Abstract
Rheumatic and musculoskeletal diseases (RMDs) usually lead to morphological and functional deficits of various extend, increased morbidity and a considerable loss of quality of life. Modern pharmacological treatment has become effective and can stop disease progression. Nonetheless, disease progression is often only slowed down. Moreover, pharmacological treatment does not improve functionality per se. Therefore, multimodal treatment of rheumatic disorders with physical therapy being a key element is of central importance for best outcomes. In recent years, research into physical medicine shifted from a sole investigation of its clinical effects to a combined investigation of clinical effects and potential changes in the molecular level (e.g., inflammatory cytokines and the cellular autoimmune system), thus offering new explanations of clinical effects of physical therapy. In this review we provide an overview of studies investigating different heat applications in RMDs, their effect on disease activity, pain and their influence on the molecular level.
Background
Facts about current care
Despite considerable drug-related therapeutic advances with anti-cytokine therapeutics (so-called biologics) in the treatment arsenal of rheumatic and musculoskeletal diseases (RMDs), some patients still have functional and participation-related limitations with inability to work and reduced earning capacity [Citation1,Citation2]. In addition, biologics are not approved as first-line therapy, so that functional health restrictions exist even when they are used. The current data from the German Collaborative Arthritis Center showed that despite standard drug therapy, only 36% of patients with rheumatoid arthritis (RA) achieve remission. About 50% of these patients suffer from moderate to severe pain and require therapy with coxibes, analgesics or opioids [Citation3]. In patients with ankylosing spondylitis (AS), the situation does not look any better. 52–70% have a mild to severe functional impairment with accompanying pain [Citation4]. The economic consequences for the welfare state vary depending on the extent of the functional restrictions. Total annual medical costs for RA and AS vary between €35.000 per patient for severe versus €8.000 for minor functional impairments [Citation5]. According to estimates by the Federal Statistical Office, the costs for osteoarthritis (OA) patients were significantly higher in 2008, at around €7.26 billion [Citation6].
Interdisciplinary international guidelines on RA, AS and OA promote improvement of functional restrictions and pain reduction. However, the potential of physical therapy prescriptions is often not fully exploited in Germany [Citation7]. Therefore, the reality of providing care for rheumatic patients with physiotherapeutic options in Germany is absolutely in need of improvement.
Physical therapy in transition to molecular physical medicine
Until the second half of the last century, methods of physical therapy played an indispensable role in the therapeutic concept of rheumatic diseases due to the limited anti-rheumatic drugs available.
Physical therapy is currently facing two major challenges: On the one hand, it is no longer necessary to rely solely on tradition and experience, but to take into account the requirements of modern evidence-based medicine and to provide scientific knowledge and proof of efficacy, if this is possible at all. On the other hand, in times of rising budgets and parallel increases in drug costs, there is hardly any financial scope, so that economic and economic regulations must be observed. Molecular medicine represents a new, future-oriented discipline and combines the content and questions of experimental medicine with the methodology of molecular biology.
Molecular medicine is indispensable for the further improvement of diagnostics and therapy of contemporary diseases. For example, the number of diseases whose cause can be defined in terms of molecular biology is constantly increasing and with it the need for research in this field. Molecular physical medicine is an interesting field of work in molecular medicine, which refers to the investigation of the effects of methods of physical medicine, for example on RMDs at the cellular and molecular levels. Of particular interest here in RMDs are changes in the pro-inflammatory cytokine milieu. A promising field of research is the interaction between bones, the immune system and the influence of physiotherapeutic measures on these biological systems.
Molecular physical medicine can make a decisive contribution to solving the two above mentioned tasks of physical therapy.
By investigating the molecular and cellular biological effects of physiotherapeutic measures on the human organism and on the pathophysiological processes of various disease patterns identified by modern immunological basic research, molecular physical medicine can succeed in providing both the required evidence and a comparison with drugs to prove economic viability.
Even today, the effects of differential-indicative physiotherapeutic procedures on specific mechanisms of the inflammatory process and on immune-competent cells are largely unknown.
This review investigates the first interesting and promising results of this still young discipline of molecular physical medicine for RMDs concerning hyperthermia as a method of action in physical therapy.
