Abstract
Objectives
To evaluate the feasibility, efficacy, and safety of radiofrequency ablation (RFA) for solitary T1N0M0 papillary thyroid carcinoma (PTC) in the danger triangle area.
Methods
94 participants (mean age 44.45 ± 13.08; 73 females) with solitary T1N0M0 PTC in the danger triangle area who underwent percutaneous RFA at the hospital from January 2018 to April 2020 were retrospectively analyzed. Key ablation procedures included sufficient paratracheal fluid isolation, low-power, and short active tip (5 mm working electrode). Tumor size changes at different time points after RFA, technical success rates, tumor disappearance, disease progression, and complications were recorded and compared.
Results
Contrast-enhanced ultrasonography revealed that complete tumor ablation was performed with a 100% success rate in these patients. Post-ablation, the maximum diameter and volume of the ablation zone increased at the first and third month (p < 0.001), followed by a gradual decrease in size, without significant difference by the 6th month. The tumor disappearance rate was 76.59% (72/94), with higher rates in the T1a group compared to the T1b group (80% [64/80] VS57.1% [8/14], p < 0.001). There were no local recurrences. The incidence of new lesions and LNM was 3.2% (3/94), limited to the T1a subgroup. Further ablation was successfully applied to all new lesions and LMN. Mild voice changes were the only complication, with a rate of 3.2% (3/94), resolved within 4 months after RFA.
Conclusions
Sufficient paratracheal fluid isolation combined with a low-power, short active tip radiofrequency ablation strategy is a safe and effective method for treating solitary T1N0M0 PTC in the danger triangle area.
SIGNIFICANCE STATEMENT
The ‘danger triangle’ area comprises the dorsal edge of the thyroid gland, the lateral tracheal wall, and the anterior edge of the esophageal wall. When PTC tumors are present within the danger triangle, there is only limited space available for ablation. Furthermore, the proximity of the tumor with the esophagus, trachea, and thyroid capsule can complicate technical treatment success, potentially increasing the chance of local tumor recurrence and nerve injury. Therefore, the most effective approach for managing PTC lesions within the danger triangle remains undetermined. The goal of this study was to clarify the viability of ultrasound-guided RFA as a means of managing solitary T1N0M0 PTC tumors within the danger triangle area, providing a foundation for future clinical decision-making efforts.
Introduction
Recent advancements in thermal ablation technologies have resulted in the growing clinical recognition of the therapeutic effectiveness of thermal ablation-based treatment for papillary thyroid carcinoma (PTC) lesions [Citation1–3]. The 2021 European guidelines recommend thermal ablation as an approach to treating low-risk papillary thyroid microcarcinoma (PTMC) and radioiodine-refractory metastases [Citation4,Citation5]. Several studies have demonstrated the efficacy and safety of microwave ablation-based treatment for PTC lesions invading or proximal to the thyroid capsule [Citation6,Citation7]. The ‘danger triangle’ area comprises the dorsal edge of the thyroid gland, the lateral tracheal wall, and the anterior edge of the esophageal wall. When PTC tumors are present within the danger triangle, there is only limited space available for ablation. Furthermore, the proximity of the tumor with the esophagus, trachea, and thyroid capsule can complicate technical treatment success, potentially increasing the chances of nerve injury and local tumor recurrence (LTR) [Citation6]. The most effective approach to managing PTC lesions within this danger triangle thus remains a matter of debate, and no in-depth studies to date have systematically explored the safety, efficacy, and feasibility of radiofrequency ablation (RFA) for tumors in this region. As such, the present study was designed to retrospectively analyze clinical data from patients exhibiting solitary T1N0M0 PTC lesions within the thyroid danger triangle that had undergone ultrasound-guided RFA consisting of sufficient tracheal-paratracheal hydro dissection combined with a low-power, short active tip ablation strategy at our hospital. The goal of these analyses was to clarify the viability of ultrasound-guided RFA as a means of managing solitary T1N0M0 PTC tumors within the danger triangle area, providing a foundation for future clinical decision-making efforts.
