?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
Pelvic recurrences from rectal cancer present a challenging clinical scenario. Hyperthermia represents an innovative treatment option in combination with concurrent chemoradiation to enhance therapeutic effect. We provide the initial results of a prospective single center feasibility study (NCT02528175) for patients undergoing rectal cancer retreatment using concurrent chemoradiation and mild hyperthermia with MR-guided high intensity focused ultrasound (MR-HIFU).
Methods
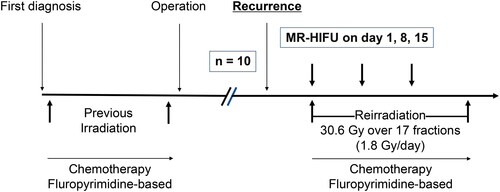
All patients were deemed ineligible for salvage surgery and were evaluated in a multidisciplinary fashion with a surgical oncologist, radiation oncologist and medical oncologist. Radiation was delivered to a dose of 30.6 Gy in 1.8 Gy per fraction with concurrent capecitabine. MR-HIFU was delivered on days 1, 8 and 15 of concurrent chemoradiation. Our primary objective was feasibility and toxicity.
Results
Six patients (total 11 screened) were treated with concurrent chemoradiation and mild hyperthermia with MR-HIFU. Tumor size varied between 3.1-16.6 cm. Patients spent an average of 228 min in the MRI suite and sonication with the external transducer lasted an average of 35 min. There were no complications on the day of the MR-HIFU procedure and all acute toxicities (no grade >/=3 toxicities) resolved after completion of treatment. There were no late grade >/=3 toxicities.
Conclusion
Mild hyperthermia with MR-HIFU, in combination with concurrent chemoradiation for appropriately selected patients, is safe for localized pelvic recurrences from rectal cancer. The potential for MR-HIFU to be applied in the recurrent setting in rectal cancer treatment requires further technical development and prospective evaluation.
Introduction
Colorectal cancer is the second leading cause of cancer mortality and third most common cancer diagnosis in the world [Citation1]. Locally advanced rectal cancer is managed with a total mesorectal excision in combination with radiotherapy and or chemotherapy (pre-operative or post-operative) as standard of care. Pelvic recurrences can carry significant morbidity depending on the location of the recurrence and often present with pain, obstruction or bleeding requiring symptom control [Citation2,Citation3]. Salvage surgery with or without reirradiation and/or chemotherapy can offer a chance for long term disease control and possible cure, however, only a minority of patients are candidates for re-resection leaving radiation and chemotherapy the only treatment option for most patients [Citation4–7]. Radical radiation is not an option as patients have generally had prior radiotherapy. New and innovative treatment options are thus needed to improve control and provide symptom relief.
Hyperthermia is a method of oncologic treatment where a target is heated to temperatures between 40–45 0C. Heat can lead to increased sensitivity of cancer cells to both radiation and chemotherapy through a variety of physiologic mechanisms including increasing tumor blood flow and oxygenation, denaturing nuclear proteins involved in DNA damage repair, and increasing drug extravasation [Citation8–11]. To date, hyperthermia has demonstrated efficacy in several tumor types including prostate, bladder, breast, head and neck, cervix and sarcoma [Citation12–18]. In these trials, a variety of heating methods from electromagnetic heating, ultrasound heating or hyperthermic perfusion have been applied. Through recent advances in technology, clinical systems can now utilize MRI to guide hyperthermia treatment with superior soft tissue target delineation and real time thermal mapping to ensure accurate heating of the target and minimizing effect to normal tissues. Preclinical studies demonstrate the technical development, safety and feasibility of hyperthermia utilizing MRI guided high intensity focused ultrasound (MR-HIFU) in ex-vivo and in-vivo models [Citation17,Citation19–22] and support the impetus for evaluation in the clinical setting.
As pelvic recurrences from rectal cancer present a challenging clinical scenario, hyperthermia can potentially enhance the therapeutic response to radiation and chemotherapy. We hypothesize that the addition of mild hyperthermia with MR-HIFU in patients being treated with concurrent re-irradiation and chemotherapy is a safe and feasible therapeutic option without increased toxicity. Herein we provide the initial results of our pilot study.