History of hyperthermia therapy
Thermotherapy, or heat therapy, has been recognized for its medicinal benefits since ancient times. Hippocrates (460–377 BC), a seminal figure in medicine, observed the beneficial effects of fever in reducing epileptic convulsions, encapsulated in his statement, ‘fever resolves spasm’. Similarly, the Roman scholar Aulus Cornelius Celsus (25 BC–AD 50) recommended heated sand and warm baths for treating edema. This therapeutic approach extended into the treatment of psychiatric disorders in the nineteenth century, as noted in the works of the renowned French psychiatrist Philippe Pinel (1745–1826). A significant milestone in the advancement of thermotherapy occurred in the early twentieth century. Austrian psychiatrist Julius Wagner-Jauregg (1857–1940) was awarded the Nobel Prize in Medicine and Physiology in 1927 for his pioneering work in fever therapy, particularly in the treatment of neurosyphilis, in the era preceding the development of penicillin [Citation8].
Hyperthermia therapy is currently a subject of significant interest in the field of oncology, with continuous advancements in knowledge. In fact, a society dedicated to hyperthermia has been established. However, when it comes to musculoskeletal diseases, the utilization of this therapy is less grounded in scientific evidence and often relies more on empirical approaches [Citation8].
Effects of different whole-body hyperthermia applications on pain and cytokines
The first pioneering studies were conducted in the 1970s and 1980s. In animal experiments, hyperthermia has an immunosuppressive effect in adjuvant-induced arthritis in rats and secondary reactions were significantly reduced [Citation9].
In the meantime, it has now been clearly demonstrated that intensive local and systemic hyperthermia (target body core temperature > 41° C) have an immunosuppressive effect, whereas a mild, moderate systemic hyperthermia (target body core temperature 38–40° C) induces immunomodulating and immunostimulatory effects [Citation9]. The systemic application of whole-body hyperthermia has the advantage that deeper tissue structures can also be reached.
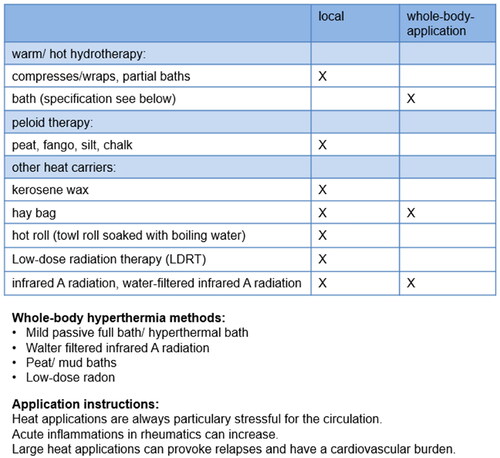
Various studies in recent years have shown that it is possible to influence the cytokine environment/milieu and other immunological mediators both through local heat therapy and through whole-body hyperthermia () [Citation10]. A reduction of prostaglandin E2 and leukotriene B4 is described by local heat applications [Citation11].
Table 1. Heat application and changes in cytokines [Citation9].
In the age of drug-based and expensive biological therapy, which is directed primarily against pro-inflammatory cytokines such as interleukin (IL)-1, IL-6 and tumor necrosis factor α (TNF-α), it is of particular interest whether and how these cytokines are influenced by physical therapy options and whether they induce an immunostimulatory or anti-inflammatory effect at the cellular and molecular level.
In the following, individual forms of whole-body thermotherapy will be presented. provides an overview.
Hyperthermal bath
Mild systemic hyperthermia in the form of a heat bath has been successfully used since the late 1960s as so-called passive mild whole-body hyperthermia (WBH) in AS. From an empirical point of view, WBH has effects on muscular detonation, blood circulation and analgesia [Citation9]. From the perspective of molecular physical medicine, however, the focus is on possible cellular and molecular mechanisms.
In an exploratory study in AS (9 serial overheating baths within 18 days, target body temperature 38.5 °C, duration 50 min), no hyperthermia-induced stress response could be objectified in the analysis of B- and T-lymphocytes and cortisol levels compared to a healthy control [Citation12]. On the other hand, the results at the molecular level were surprising. All pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) presented a significant reduction by 40- 50% (24 h after the last serial bath until the start of the last bath) compared to a healthy control [Citation13]. Thus, serial mild whole-body hyperthermia in AS results in heat-induced changes of the pro-inflammatory cytokine network.
Serial water-filtered infrared a radiation
This physical therapy application was analyzed as add-on to multimodal physical therapy for possible molecular changes and pain in two randomized, prospective trials in psoriatic arthritis (PSA) and AS (6 irradiations in 9 days, duration 45–60 min, target value rectal temperature 38.5° C), with controls receiving only multimodal physical therapy. PSA showed significant pain relief up to 3 months, which was reflected in a decrease in pain medication. The pro-inflammatory cytokines remained stable without significant changes and corresponded to the clinical course without exacerbation [Citation14]. A significant pain relief of up to 6 months could be objectified in AS with reduced pain medication. At the molecular level a significant decrease of IL-1ß and TNF-α was observed after 3 months compared to baseline [Citation15]. In parallel, both studies demonstrated significant improvements in disease activity and functional parameters.