Materials and methods
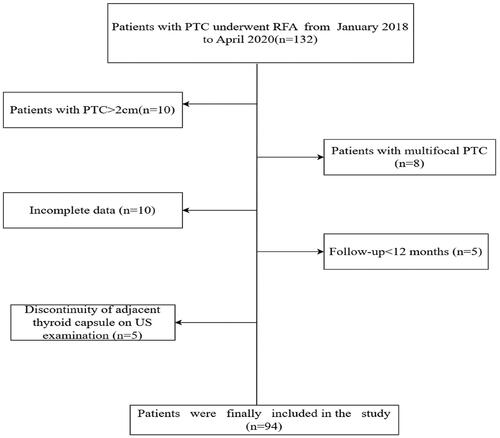
Patients
This retrospective study was approved by the Ethics Committees of Fujian Provincial hospital (K2023-07017) and was carried out according to the Declaration of Helsinki. The need to obtain informed consent to publish the data was waived for this retrospective investigation. Written informed consent was obtained from each patient before RFA treatment. In total, clinical data from 132 PTC patients with lesions located in the danger triangle who had undergone percutaneous RFA at Fujian Provincial Hospital between January 2018 and April 2020 were collected for retrospective analyses. Patients eligible for study inclusion were: (1) individuals who were not eligible for surgery or who had refused to undergo surgery; (2) papillary thyroid carcinoma or suspected papillary thyroid carcinoma up to 2 cm diagnosed by FNA or core needle biopsy; (3) patients exhibiting unifocal PTC tumors in the danger triangle area (maximum diameter: 2.0 cm) adjacent to or abutting the intact thyroid capsule, with continuity of the proximal thyroid capsule as confirmed via US examination; (4) patients with imaging results that confirmed the absence of distant metastasis or lymph node metastasis (LNM); (5) patients without any history of neck irradiation; and (6) patients with a post-RFA follow-up duration > 12 months. (7) normal vocal cord mobility on flexible endoscopy preoperatively. Patients were excluded if: (1) US examination revealed a discontinuity of the adjacent thyroid capsule; (2) the maximum tumor diameter exceeded 2.0 cm; (3) multifocal PTC was detected; (4) LNM or distant metastasis was evident; (5) follow-up was performed for <12 months after ablation; or (6) there was any history of other malignancies, renal failure, respiratory failure, severe cardiac failure, or coagulatory disorders; (7) abnormal vocal cord mobility on flexible endoscopy preoperatively. Patients were considered lost to follow-up and excluded from these analyses if efforts to obtain complete follow-up data were not successful. All patients meeting the inclusion criteria were enrolled in these analyses. Following the exclusion of 38 patients, the remaining 94 patients (71.2%, 94/132) were enrolled (). Based on thyroid nodule diameter measurements, these patients were classified into the T1a (diameter ≤ 1 cm) and T1b (1 cm < nodule diameter ≤ 2 cm).
Pretreatment analyses
The distance between the PTC nodule and the thyroid capsule was measured as the shortest distance between the nodule and the nearest site on the thyroid capsule. Based on US examination results, adjacency was defined by a distance >0 mm but ≤2 mm, whereas the PTC nodule was considered to abut the thyroid capsule if this distance was 0 mm but there was no evidence of the invasion of the surrounding tissue and a thyroid capsule that was confirmed to be complete and continuous by the US [Citation6,Citation7].
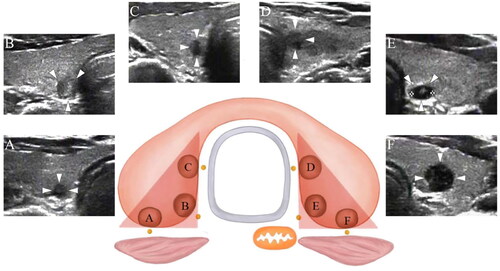
Based on the transverse ultrasonographic view of adjacent structures, the thyroid capsule within the danger triangle area was separated into the posterior medial (close to the retropharyngeal space/tracheoesophageal groove), posterior (close to the retropharyngeal space), and medial (close to the trachea) capsules ().
Before ablation, US examination, US-guided FNA or core needle biopsy, and laboratory testing were performed for all patients. Conventional US was used to measure tumor size (including three diameters), characteristics, location, and vascularity. Tumor volume was computed with the formula V = Πabc/6, where a denotes the maximum transverse diameter, while b and c denote the two perpendicular diameters. The volume reduction ratio (VRR) was computed as VRR = [(initial volume-final volume)]/initial volume computed tomography (CT) and US examinations were employed to assess patients for LNM and distant metastases. Samples were submitted for histopathological or cytological analyses and for the detection of BRAFV600E mutations to enhance diagnostic accuracy and minimize the risk of missed cases biopsied. Laboratory testing included analyses of complete blood count, thyroid function, and coagulatory function. Two physicians exhibiting > 10 years of experience with interventional ultrasonography performed all US examinations.