Materials and methods
We conducted a research ethics board approved, single center, prospective safety and feasibility study. This trial was registered with clinicaltrials.gov (NCT02528175). All potentially eligible patients were discussed at our local multi-disciplinary cancer case conference and assessed by a surgical oncologist, radiation oncologist and medical oncologist. Planned accrual was 10 patients. All patients had a baseline pelvic MRI and staging CT scan of the chest, abdomen and pelvis. Patients were eligible if they were at least 18 years of age with a biopsy proven recurrent rectal adenocarcinoma in the pelvis, had unresectable or inoperable disease, received prior pelvic radiotherapy, were fit for reirradiation and chemotherapy, target was visible on MRI, target was accessible to MR-HIFU procedure, lesion was 8 cm in depth, and were able to communicate during MR-HIFU procedure. Patients were excluded if they had any recurrent disease involving small bowel, had an orthopedic implant that would interfere with the MR-HIFU beam, were unable to tolerate the MR-HIFU treatment position or had serious medical comorbidities precluding treatment. All patients provided informed consent. The study treatment workflow is detailed in .
Radiotherapy and chemotherapy
Radiotherapy was delivered to encompass only the recurrent disease in the pelvis identified on MR and CT. Treatment was planned and delivered using standard institutional technique. Gross tumor volume (GTV) was delineated by the treating radiation oncologist and a clinical tumor volume (CTV) of at least 1 cm encompassed areas at risk of microscopic disease. The planning target volume (PTV) was a standard 1 cm expansion around the CTV. Radiation was delivered to a dose of 30.6 Gy in 1.8 Gy per fraction. Radiation planning occurred two weeks prior to the start date of the MR-HIFU procedure. Concurrent capecitabine was administered starting on the first day of radiation.
MRI guided focused ultrasound device description
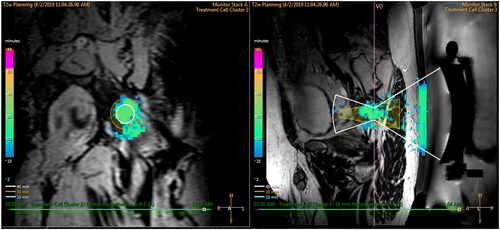
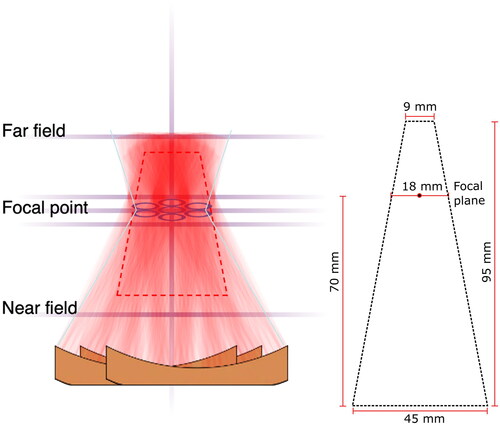
The MR-HIFU system (Sonalleve V2, Profound Medical, Mississauga, Canada) uses an external therapeutic ultrasound (US) transducer integrated into the treatment bed. It is mounted on a positioning system that allows for orienting the transducer toward the intended target. The transducer focuses a beam of US energy, which in situ is converted to heat, into a specified volume at a target site in the body. The US is applied in combination with MRI for superior soft tissue contrast and tumor visualization to deliver volumetric hyperthermia for target coverage and delivery of thermal dose. Real-time temperature maps were calculated from phase images of a dynamic multiple 2D Fast Field Echo - Echo Planar Image acquisition with in-plane resolution of 2.5 × 2.5 mm, updated every 3 s. The MR-HIFU system displays temperature data overlaid on 3D T2-weighted planning images. Artifacts in MR-thermometry introduced by temporal changes in the magnetic field inhomogeneity (drift) were compensated by a 3D linear fit using all voxels in the monitoring slices that were outside the beam path [Citation23]. In addition, it displays accumulated thermal dose information in the treated volume and the US parameters used to generate the thermal exposure (ex. transmitted output power). Temperatures are monitored at the near field, at the treatment plane, at planes above and below the treatment plane, and at the far field. Temperature is also monitored along the central beam path (). Based on our previous work, the average ± SD of temperature precision and stability were 1.0 ± 0.5 °C and 0.5 ± 0.2 °C [Citation19]. The device is capable of performing HT delivery to cover regions with a cross-section area ranging from a diameter of 18 mm, using only electronic steering, to diameters of 32, 44 and 58 mm using a combination of electric steering and mechanical movement of the therapeutic device [Citation14]. Each diameter option for HT delivery is identified as a treatment cell size. Each treatment cell has a predefined planned treatment volume (PTV) that is calculated based on the expected coverage of thermal energy deposition with the therapeutic device [Citation14]. illustrates the combination of HT delivery combining mechanical and electronic steering and an illustration of the PTV for the treatment cell of 18 mm, which corresponds to a volume of 63 cc.