The additive locoregional serial application of water-filtered infrared A radiation (2 applications daily over 6 days with a duration of 30 min) was recently examined in the back area of patients with AS. It was also possible to objectify a significant pain reduction at the end of the radiation series versus the initial value, which could also be observed on days 1, 2 and 6 on the respective evening vs. the daily starting value. There was a significant decrease in TNF-α levels at the end of the radiation series compared to the proven baseline value [Citation16].
Serial whole-body radon hyperthermia
The effects of serial radon hyperthermia-exposure (12 applications in 3 weeks, 60 min per application) were analyzed in RA, AS and OA. In RA, pain relief could be objectified, lasting up to 3 months after the end of therapy, in parallel with a clinically relevant pain reduction of at least 30% with a significant decrease in pain medication and a significant decrease in TNF-α levels at the end of therapy compared to baseline [Citation17,Citation18]. In AS patients, a significant reduction in pain could be observed directly at the end of the therapy, which lasted up to 3 months, in addition to a tendency toward a decrease in pro-inflammatory TNF-α at the end of the therapy compared to baseline [Citation19]. Patients with osteoarthritis showed no pain reduction at the end of therapy but did so 3 months after the end of therapy. However, at the end of the therapy there were already significant decreases in the TNF-α serum levels compared to baseline [Citation17,Citation18].
Serial mud baths and packs
The application of 9 serial mud baths (temperature 44 °C, 20 min) in 21 days in patients with osteoarthritis resulted in a significant pain reduction, which lasted up to 3 months [Citation20,Citation21]. Serial mud baths also caused a significant decrease in pain in RA and AS, in addition to a significant decrease in IL-1ß and a significant increase in IL-10 [Citation21,Citation22].
It is noteworthy that serial mud packs (9 mud packs in 21 days, temperature 44 °C, 20 min, thickness about 5 cm on both knees) also showed a significant decrease in pain in patients with knee osteoarthritis, in addition to a significant decrease in IL-1ß and a significant increase in IL-10 levels [Citation23].
Low dose radiation therapy
Low dose radiation therapy (LDRT) is used in chronic inflammatory and degenerative diseases, particularly in patients who have not responded to traditional therapies. The main goal of LDRT is to reduce pain and improve mobility. LDRT has been used for over 100 years to relieve pain in joint diseases, with effects lasting up to one year [Citation24]. It is typically applied as a localized (targeting one joint) irradiation using X-Rays, with a single dose ranging between 0.5 and 1.0 Gray (Gy) and a total dose not exceeding six Gy [Citation25]. Side effects from radiation are minimal in LDRT [Citation26].
Although the analgesic effects of LDRT are well-established, the specific molecular mechanisms of action are still being researched. Researchers believe that LDRT modulates the inflammatory reaction, as pain often correlates with inflammation. Preclinical and clinical studies have revealed that LDRT can suppress existing inflammatory and bone degeneration processes. LDRT impacts the secretion of pro-inflammatory and anti-inflammatory cytokines, creating a more favorable and anti-inflammatory microenvironment in the affected tissue [Citation27]. Additionally, LDRT positively affects bone metabolism by slowing down degradation and promoting new bone formation, thereby reducing degenerative processes [Citation28].
Overall, LDRT offers promising results for patients suffering from chronic inflammatory and degenerative diseases. Its ability to alleviate pain and improve mobility, combined with minimal side effects, make it an attractive treatment option for those who have not responded to traditional therapies.
Conclusion
In summary, the results of the described heat therapy studies show a clinically significant pain reduction and that this effect has a molecular correlate. Different modalities of heat therapy obviously have different effects on different molecular inflammation mediators.