Radiofrequency ablation
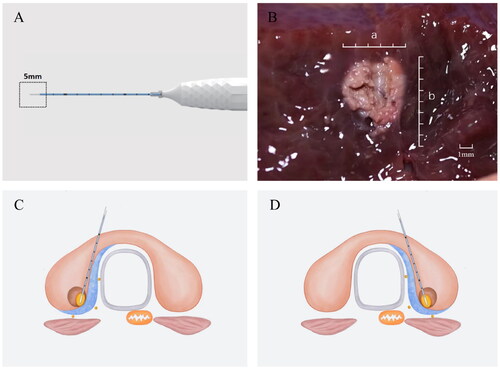
A monopolar RFA ablation needle with a 5 mm working electrode (Hangzhou, Canwell Radiofrequency Ablation Device CRS2000) was utilized to conduct the procedures in this study (). Patients were placed in the supine position with their necks extended, and 1% lidocaine was used for local anesthesia. Hydrodissection was performed by inserting a needle from the isthmus and introducing a 21 G needle into the posterior medial thyroid space (between the posterior medial thyroid capsule, esophagus, and recurrent laryngeal nerve) to enable the continuous injection of 5% glucose to effectively separate the target tumor from critical structures by a minimum of 5 mm to minimize the risk of thermal injury. A trans-isthmic approach was used to perform RFA with a moving ablation technique, initially ablating the tumor tissue closest to the posterior capsule and trachea followed by superficial movement. The initial RFA power was 15 W, and this power was increased to 20 W if no transient hyperechoic region was evident at the electrode tip within 5–10 s, with a 15 s needle ablation time at each site before moving to a non-ablated area and awaiting a pulse. To minimize the potential for residual tumor tissue and tumor recurrence, the ablated area extended 3 mm beyond the tumor edge on all sides other than the capsule side. Post-RFA contrast-enhanced US (CEUS) was conducted to assess the ablation volume, with supplementary ablation being performed if abnormal enhancement was detected within the ablation necrosis zone. During this procedure, efforts were made to protect critical cervical structures to protect against hematoma, nerve injuries, or other potentially serious complications. All patients underwent in-hospital monitoring for 12 h and were assessed for intra- and post-operative RFA complications. For further details regarding the RFA procedure, see .
Post-ablation evaluation
Laboratory tests of thyroid function were performed for all patients at 1 month post-RFA. Changes in nodule size were assessed via US at 1, 3, 6, 12, 18, 24, 30, and 36 months post-ablation. During postoperative follow-up, patients underwent neck US examinations, while annual chest CT scans were conducted to monitor for distant metastasis. If the volume of the ablation zone subsequently increased compared to the previous visit, Ultrasound-guided CNB/FNA was conducted at both the central and periphery of the ablation zone, as well as the surrounding thyroid parenchyma, under the assumption that viable neoplastic cells could be present. A biopsy was additionally conducted in cases where there was suspicion of tumor recurrence or LNM. If symptoms of distant metastasis were found, CT, PET, or bone scans were conducted. Flexible endoscopy was used to evaluate vocal cord movement in any patients exhibiting postoperative hoarseness.
Study endpoints
The rate of disease progression was the primary endpoint for this study and included tumor recurrence, local recurrence, LNM, and PTC-related death. Secondary endpoints included changes in tumor size and volume, tumor disappearance, technical success rates, and complication rates. Technical success was defined by the absence of any enhancement upon CEUS examination following RFA procedure completion.
Statistical methods
SPSS 27.0 (IBM, NY, USA) was used for all statistical testing. Continuous data are given as medians or means ± standard deviations and compared with Mann-Whitney U tests (skewed data) or Student’s t-tests (normally distributed data), whereas categorical data are given as frequencies and percentages and compared with chi-square tests or Fisher’s exact test. Kaplan-Meier curves and log-rank tests were used to compare tumor disappearance and disease progression between groups. p < 0.05 serves as the threshold to define significance.
Results
Patient characteristics
Participant and tumor characteristics for all subjects included in this study are summarized in .
Table 1. Demographic characteristics of patients with PTC in the danger triangle area.