Figure 2. Temperatures are monitored at the near field, at the treatment plane (in the plane of the focal point), at planes above and below the treatment plane, and at the far field. Temperature is also monitored along the Central beam path. Left - In this example, the combination of mechanical motion of the therapeutic device and electronic steering (individual circles in the focal plane) is illustrated. Right - Illustration of PTV for a treatment cell of 18 mm.
MRI guided focused ultrasound procedure
MR-HIFU mediated mild hyperthermia was delivered prior to scheduled radiation treatments on days 1, 8 and 15 of concurrent chemoradiation. Patients were brought into the procedure room and given an intravenous line for conscious sedation and analgesics. Patients’ baseline body temperature was measured orally and recorded. Patients were prepped and setup as previously reported [Citation18]. The rectum was filled with 200–300cc of normal saline. Patients were positioned in a rotated supine manner on the treatment table, with an ultrasound coupling gel pad placed between the MR-HIFU table and the participant’s skin. This placed the greater sciatic foramen parallel with the external transducer so that no intervening bone would be in the beam path. The radiation oncologist was responsible for locating and delineating the target tissue on the MR images. The operator then started the hyperthermia treatment and monitored the progress with MR based thermometry to ensure adherence to the treatment plan. Throughout the procedure, patients were able to provide verbal feedback to pause treatment upon feeling a heating sensation at skin or nerve. In a single weekly session, the hyperthermia delivery could be split into separate focused ultrasound sonications. Following completion of treatment, the patients were transported to the recovery room for supervision.
Outcomes
Our primary objective was to assess feasibility of treatment and toxicity. Acute toxicity was reported using CTCAE v4.0. Unacceptable toxicity was defined as any grade 3 toxicity within the first 3 months. The proposed treatment was deemed too toxic if more than 20% of patients experience unacceptable toxicity. Secondary objectives included MR-HIFU treatment accuracy, radiologic response, pain response, and patient quality of life (QOL). Accuracy of MR-HIFU treatment was determined by the average temperature during the best hyperthermia session. We also recorded the cumulative time the temperature was maintained within the range of 40–45 °C (time in range, TIR), the cumulative thermal dose at a reference temperature of 43 °C (CEM43), the 10th and 90th percentile (T10 and T90, respectively) of temperature values calculated over the area of the treatment cell size in the coronal plane at the focal distance perpendicular to the acoustic beam. We performed an approximation of the effective treated volume using cumulative TIR maps from the thermal monitoring at the beam plane, and from them, we segmented the regions showing TIR
5 min and eliminated the region corresponding to the ultrasound coupling pad. An ellipse was fit with the largest segmented region, and the effective treated volume (TIR volume) was estimated by the volume given by extrapolating the ellipse dimensions into an ellipsoid volume. Radiologic response was assessed with a CT and MRI at 90 days using response evaluation criteria in solid tumors (RECIST). Pain response was evaluated using the brief pain inventory (BPI) and QOL was evaluated with the functional assessment of cancer therapy-colorectal (FACT-C) questionnaire.
Results
A total of 11 patients were evaluated for possible inclusion in this pilot study. One patient was excluded as he was ultimately felt to be a candidate for upfront pelvic exenteration and underwent this operation; one patient was excluded due to an inaccessible tumor location in the presacral and coccygeal region which could not be targeted by the US transducer; one patient was ineligible due to involvement of the small bowel with concern for bowel perforation; one patient ultimately refused treatment and one patient was ineligible due to having received 2 prior courses of radiation therapy to the pelvis (45 Gy in 25 fractions and a further 30 Gy in 10 fractions). A total of six patients were eligible for hyperthermia treatment with MR-HIFU as part of their rectal cancer retreatment protocol. All patients were treated with prior pelvic long course chemoradiotherapy followed by total mesorectal excision. Patient demographics including lesion characteristics are detailed in . Tumor size varied from 3.1 cm to 16.6 cm in the greatest dimension. All patients were treated with a treatment cell size of 18 mm, given the available opening through the sciatic notch to the target tumor region. No complications were experienced on the day of the MR-HIFU procedure. Patients remained conscious throughout and provided verbal feedback. Patient 4 had severe pain from his recurrent tumor that persisted while on study protocol and could only complete one hyperthermia session. The patient was unable to tolerate his second session due to positioning and treatment had to be abandoned. He then decided to drop out of the trial completely.