Explanations of the molecular changes in pain sensation
The pro-inflammatory cytokines TNF-α, IL-1, IL-6 play a central role in the rheumatic inflammatory process. They not only act on the nociceptor, but also induce systemic effects. For example, IL-6 induces hepatic CRP synthesis. It is known that immunological mechanisms are directly related to nociception [Citation29,Citation30]. Glycoprotein 130 acts as a receptor for cytokines on polymodal nociceptors. In so-called inflammatory pain, there is an interaction between analgesic (including IL-1, −4, −10, −13) and hyperalgesic (including bradykinin, prostaglandins, IL-1, −6, −8, TNF-α) mediators. At the onset of inflammation, the hyperalgesic mediators dominate, while analgesic cytokines are simultaneously induced in the immune system [Citation29]. After binding to the receptor of the nociceptors, pro-inflammatory cytokines induce depolarization with consecutive transmission of impulses/stimuli to the dorsal horn of the spinal cord and from there to the cortex with central pain perception. If the pro-inflammatory cytokines are decreased, it is conceivable that the receptors of the nociceptors are less depolarized, and that less input to the cortical pain matrix is achieved via the pain pathways with corresponding aging of the conscious pain sensation [Citation31]. In addition, some of the sensory C-fibers, which act as silent nociceptors, are sensitized to mechanical and thermal stimuli during the inflammatory process and are therefore sensitive to nano-mechanics. This means that an influence on this process is conceivable even if inflammatory mediators are removed.
Finally, it should be mentioned that pro-inflammatory cytokines cause an increased production of nerve growth factor (NGF) in the tissue. Among other things, NGF causes tropomyosin receptor kinase (TrkA)-positive sensitive neurons to be activated and sensitized to mechanical, chemical and thermal stimuli, which among other things changes/alters the properties of A-delta fibers (sensitization). Blocking NGF-TrkA is known to reduce skeletal pain [Citation32]. It can therefore also be discussed that the cytokine changes/alterations shown in the studies induce a reduced production of NGF with consecutive desensitization of TrkA-positive sensitive neurons.
Taken together, the studies on heat therapy at the molecular level in rheumatic and musculoskeletal disorders demonstrated an influence on pro- and anti-inflammatory cytokines for the first time. A molecular correlate was thus demonstrated for the first time for the objective reduction of pain. Particular attention should be paid to the fact that different intensities and modalities of heat therapy seem to have different effects on different molecular markers. For the future, the research field ‘heat therapy and effects on the cytokine network in rheumatic diseases’ offers many interesting questions, also regarding the therapeutic spectrum and dosage.
Research needs and future perspectives
While significant advancements have been achieved, considerable gaps in our understanding persist. Addressing these gaps is crucial for advancing the application of hyperthermia in clinical practice.
A primary area requiring further exploration is the molecular mechanisms underlying hyperthermia treatment. Despite promising findings regarding cytokine modulation and pain reduction, the specific pathways and interactions at a molecular level remain inadequately defined. This lack of clarity hinders the development of more effective and targeted hyperthermia treatments.
Another significant gap is the limited scope of research on specific RMDs and patient populations. Current studies have predominantly focused on a subset of conditions and demographics. Expanding research to include a broader range of RMDs and diverse patient groups is essential to ascertain the generalizability and efficacy of hyperthermia across different clinical scenarios.
Safety concerns also warrant careful consideration. The long-term effects and potential adverse outcomes of hyperthermia treatment, especially in patients with complex medical histories or comorbidities, are not thoroughly understood. Systematic investigation into these aspects is imperative to ensure the safe integration of hyperthermia into therapeutic regimens.
Considering future research directions, there is a compelling need to explore novel hyperthermia techniques, such as advanced delivery methods, optimized treatment intensities and durations, and the potential of combination therapies. Personalized hyperthermia approaches, tailored to individual patient profiles or specific molecular markers, also present a promising area of investigation. Such personalized strategies could lead to more effective and efficient treatment modalities, potentially enhancing clinical outcomes for patients with RMDs.
In summary, while the field of hyperthermia treatment for RMDs has made notable strides, addressing the current gaps through targeted and comprehensive research is imperative. Such efforts could significantly enhance our understanding and application of hyperthermia, ultimately benefiting patients afflicted with these challenging conditions.
Conclusions for practice
With methods of molecular physical medicine it is now possible to analyze also central messengers of inflammation and pain development as well as their influence through heat therapy.
Different/various methods of serial heat therapy induce measurable influences on inflammatory processes and subjective pain sensation.
The results on a molecular level provide an explanation for the clinically detectable pain reduction for the first time.
The studies demonstrate a further development of heat therapy from an empirical, deductive to an evidence-based form of treatment.
The additive effect of heat therapy should be used in the therapy concept of rheumatic diseases, possibly a potential cost reduction of the expensive antirheumatic medication can be achieved.
Authors’ contributions
PK, NS, PB and UL made substantial contributions to the conception and design of the work; PK, NS, PB and UL made the acquisition, analysis, and interpretation of data; PK, NS, PB and UL have drafted the work and substantively revised it.