The association between PTC nodules and the thyroid capsule
In total, 16 PTC nodules were found to be adjacent to the thyroid capsule, of which 13 and 3 were respectively classified as T1a and T1b nodules. In addition, 78 PTC nodules were found to abut the capsule, including 67 and 11 T1a and T1b nodules, respectively. Of these, 35 abutted one side of the thyroid capsule (18 and 17 for the posterior and medial capsules, respectively), whereas 43 abutted both the posterior and medial capsules.
Technical feasibility and technical success
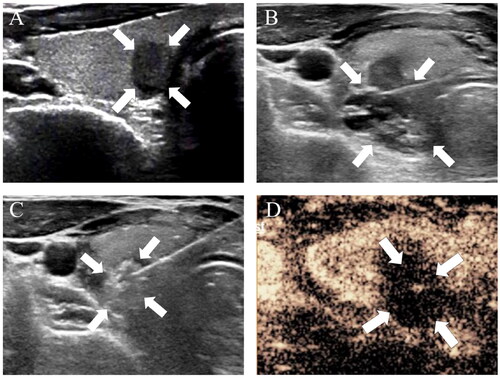
All ablation procedures were conducted utilizing a protocol that consisted of a combination of sufficient peritracheal hydrodissection and low-power short-electrode ablation. The mean duration of this ablation procedure was 151.9 ± 75.3 s (range: 40–431 s), with respective median ablation times for T1a PTC and T1b PTC patients of 143.6 ± 74.5 s (range: 33–431 s) and 199.4 ± 63.5 s (range: 101–331 s). Post-ablation CEUS results confirmed the achievement of complete ablation in all cases for a 100% technical success rate.
Pre- and post-ablation tumor changes
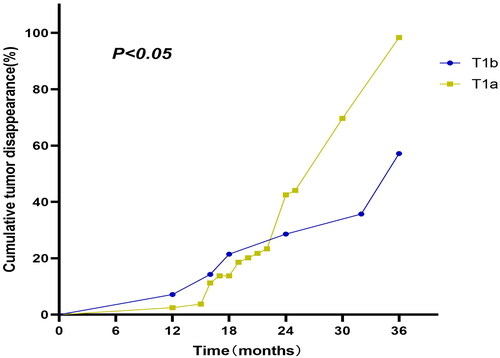
No significant differences in measured T3, fT4, or TSH levels were observed when comparing the pre- and post-RFA time points. As of the most recent follow-up, 72/94 tumors (76.6%) were completely absent on US examination, including 80% (64/80) of T1a tumors and 57.1% (8/14) T1b tumors. Kaplan-Meier curves revealed pronounced differences in tumor disappearance rates between these two patient groups (log-rank test = 9.367, p = 0.002) (), with T1a tumors disappearing more quickly than T1b tumors (27 vs. 30 months).
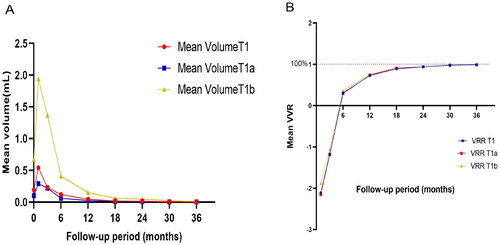
The mean maximum tumor diameter in PTC patients before RFA was 0.73 ± 0.34 cm (range: 0.24–2.0 cm), and the mean target lesion volume was 0.18 ± 0.27 ml (range: 0.02–0.78 ml). Due to the expansion of the ablation zone at 1- and 3-months post-RFA, the volume and maximum diameter of this zone were both elevated as compared to the primary tumor (p < 0.001), although they declined such that they were smaller than those of the primary tumor after 3 months. Significant differences in maximum diameter and mean tumor volume measurements were observed when comparing tumors before RFA and the ablation zone at all follow-up time points (p < 0.05), except comparisons of the maximum diameter at baseline and 6 months (p > 0.05). Throughout the 36-month follow-up period, mean tumor volume declined from a pre-ablation value of 0.18 ± 0.27 ml (range: 0.022–0.783 ml) to 0.001 ± 0.005 ml (range: 0–0.012 ml) after ablation. A significant difference in VRR was detected for all follow-up time points (p < 0.05). For further details regarding changes in thyroid function and size following these RFA procedures, see and ; .
Table 2. Indicators of thyroid function before RFA and after RFA.
Table 3. Tumor size before RFA and at each follow-up time-point after RFA.