Table 1. Patient demographics and lesion characteristics.
There was no acute or late skin/soft tissue damage observed in any of the patients. Three patients experienced grade 1 acute diarrhea. Grade 1 mucositis, tenesmus and abdominal distention was experienced by one patient each. All acute toxicities resolved after completion of treatment. Patient 1 originally presented with a left pelvic sidewall mass that was encasing the left distal ureter causing mild/moderate hydronephrosis at presentation. After completion of his treatment protocol, the mass reduced in size and hydronephrosis resolved. One year after completion of the trial protocol, he was noted to have new moderate hydronephrosis that was asymptomatic. It was unclear if this was secondary to malignant progression or progressive fibrosis. The obstruction resolved with the placement of a ureteric stent. As part of data safety monitoring, an independent radiation oncologist not associated with the study, determined this to be possibly related to protocol treatment and deemed this a grade 2 event. Patient 3 experienced an episode of somnolence 3 months after completing his MR-HIFU treatment and this was assessed by his physician to be due to newly started methadone and not due to his rectal cancer retreatment. Patient 4 experienced two adverse events within 4 months of his MR-HIFU treatment that were deemed unrelated to his rectal cancer retreatment. The first was an admission to hospital with opioid toxicity from methadone and hydromorphone that required dosage adjustment. The second was an admission to hospital for sepsis secondary to an infected nephrostomy tube requiring IV antibiotics and nephrostomy tube change.
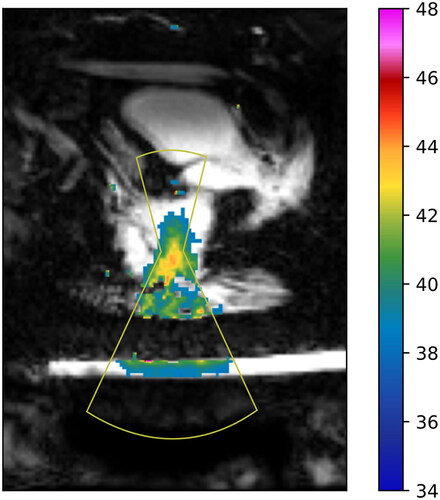
As shown in , on average it took 149 min for treatment planning (includes patient positioning). For most patients, subsequent sessions led to improvement and less time spent on this aspect of the treatment. On average, total time spent in the MRI suite was 228 min (measured from planning to the post-treatment imaging). Sonication (HIFU on) was maintained for an average of 35 min each session. Patients were observed in the recovery suite post MR-HIFU for an average of 49 min and subsequently went for radiation treatment, which lasted on average 13 min. and display an example of the thermal distribution of a patient during the MRI-HIFU procedure and TIR between 40–45 °C in minutes for patient 1. Overall, the thermal distribution for patients was within the desired temperature range at the target for the duration of the treatment. The best hyperthermia session (longest TIR) for each patient is displayed in . shows representative examples (patients 2, 3 and 6) of the average temperature measured for monitoring and control as a function of time. As different cooling periods with active MR thermometry were observed, metrics in were calculated until the time point when the ultrasound was stopped and the cooling period initiated. Patients averaged a hyperthermia treatment temperature between 39.4–42.5 °C. The average difference between the T90 and T10 temperature was 2.2 °C. Radiologic response is also noted in . Three patients experienced stable disease (SD), one patient experienced a partial response (PR) and 1 patient had progressive disease (PD) on surveillance MRI and CT scan at 90 days post treatment. demonstrates an example of a partial radiologic response post completion of re-irradiation with concurrent chemo and MR-HIFU treatment for patient 2.
Figure 3. Time (min) for each component of hyperthermia treatment days. Day 1, day 8 and day 15 displayed from top to bottom.