Competing interest
All authors declare no conflicts of interest.
Philipp Klemm declares no conflicts of interest.
Nils Schulz declares no conflicts of interest.
Priyanka Boettger declares no conflicts of interest.
Uwe Lange declares no conflicts of interest.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
| Abbreviations | ||
| AS | = | ankylosing spondylitis |
| IL | = | interleukin |
| NGF | = | nerve growth factor |
| OA | = | osteoarthritis |
| PSA | = | psoriatic arthritis |
| RA | = | rheumatoid arthritis |
| RMDs | = | Rheumatic and musculoskeletal diseases |
| TNF-α | = | tumor necrosis factor α |
| TrkA | = | tropomyosin receptor kinase |
| WBH | = | whole-body hyperthermia |
Acknowledgements
None
Availability of data and materials
Not applicable
References
- Mau W, Thiele K, Lamprecht J. Trends of work force participation of patients with rheumatic diseases. Z Rheumatol. 2014;73(1):1–6. doi: 10.1007/s00393-013-1205-y.
- Mau W, Beyer W, Ehlebracht-König I, et al. Trends in rehabilitation of patients with rheumatic diseases in Germany. Z Rheumatol. 2014;73(2):139–148. doi: 10.1007/s00393-013-1259-x.
- Albrecht K, Huscher D, Eidner T, et al. Medical treatment of rheumatoid arthritis in 2014 - Current data from the german collaborative arthritis centers. Z Rheumatol. 2017;76(1):50–57. doi: 10.1007/s00393-016-0156-5.
- Huscher D, Thiele K, Rudwaleit M, et al. Trends in treatment and outcomes of ankylosing spondylitis in outpatient rheumatological care in Germany between 2000 and 2012. RMD Open. 2015;1(1):e000033–e000033. doi: 10.1136/rmdopen-2014-000033.
- Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65(9):1175–1183. doi: 10.1136/ard.2005.046367.
- Lange U, Klemm P, Dischereit G. Physical therapy in rheumatic diseases – what is evidence-based? Arthritis Und Rheuma. 2020;40(01):9–14. doi: 10.1055/a-1071-5833.
- Albrecht K, Huscher D. Do we prescribe physical medicine sufficiently? Current data from the national database of the german collaborative arthritis centers. Aktuelle Rheumatologie. 2017;42:118–121. doi: 10.1055/s-0042-116425.
- Papaioannou TG, Karamanou M, Protogerou AD, et al. Heat therapy: an ancient concept re‐examined in the era of advanced biomedical technologies. J Physiol. 2016;594(23):7141–7142. doi: 10.1113/JP273136.
- Schmidt KL. Effects of whole body hyperthermia on inflammations and immune reactions: experimental aspects. Phys Rehab Kur Med. 2004;14(5):227–235. doi: 10.1055/s-2003-815023.
- Lange U, Müller-Ladner U, Schmidt KL. Balneotherapy in rheumatic diseases - An overview of novel and known aspects. Rheumatol Int. 2006;26(6):497–499. doi: 10.1007/s00296-005-0019-x.
- Huang YH, Haegerstrand A, Frostegård J. Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin Exp Immunol. 1996;103(1):61–66. doi: 10.1046/j.1365-2249.1996.00932.x.
- Lange U, Thielen G, Neeck G, et al. Effects of whole body hyperthermia on plasma cortisol, total lymphocytes and -Subpopulation in patients with ankylosing spondylitis and healthy controls. Phys Rehab Kur Med. 2005;15(1):44–47. doi: 10.1055/s-2004-834601.
- Tarner IH, Müller-Ladner U, Uhlemann C, et al. The effect of mild whole-body hyperthermia on systemic levels of TNF-alpha, IL-1beta, and IL-6 in patients with ankylosing spondylitis. Clin Rheumatol. 2009;28(4):397–402. doi: 10.1007/s10067-008-1059-x.
- Lange U, Schwab F, Müller-Ladner U, et al. Effectiveness of whole-body hyperthermia by mild water-filtered infrared A radiation in psoriatic arthritis: a controlled, randomised, prospective trial. Aktuelle Rheumatol. 2014;39:310–316. doi: 10.1055/s-0034-1383589.
- Lange U, Müller-Ladner U, Dischereit G. Effectiveness of whole-body hyperthermia by mild water-filtered infrared A radiation in ankylosing spondylitis – A controlled, randomised, prospective study. Aktuelle Rheumatol. 2017;42:122–128. doi: 10.1055/s-0042-116945.