Post-RFA recurrence and metastasis
No patients exhibited any evidence of local recurrence during the 36-month follow-up period. The overall disease progression rate in this study, including both LNM and new tumor formation, was 3.2% (3/94). Of these three patients, one exhibited a metastatic lymph node that appeared in the ipsilateral neck at 6 months post-RFA, while another presented with a new PTC nodule in the contralateral thyroid lobe at 12 months post-RFA, and the third exhibited an ipsilateral neck metastatic lymph node at 18 months post-RFA. Since all three patients with disease progression refused to undergo surgery, further ablation was performed to eradicate these lesions. Primary nodules in all patients in the T1a subgroup were positive for the BRAF V600E mutation. None of the T1b PTC patients exhibited LNM or new malignant thyroid nodules. Relative to patients in the T1b subgroup (0/14, 0%), higher rates of adverse events were detected in the T1a subgroup (3/80, 3.8%), although the difference was non-significant (p > 0.05). Of the three patients that did experience adverse events, two presented with lesions adjacent to the posterior medial capsule whereas one exhibited a lesion at the medial capsule. Following further ablation, no additional instances of recurrence or distant metastasis were observed throughout the follow-up.
Treatment complications
This RFA treatment approach was well tolerated by all participants included in this study, with no evidence of any major complications. Three patients did experience mild voice changes, including 2 and 1 patients in the T1a and T1b PTC subgroups, respectively, with no significant difference between the two groups (p > 0.05). Of the nodules in these three patients, one abutted the posterior capsule of the left thyroid, while another abutted the posterior capsule of the right thyroid, and the third abutted the posterior medial capsule of the right thyroid. In all cases, complete symptomatic resolution occurred within 4 months without the need for any specific treatments, and no patients exhibited severe hematomas or skin burns.
Discussion
All patients enrolled in this study underwent curative RFA procedures performed with an established protocol, achieving a technical success rate of 100%. This suggests that RFA is a viable approach to achieving positive therapeutic outcomes in PTC patients exhibiting lesions situated within the thyroid danger triangle area. Over the average 29.06 ± 7.77 months follow-up period, 72 of the target nodules (76.5%) disappeared completely, with this disappearance rate being similar to that reported in a recent meta-analysis of 12 studies focused on RFA (combined n = 1187) [Citation8]. T1a tumors disappeared with greater frequency than did T1b patients, in line with the results of retrospective comparisons of RFA treatment outcomes for simple T1aN0M0 and T1bN0M0 PTC outcomes reported previously [Citation9]. There was no evidence of local recurrence over the follow-up period. The overall rate of disease progression was 3.2% (3/94), including 2 cases (2.1%) of LNM and one case (1.1%) of new tumor formation, in line with the low 0.5% and 0.6-2.0% rates of local recurrence and new tumor formation/LNM, respectively, that have been reported in prior studies [Citation8, Citation10]. This suggests that RFA may represent an effective and feasible means of T1N0M0 PTC nodules treatment within the thyroid danger triangle area.
Just 3.2% of the patients in this study (3/94) experienced mild voice changes following RFA, and these were the only major complications detected throughout follow-up. None of these patients experienced permanent hoarseness, with all symptoms resolving on their own within 4 months. As such, the overall complication rate for this procedure was lower than the 7.1 and 6.0% rates reported previously for surgery and microwave ablation [Citation11,Citation12]. None of the enrolled patients developed hypothyroidism, suggesting that RFA has no impact on thyroid functionality, in line with past reports [Citation13]. These data thus offer strong evidence that RFA is a safe and effective means of treating T1N0M0 PTC lesions within the danger triangle area.
The low complication rates and absence of disease recurrence following RFA-based removal of T1N0M0 PTC lesions in the thyroid danger triangle in this study may be attributable to some of the innovative aspects of the employed ablation strategy. RFA procedures for these patients were performed using a strategy entailing sufficient paratracheal hydro dissection combined with low-power, short active tip ablation performed via isthmus movement. One key aspect of this approach is that the isthmus pathway can enable the continuous monitoring of the relationship between the electrode, target lesion, and recurrent laryngeal nerve, which is particularly important as a means of preventing thermal injury in the danger triangle area. In addition, short active tip electrodes generate a thermal field that is smaller and more readily controlled as compared to the field generated by conventional electrodes, reducing the potential for normal tissue or neural injury and thereby facilitating precision treatment [Citation14,Citation15]. An optimized approach to ablation needle placement was also employed herein by first inserting needles into the isthmus before positioning these needles at the posterior capsule and the inner capsule closest to the nerve, without puncturing the posterior capsule at any point during the RFA process. Ablation was performed from the deepest to the shallowest depth to minimize the potential for nerve damage. The injection of 5% glucose was also successful as an approach to isolating the thyroid lobe from surrounding critical structures, generating a hydro dissection barrier that simultaneously facilitated complete ablation while protecting against thermal injury to any surrounding structures. During the ablation procedure, the target lesion was also pulled away from adjacent nerves and other key structures via lifting the electrode, thus providing additional separation conducive to the abrogation of injury risk.