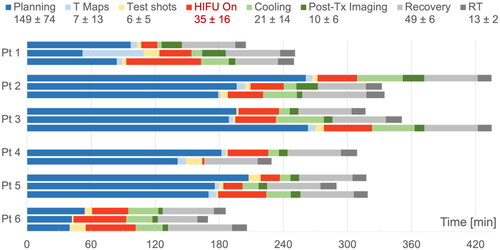
Figure 6. Temperature profiles at monitoring region for patients 2 (top), 3 (Middle) and 6 (bottom). Each profile corresponds to a combination of weekly session and sonication number; for example, ‘w1-s1’ corresponds to week 1 and sonication 1. The temperature profiles were calculated from the spatial average of the region of interest in the coronal view, which is perpendicular to the acoustic propagation and corresponds to the diameter of the acoustic beam of 18 mm.
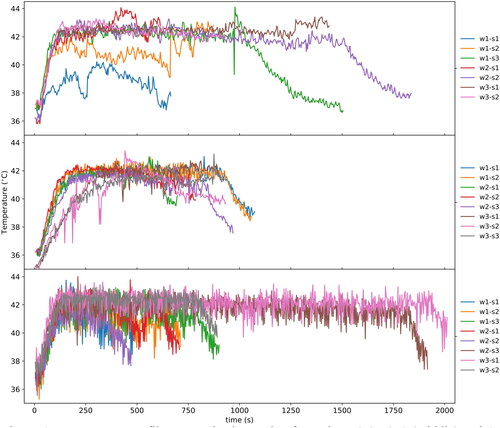
Figure 7. T2 weighted axial and sagittal MR images of patient 2. The images represent the maximum tumor extension in each orientation. Images A and B demonstrate pretreatment tumor volume. The tumor recurrence with large exophytic component shows a partial clinical response 3 months post treatment, as shown in images C and D.
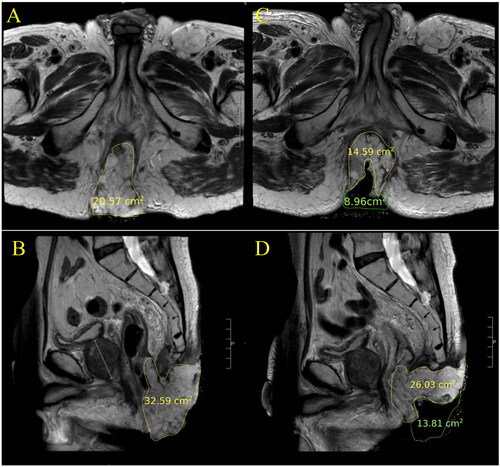
Table 2. Hyperthermia quality and radiologic response. T90 : temperature above 90th percentile, T10 = temperature below 10th percentile. TIR = time in range. CEM43 = cumulative equivalent minutes at the reference temperature of 43 °C. PR: partial response; SD: stable disease; PD: progressive disease.
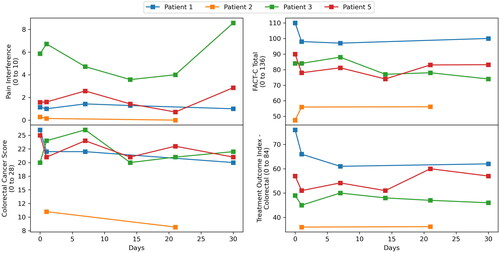
Pain interference and QOL scores are displayed in . Patient 4 dropped out of the trial after one session due to preexisting pain from his tumor and inability to tolerate the hyperthermia treatment position. As he formally dropped out of the study, no follow up imaging, BPI or QOL questionnaires were completed. Patient 6 was not compliant with filling out either the BPI or QOL questionnaires. From the limited data, there does not appear to be a strong trend in pain scores. Patient 3 initially appeared to have a response in pain score however it would subsequently rise again by day 30, consistent with the fact that post treatment MRI evaluation at 90 days demonstrated progressive disease. Scores on the FACT-C domains largely remained the same throughout treatment, demonstrating no adverse effect of MR-HIFU mild hyperthermia on patient QOL.
Figure 8. Pain interference and quality of life scores using FACT-C measured on day 1, day 7, day 14, day 21 and day 30. Patient 4 dropped out of the study after first hyperthermia session, hence did not complete follow up questionnaires. Patient 6 was not compliant with completing pain questionnaire.
