. Symptoms of uterine myomas: data of an epidemiological study in Germany. Arch Gynecol Obstet. 2017;295(2):415–426. doi:10.1007/s00404-016-4239-y.
- Scheurig-Muenkler C, Koesters C, Powerski MJ, et al. Clinical long-term outcome after uterine artery embolization: sustained symptom control and improvement of quality of life. J Vasc Interv Radiol. 2013;24(6):765–771. doi:10.1016/j.jvir.2013.02.018.
- Moss JG, Cooper KG, Khaund A, et al. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118(8):936–944. doi:10.1111/j.1471-0528.2011.02952.x.
- Chen J, Li Y, Wang Z, et al. Evaluation of high-intensity focused ultrasound ablation for uterine fibroids: an IDEAL prospective exploration study. BJOG. 2018;125(3):354–364. doi:10.1111/1471-0528.14689.
- Jacoby VL, Kohi MP, Poder L, et al. PROMISe trial: a pilot, randomized, placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril. 2016;105(3):773–780. doi:10.1016/j.fertnstert.2015.11.014.
- Ikink ME, Nijenhuis RJ, Verkooijen HM, et al. Volumetric MR-guided high-intensity focused ultrasound versus uterine artery embolisation for treatment of symptomatic uterine fibroids: comparison of symptom improvement and reintervention rates. Eur Radiol. 2014;24(10):2649–2657. doi:10.1007/s00330-014-3295-6.
- Stewart EA, Rabinovici J, Tempany CM, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril. 2006;85(1):22–29. doi:10.1016/j.fertnstert.2005.04.072.
- Chen J, Chen W, Zhang L, et al. Safety of ultrasound-guided ultrasound ablation for uterine fibroids and adenomyosis: a review of 9988 cases. Ultrason Sonochem. 2015;27:671–676. doi:10.1016/j.ultsonch.2015.05.031.
- Radosa MP, Owsianowski Z, Mothes A, et al. Long-term risk of fibroid recurrence after laparoscopic myomectomy. Eur J Obstet Gynecol Reprod Biol. 2014;180:35–39. doi:10.1016/j.ejogrb.2014.05.029.
- Li W, Jiang Z, Deng X, et al. Long-term follow-up outcome and reintervention analysis of ultrasound-guided high intensity focused ultrasound treatment for uterine fibroids. Int J Hyperthermia. 2020;37(1):1046–1051. doi:10.1080/02656736.2020.1807617.
- Gomez E, Nguyen MT, Fursevich D, et al. MRI-based pictorial review of the FIGO classification system for uterine fibroids. Abdom Radiol . 2021;46(5):2146–2155. doi:10.1007/s00261-020-02882-z.
- Zhao WP, Chen JY, Zhang L, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol. 2013;82(1):e43-9–e49. doi:10.1016/j.ejrad.2012.08.020.
- Duc NM, Keserci B. Emerging clinical applications of high-intensity focused ultrasound. Diagn Interv Radiol. 2019;25(5):398–409. doi:10.5152/dir.2019.18556.
- Li W, Yang Z, Gao B, et al. Comparison of ultrasound-guided high-intensity focused ultrasound ablation and hysteroscopic myomectomy for submucosal fibroids: a retrospective study. Int J Hyperthermia. 2021;38(1):1609–1616. doi:10.1080/02656736.2021.1995053.
- Sandberg EM, Tummers F, Cohen SL, et al. Reintervention risk and quality of life outcomes after uterine-sparing interventions for fibroids: a systematic review and meta-analysis. Fertil Steril. 2018;109(4):698–707.e1. doi:10.1016/j.fertnstert.2017.11.033.
- Fauconnier A, Chapron C, Babaki-Fard K, et al. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602. doi:10.1093/humupd/6.6.595.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881. doi:10.1097/01.AOG.0000156298.74317.62.
- Englund K, Blanck A, Gustavsson I, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metabolism. 1998;83(11):4092–4096. doi:10.1210/jc.83.11.4092.
- Stewart EA, Gostout B, Rabinovici J, et al. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110(2 Pt 1):279–287. doi:10.1097/01.AOG.0000275283.39475.f6.
- Keserci B, Duc NM. The role of T1 perfusion-based classification in magnetic resonance-guided high-intensity focused ultrasound ablation of uterine fibroids. Eur Radiol. 2017;27(12):5299–5308. doi:10.1007/s00330-017-4885-x.
- Lénárd ZM, McDannold NJ, Fennessy FM, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery–imaging predictors of success. Radiology. 2008;249(1):187–194. doi:10.1148/radiol.2491071600.
- Park MJ, Kim YS, Rhim H, et al. Safety and therapeutic efficacy of complete or near-complete ablation of symptomatic uterine fibroid tumors by MR imaging-guided high-intensity focused US therapy. J Vasc Interv Radiol. 2014;25(2):231–239. doi:10.1016/j.jvir.2013.11.011.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25(5):1317–1328. doi:10.1007/s00330-014-3538-6.
- Wang Y, Wang ZB, Xu YH. Efficacy, efficiency, and safety of magnetic resonance-guided high-intensity focused ultrasound for ablation of uterine fibroids: comparison with ultrasound-guided method. Korean J Radiol. 2018;19(4):724–732. doi:10.3348/kjr.2018.19.4.724.
- Gong X, Zhang X, Liu D, et al. Physician experience in technical success of achieving NPVR ≥ 80% of high-intensity focused ultrasound ablation for uterine fibroids: a multicenter study. Front Med Technol. 2021;3:790956. doi:10.3389/fmedt.2021.790956.
- Gong X, Liu D, Yang MJ, et al. Tolerance and efficacy of HIFU ablation for uterine fibroids NPVR ≥ 90%: a nested case-control study. Int J Hyperthermia. 2022;39(1):946–951. doi:10.1080/02656736.2022.2093414.
- Jiang L, Yu JW, Yang MJ, et al. Ultrasound-guided HIFU for uterine fibroids of hyperintense on T2-weighted MR imaging with or without GnRH-analogue-pretreated: a propensity score matched cohort study. Front Surg. 2022;9:975839. doi:10.3389/fsurg.2022.975839.
- Yang Z, Zhang Y, Zhang R, et al. A case-control study of high-intensity focused ultrasound combined with sonographically guided intratumoral ethanol injection in the treatment of uterine fibroids. J Ultrasound Med. 2014;33(4):657–665. doi:10.7863/ultra.33.4.657.
- Panganamamula UR, Harmanli OH, Isik-Akbay EF, et al. Is prior uterine surgery a risk factor for adenomyosis? Obstet Gynecol. 2004;104(5 Pt 1):1034–1038. doi:10.1097/01.AOG.0000143264.59822.73.
- Yu O, Schulze-Rath R, Grafton J, et al. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am J Obstet Gynecol. 2020;223(1):94.e1–e10. doi:10.1016/j.ajog.2020.01.016.