There are some limitations to this study. For one, as a retrospective analysis, selection bias may have influenced study results. Second, the ablation of the danger triangle area remains controversial; it is limited to individuals with technical expertise and is not usually recommended for beginners. The third 36-month follow-up duration for this study is relatively short, given the fact that PTC progression rates rise with the extension of follow-up. Future long-term prospective research will thus be important to definitively confirm the utility of thermal ablation as an approach to eliminating PTC lesions present within the danger triangle area.
Conclusion
In summary, these results demonstrate that paratracheal hydro dissection combined with low-power short active tip RFA represents a safe and effective treatment strategy for T1N0M0 PTC lesions situated within the thyroid danger triangle area.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request. Please contact the corresponding author ([email protected]).
Additional information
Funding
References
- Cao XJ, Wang SR, Che Y, et al. Efficacy and safety of thermal ablation for treatment of solitary T1N0M0 papillary thyroid carcinoma: a multicenter retrospective study. Radiology. 2021;300(1):209–216. doi: 10.1148/radiol.2021202735.
- Baek JH, Cho SJ. Thermal ablation for small papillary thyroid cancer: a potential game changer. Radiology. 2021;300(1):217–218. doi: 10.1148/radiol.2021210424.
- Hahn SY, Shin JH, Na DG, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2019;20(4):609–620. doi: 10.3348/kjr.2018.0696.
- Mauri G, Hegedüs L, Cazzato RL, et al. Minimally invasive treatment procedures have come of age for thyroid malignancy: the 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Cardiovasc Intervent Radiol. 2021;44(9):1481–1484. doi: 10.1007/s00270-021-02870-w.
- Mauri G, Hegedüs L, Bandula S, et al. European thyroid association and cardiovascular and interventional radiological society of Europe 2021 clinical practice guideline for the use of minimally invasive treatments in malignant thyroid lesions. Eur Thyroid J. 2021;10(3):185–197. doi: 10.1159/000516469.
- Wu J, Zhao ZL, Cao XJ, et al. A feasibility study of microwave ablation for papillary thyroid cancer close to the thyroid capsule. Int J Hyperthermia. 2021;38(1):1217–1224. doi: 10.1080/02656736.2021.1962549.
- Zheng L, Dou JP, Han ZY, et al. Microwave ablation for papillary thyroid microcarcinoma with and without US-detected capsule invasion: a multicenter prospective cohort study. Radiology. 2023:1–11.
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278–1286.
- Xiao J, Zhang Y, Yan L, et al. Ultrasonography-guided radiofrequency ablation for solitary T1aN0M0 and T1bN0M0 papillary thyroid carcinoma: a retrospective comparative study. Eur J Endocrinol. 2021;186(1):105–113. doi: 10.1530/EJE-21-0580.
- Wang L, Xu D, Yang Y, et al. Safety and efficacy of ultrasound-guided percutaneous thermal ablation in treating low-risk papillary thyroid microcarcinoma: a pilot and feasibility study. J Cancer Res Ther. 2019;15(7):1522–1529. doi: 10.4103/jcrt.JCRT_214_19.
- Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–276. doi: 10.1007/s00268-003-6903-1.
- Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712–717. doi: 10.1016/j.crad.2019.05.012.
- Cao XJ, Liu J, Zhu YL, et al. Efficacy and safety of thermal ablation for solitary T1bN0M0 papillary thyroid carcinoma: a multicenter study. J Clin Endocrinol Metab. 2021;106(2):e573–e581. doi: 10.1210/clinem/dgaa776.
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol. 2015;25(1):163–170. doi: 10.1007/s00330-014-3405-5.
- Lee YH, Baek JH, Jung SL, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society Of Thyroid Radiology. Korean J Radiol. 2015;16(2):391–401. doi: 10.3348/kjr.2015.16.2.391.