Discussion
To our knowledge, we are the first to describe a prospective pilot study to test the safety and feasibility of MR-HIFU mild hyperthermia for recurrent rectal cancer patients. The feasibility of this approach using the same MR-HIFU system was first tested in a proof-of-concept study at our center using a swine model and patient volunteers for MR thermometry [Citation22]. Encouragingly, this patient trial demonstrated no soft tissue or radiologic damage from the addition of MR-HIFU mild hyperthermia to reirradiation and concurrent chemotherapy. In addition, acute toxicity was limited to grade 1 events. There were no serious adverse events (>/= grade 3) related to treatment despite the extensive tumors, suggesting the addition of MR-HIFU mild hyperthermia is a safe therapeutic strategy. Interpretation of outcomes for pain response and QOL are limited given the small sample size, short duration of treatment for patients 4 and 5, and incomplete data, however it appears that MR-HIFU mild hyperthermia did not lead to clear detriment in any domain. Future prospective studies would need to further investigate the impact of this modality on clinical outcomes, pain response and QOL.
We examined the feasibility of delivering MR-HIFU mild hyperthermia therapy on day 1, 8 and 15 of our reirradiation protocol. On average, approximately three and a half hours were required in the MRI suite per session for all patients. It is worth noting that the TIR volume () for all six participants exceeded the PTV (63 cc) associated with the prescribed treatment cell of 18 mm, indicating that the MR-HIFU system was capable of meeting the PTV as a minimum. We did observe a trend toward decreasing planning time with subsequent sessions and we would expect further refinement with increasing clinical experience and utilization of a virtual pre-planning workflow with diagnostic MRIs. We were successful in accurately heating the target to the desired temperature range. The average difference between the T90 and T10 temperature of 2.2 °C indicates good temperature uniformity. Our study is superior to previous hyperthermia studies in rectal cancer by overcoming limitations of inadequate tumor and soft tissue visualization, superficial and non-uniform heating, lack of quantification of thermal dose over space and time and overall lack of hyperthermia treatment detail [Citation23–29]. The clear target delineation of MRI enables accurate thermal dose delivery and sparing of normal tissues. This along with real time monitoring of the temperature and thermal dose distribution and ability for both patient and controller to stop treatment makes for an operational clinical system. Despite this, several technical limitations do exist. Specifically, limits to the heating depth due to transducer focal length, the potential for patient motion that may interfere with efficacy and accuracy of MR thermometry, and patient positioning requirements for an acoustic pathway into soft tissue can be challenging. These are significant factors that play a major role in the patient selection process and exclude patients who would otherwise benefit from the addition of MR-HIFU mild hyperthermia. One patient who was evaluated for enrollment on this trial was ultimately ineligible due to tumor location in the lower presacral and coccygeal region that could not be accessed by MR-HIFU system. In addition, certain clinical scenarios prevent us from delivering MR-HIFU mild hyperthermia due to the potential for life threatening complications. For example, one patient who was evaluated for enrollment on this trial was found to have tumor involving his small bowel which unfortunately could have put him at high risk of bowel perforation.
One limitation of the study was the lack of co-registration of the treated volume to planning images for radiation therapy. Radiation simulation and planning CT/MR scans were acquired in the supine position. The MRI planning scans for hyperthermia were acquired in a different treatment position and at a different time; hence, retrospectively generating a fused image in the exact same plane for radiation and heat was not feasible. Ideally, radiation and hyperthermia treatment plans would be generated from the same MR planning image datasets.