- Klemm P, Eichelmann M, Aykara I, et al. Serial locally applied water-filtered infrared a radiation in axial spondyloarthritis – a randomized controlled trial. Int J Hyperthermia. 2020;37(1):965–970. doi: 10.1080/02656736.2020.1804079.
- Lange U, Dischereit G, Tarner I, et al. The impact of serial radon and hyperthermia exposure in a therapeutic adit on pivotal cytokines of bone metabolism in rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2016;35(11):2783–2788. doi: 10.1007/s10067-016-3236-7.
- Lange U, Dischereit G, Müller-Ladner U, et al. The impact of serial radon and hyperthermia exposure in a therapeutic adit on clinical parameters and pivotal cytokines in rheumatoid arthritis and osteoarthritis. Phys Med Rehab Kuror. 2017;27(02):87–94. doi: 10.1055/s-0043-104052.
- Dischereit G, Neumann N, Müller-Ladner U, et al. The impact of serial low-dose radon hyperthermia exposure on pain, disease activity and pivotal cytokines of bone metabolism in ankylosing spondylitis – A prospective study. Akt Rheumatol. 2014;39(05):304–309. doi: 10.1055/s-0034-1384554.
- Dischereit G, Fetaj S, Goronzy J-E, et al. Effects of serial mud baths in osteoarthritis on parameters of functional health and cytokines – A controlled, randomised, prospective trial. Aktuelle Rheumatol. 2017;42:129–136. doi: 10.1055/s-0042-118383.
- Dischereit G, Goronzy J-E, Müller-Ladner U, et al. Effects of serial mud baths on inflammatory rheumatic and degenerative diseases. Z Rheumatol. 2019;78(2):143–154. doi: 10.1007/s00393-018-0582-7.
- Lange U, Goronzy J-E, Müller-Ladner U, et al. Anti-inflammatory effect of serial mud baths in rheumatoid arthritis and ankylosing spondylitis. IOSR J Pharm Biol Sci. 2018;13:59–63. doi: 10.9790/3008-1302045963.
- Lange U, Fetaj S, Ehnert M, et al. Serial mud packs induce Anti-inflammatory effects in knee osteoarthritis – A randomized, prospective clinical study. Physikal Mediz Rehabilitationsmedizin Kurortmed. 2020;31(01):20–24. doi: 10.1055/a-1217-0749.
- Dove APH, Cmelak A, Darrow K, et al. The use of low-dose radiation therapy in osteoarthritis: a review. Int J Radiat Oncol Biol Phys. 2022;114(2):203–220. doi: 10.1016/J.IJROBP.2022.04.029.
- Ott OJ, Niewald M, Weitmann HD, et al. DEGRO guidelines for the radiotherapy of non-malignant disorders. Part II: painful degenerative skeletal disorders. Strahlenther Onkol. 2015;191(1):1–6. doi: 10.1007/s00066-014-0757-3.
- Torres Royo L, Antelo Redondo G, Árquez Pianetta M, et al. Low-dose radiation therapy for benign pathologies. Rep Pract Oncol Radiother. 2020;25(2):250–254. doi: 10.1016/J.RPOR.2020.02.004.
- Donaubauer AJ, Becker I, Weissmann T, et al. Low dose radiation therapy induces Long-Lasting reduction of pain and immune modulations in the peripheral blood – interim analysis of the IMMO-LDRT01 trial. Front Immunol. 2021;12:740742. doi: 10.3389/FIMMU.2021.740742.
- Deloch L, Derer A, Hueber AJ, et al. Low-Dose radiotherapy ameliorates advanced arthritis in hTNF-α tg mice by particularly positively impacting on bone metabolism. Front Immunol. 2018;9:1834. doi: 10.3389/FIMMU.2018.01834.
- Rittner HL, Brack A, Stein C. Pain and the immune system: friend or foe? Anaesthesist. 2002;51(5):351–358. doi: 10.1007/s00101-002-0306-9.
- Boettger MK, Hensellek S, Richter F, et al. Antinociceptive effects of tumor necrosis factor α neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58(8):2368–2378. doi: 10.1002/art.23608.
- Kulkarni B, Bentley DE, Elliott R, et al. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56(4):1345–1354. doi: 10.1002/art.22460.
- Peuker ET. Neuroanatomical basics of joint pain. Akt Rheumatol. 2016;41(04):300–305. doi: 10.1055/s-0042-111315.