With regards to future applications, MR-HIFU mild hyperthermia may be an option for intensification of rectal cancer therapy not just in the recurrent setting but also in the upfront definitive setting. There is new drive in the last several years to maximize the opportunity for a clinical complete response (cCR). Several prospective studies have shown that surgery can be omitted in appropriately selected patients who have a cCR to neoadjuvant therapy [Citation30–32]. A non-operative approach has been included in guidelines published by the American Society of Colon and Rectal Surgeons and the National Comprehensive Cancer Network for appropriately selected patients managed in a multidisciplinary setting [Citation33,Citation34]. Recently published randomized phase III trials RAPIDO, STELLAR, and PRODIGE 23 [Citation35–38] reported the use of total neoadjuvant therapy (TNT) compared to long course neoadjuvant chemoradiation. The pathologic complete response (pCR) rate in the TNT arm of all three studies was increased compared to the standard arm, suggesting treatment intensification in the neoadjuvant setting can allow for a non-operative approach. We hypothesize that the addition of hyperthermia could be another modality to further intensify upfront rectal cancer therapy to maximize cCR. A recent phase II trial using hyperthermia with the BSD 2000/3D and BSD 2000 3D/MRI system (BSD Medical Systems, Salt Lake City, Utah, USA) in the neoadjuvant setting for rectal cancer was reported by Ghani et al. [Citation39]. This trial demonstrated a pCR rate of 14% with neoadjuvant long course chemoradiation only but patients with higher hyperthermia related cumulative temperatures showed greater tumor regression.
In conclusion, we were able to successfully treat patients with locally recurrent unresectable rectal cancer using a combination of MR-HIFU mediated mild hyperthermia, re-irradiation, and chemotherapy without unacceptable toxicity. Future research would aim to focus on validating the clinical benefit of MR-HIFU mild hyperthermia in rectal cancer treatment.
Disclosures statetment
No potential conflict of interest was reported by the author(s). Ari Partanen and Robert Staruch are paid employees of Profound Medical. All other authors on this manuscript have no financial or non-financial competing interests.
Acknowledgements
We would like to thank all the patients and families for their generosity in offering their time to participate in this study.
Data availability statement
All data generated and analyzed during this study are included in this published article.
Additional information
Funding
References
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: cancer Today. Lyon International Agency for Research on Cancer; 2020.
- Enríquez-Navascués JM, Borda N, Lizerazu A, et al. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17(13):1–11. doi: 10.3748/wjg.v17.i13.1674.
- Cottet V, Bouvier V, Rollot F, et al. Incidence and patterns of late recurrences in rectal cancer patients. Ann Surg Oncol. 2015;22(2):520–527. doi: 10.1245/s10434-014-3990-1.
- Guren MG, Undseth C, Rekstad BL, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol. 2014;113(2):151–157. doi: 10.1016/j.radonc.2014.11.021.
- Ng MK, Leong T, Heriot AG, et al. Once-daily reirradiation for rectal cancer in patients who have received previous pelvic radiotherapy. J Med Imaging Radiat Oncol. 2013;57(4):512–518. doi: 10.1111/1754-9485.12057.
- Sun DS, Zhang JD, Li L, et al. Accelerated hyperfractionation field involved re-irradiation combined with concurrent capecitabine chemotherapy for locally recurrent and irresectable rectal cancer. Br J Radiol. 2012;85(1011):259–264. doi: 10.1259/bjr/28173562.
- Koom WS, Choi Y, Shim SJ, et al. Reirradiation to the pelvis for recurrent rectal cancer. J Surg Oncol. 2012;105:637–642.
- Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77(4):399–408. doi: 10.1080/09553000010024687.
- Landon C, Park JY, Needham D, et al. Nanoscale drug delivery and hyperthermia: the materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J. 2011;3(1):38–64. doi: 10.2174/1875933501103010038.
- Lyon P, Gray M, Mannaris C, et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): a single-centre, open-label, phase 1 trial. The Lancet Oncology. 2018;19(8):1027–1039. doi: 10.1016/S1470-2045(18)30332-2.
- Rolf ID. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44(17):2546–2554. doi: 10.1016/j.ejca.2008.07.038.
- Van der Zee J, González D. The Dutch deep hyperthermia trial: results in cervical cancer. Int J Hyperthermia. 2011;18(1):1–12.
- Vernon C, Hand JW, Field S, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. international collaborative hyperthermia group. Int J Radiat Oncol, Biology, Physics. 2011;35(4):731–744.
- Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther. 2010;6(4):492–496. doi: 10.4103/0973-1482.77101.
- Issels RD, Lars HL, Jaap V, et al. Effect of neoadjuvant chemotherapy plus regional hyperthermia on long-term outcomes among patients with localized high-risk soft tissue sarcoma: the EORTC 62961-ESHO 95 randomized clinical trial. JAMA Oncol. 2018;4(4):483–492. doi: 10.1001/jamaoncol.2017.4996.
- Hurwitz M, Irving D, Jorgen LH, et al. Hyperthermia combined with radiation in treatment of locally advanced prostate cancer is associated with a favourable toxicity profile. int j Hyperthermia. 2005;21(7):649–656. doi: 10.1080/02656730500331967.
- Guillemin PC, Sinden D, M’Rad Y, et al. A novel concept of transperineal focused ultrasound transducer for prostate cancer local deep hyperthermia treatments. Cancers. 2022; 15(1):163. doi: 10.3390/cancers15010163.
- Van Valenberg H, Colombo R, Witjes F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32(4):351–362. doi: 10.3109/02656736.2016.1140232.
- Chu W, Staruch RM, Pichardo S, et al. Magnetic resonance-guided high-intensity focused ultrasound hyperthermia for recurrent rectal cancer: MR thermometry evaluation and preclinical validation. Int J Radiat Oncol Biol Phys. 2016;95(4):1259–1267. doi: 10.1016/j.ijrobp.2016.03.019.
- Guillemin PC, Gui L, Lorton O, et al. Mild hyperthermia by MR-guided focused ultrasound in an ex vivo model of osteolytic bone tumour: optimization of the spatio-temporal control of the delivered temperature. J Transl Med. 2019;17(1):350. doi: 10.1186/s12967-019-2094-x.
- Zhu L, et al. Characterization of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-induced large-volume hyperthermia in deep and superficial targets in a porcine model. Int J Hyperthermia. 2020;37(1):1159–1173.
- Zhu L, et al. Feasibility and safety assessment of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-mediated mild hyperthermia in pelvic targets evaluated using an in vivo porcine model. Int J Hyperthermia. 2019;36(1):1147–1159.
- Tillander M, Steffen H, Julius K, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Med Phys. 2016;43(3):1539–1549. doi: 10.1118/1.4942378.
- Berdov BA, Menteshashvili GZ. Thermoradiotherapy of patients with locally advanced carcinoma of the rectum. Int J Hyperthermia. 1990; 6(5):881–890. doi: 10.3109/02656739009140970.
- Rau B, Wust P, Hohenberger P, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: a phase II clinical trial. Ann Surg. 1998;227(3):380–389. doi: 10.1097/00000658-199803000-00010.
- Wust P, Rau B, Gellerman J, et al. Radiochemotherapy and hyperthermia in the treatment of rectal cancer. Recent Results Cancer Res. 1998;146:175–191. doi: 10.1007/978-3-642-71967-7_16.
- Kang MK, Kim MS, Kim JH. Clinical outcomes of mild hyperthermia for locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Hyperthermia. 2011;27(5):482–490. doi: 10.3109/02656736.2011.563769.
- Milani V, Pazos M, Issels RD, et al. Radiochemotherapy in combination with regional hyperthermia in preirradiated patients with recurrent rectal cancer. Strahlenther Onkol. 2008;184(3):163–168. doi: 10.1007/s00066-008-1731-8.
- Schaffer M, Krych M, Pachmann S, et al. Feasibility and morbidity of combined hyperthermia and radiochemotherapy in recurrent rectal cancer–preliminary results. Onkologie. 2003;26(2):120–124. doi: 10.1159/000069830.
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, et al. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev. 2009;3(3):CD006269. doi: 10.1002/14651858.CD006269.pub2.
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi: 10.1016/S0140-6736(18)31078-X.
- Kim H, Pedersen K, Olsen JR, et al. Nonoperative rectal cancer management with short-course radiation followed by chemotherapy: a nonrandomized control trial. Clin Colorectal Cancer. 2021;20(3):e185–e193. doi: 10.1016/j.clcc.2021.03.003.
- Garcia Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38(15_suppl):4008–4008. doi: 10.1200/JCO.2020.38.15_suppl.4008.
- You YN, Hardiman KM, Bafford A, et al. The American Society of colon and rectal surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020;63(9):1191–1222. doi: 10.1097/DCR.0000000000001762.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls.
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6.
- Jin J, Tang Y, Hu C, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. 2022;40(15):1681–1692.
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–715. May doi: 10.1016/S1470-2045(21)00079-6.
- Gani C, Lamprecht U, Ziegler A, et al. Deep regional hyperthermia with preoperative radiochemotherapy in locally advanced rectal cancer, a prospective phase II trial. Radiother Oncol. 2021;159:155–160. doi: 10.1016/j.radonc.2021.03.011.