Abstract
Introduction
There is an ongoing scientific discussion, that anti-cancer effects induced by radiofrequency (RF)-hyperthermia might not be solely attributable to subsequent temperature elevations at the tumor site but also to non-temperature-induced effects. The exact molecular mechanisms behind said potential non-thermal RF effects remain largely elusive, however, limiting their therapeutical targetability.
Objective
Therefore, we aim to provide an overview of the current literature on potential non-temperature-induced molecular effects within cancer cells in response to RF-electromagnetic fields (RF-EMF).
Material and Methods
This literature review was conducted following the PRISMA guidelines. For this purpose, a MeSH-term-defined literature search on MEDLINE (PubMed) and Scopus (Elsevier) was conducted on March 23rd, 2024. Essential criteria herein included the continuous wave RF-EMF nature (3 kHz − 300 GHz) of the source, the securing of temperature-controlled circumstances within the trials, and the preclinical nature of the trials.
Results
Analysis of the data processed in this review suggests that RF-EMF radiation of various frequencies seems to be able to induce significant non-temperature-induced anti-cancer effects. These effects span from mitotic arrest and growth inhibition to cancer cell death in the form of autophagy and apoptosis and appear to be mostly exclusive to cancer cells. Several cellular mechanisms were identified through which RF-EMF radiation potentially imposes its anti-cancer effects. Among those, by reviewing the included publications, we identified RF-EMF-induced ion channel activation, altered gene expression, altered membrane potentials, membrane oscillations, and blebbing, as well as changes in cytoskeletal structure and cell morphology.
Conclusion
The existent literature points toward a yet untapped therapeutic potential of RF-EMF treatment, which might aid in damaging cancer cells through bio-electrical and electro-mechanical molecular mechanisms while minimizing adverse effects on healthy tissue cells. Further research is imperative to definitively confirm non-thermal EMF effects as well as to determine optimal cancer-type-specific RF-EMF frequencies, field intensities, and exposure intervals.
1. Introduction
Heating of solid tumors to temperatures of 39 – 44° C (hyperthermia-therapy) by non-ionizing radiofrequency electromagnetic fields (RF-EMF) has been proposed as an additive cancer treatment to established therapy regimes, such as conventional irradiation and chemotherapy [Citation1, Citation2]. However, there are technical limitations to reaching the required temperatures deep within the body in clinical practice. Especially patients’ thermoregulation, thus perfusion-related heat dissipation from the targeted areas, still poses a significant limitation to efficient hyperthermia [Citation3].
Anti-cancer effects of RF-EMF sensitizing to chemo- and radiation therapy have thus far been attributed to induced temperature rises at the tumor site and the subsequent effects thereof, i.e., resulting in enhanced perfusion of tumor tissue, inhibition of DNA damage repair in tumor cells and immunomodulation, among others [Citation4]. However, recently, a growing body of research is pointing to the existence of RF-EMF-induced tumor-damaging effects beyond local temperature elevation [Citation5, Citation6]. There is more and more clinical data, for instance, emerging for the tumor-treating fields method (Novocure, Switzerland) using intermediate-frequency low-intensity RF-EMF at reported intra-tumoral temperatures below 38 °C [Citation7, Citation8] as well as reports of negligibly small temperature elevations (+ 1.58 °C) in cancer cells treated with RF-EMF of the GHz spectrum achieving significant cancer cell death [Citation9–11], pointing toward the clinical relevance of non-temperature-induced effects.
While the exact mechanisms by which RF-EMF might impose non-temperature-induced anti-cancer effects remain largely elusive, there is consensus within the scientific community regarding the existence of cancer cell-specific features exclusive to tumor cells that cannot be found in healthy tissue cells [Citation12–16]. Besides altered oncogene-, tumor promoter- and suppressor gene expression presented ubiquitously in cancer cells, these special cancer features are reported to lie in their distinct bio-electrical characteristics, such as their display of aberrant ion channel expression levels and membrane potentials, as well as their specific mechanical characteristics, i.e., altered cell membrane elasticity and aberrant cytoskeletal organization [Citation12–18].
Recently, it was proposed these cancer-specific characteristics might lay the ground for RF-EMF-induced cancer cell damage. Wust et al. [Citation6], for instance, hypothesize the overexpression of oncochannels in cancer cells, the specific tumor microenvironment (i.e., enhanced contact area between cells, due to higher cell density) [Citation6], as well as altered membrane elasticities [Citation5], might contribute to the enhancement of a potentially stimulative RF-EMF effect on ion channels and the cancer cell’s membranes. These phenomena may then result in potentially cancer cell fatal membrane oscillations, suggesting electro-mechanical consequences to cancer cells specifically [Citation6], as observed by i.e., Lin et al. [Citation19].
Electrical stimulation physiologically plays a vital role in regulating many cellular processes, such as i.e., metabolic and intracellular signaling pathways, osmotic regulation, differentiation, gene expression, migration, proliferation, and programmed cell death [Citation20, Citation21]. Exogenous electrical stimulation is already established in medical fields like ophthalmology and otolaryngology (retinal and cochlear implants) [Citation22], cardiology (cardioversion, cardiogenic defibrillation), and neurology (electrical neuronal stimulation, e.g., for treatment of depression, Parkinson’s, Epilepsy) [Citation23].
Albeit being an established therapeutic approach across many medical disciplines, targeted electrical stimulation of cancer tissue has not yet been explored as a potential addition to the established treatment regimens (as RF-EMF treatments of cancer patients so far solely rely on electrically induced tumor tissue heating, not the electrical stimulation of distinct cancer cell features).
However, due to their aberrant ion channel expression, altered membrane potentials, and distinct mechanical properties, such as altered membrane elasticities and cell morphology, the question arises whether the abovementioned features may render cancer cells particularly susceptible to the electrical effects of RF-EMF radiation, as proposed by i.e., Wust et al. [Citation5, Citation6].
The precise nature of molecular mechanisms activated upon RF-EMF exposure, more precisely the mode of action with which RF-EMF might induce the observed non-temperature induced anticancer effects needs to yet be examined and established. This review, therefore, aims to comprehensively gather and analyze the existing preclinical data on non-temperature induced molecular effects of RF-EMF on cancer cells to encourage scientific discussion and further examination of the RF-EMF treatment modality for cancer beyond its thermal use.
To ensure the non-temperature-induced nature of the RF-EMF effects analyzed throughout this review, temperature control was maintained across all trials in this review. This involved either the maintenance of isothermal conditions or RF exposure with direct comparison to traditional hyperthermia modalities, notably water-bath hyperthermia (WB-HT). It is well-established that hyperthermia monotherapies necessitate intra-tumoral temperatures exceeding 43 °C to invoke significant cancer cell death, encompassing cancer cell apoptosis and a general reduction in cell survival rates, as corroborated by Wust [Citation3]. Below this thermal threshold, cells are likely to exhibit thermotolerance, a phenomenon whereby a cell adapts to temperature elevations of up to 42.5 °C, thereby mitigating the occurrence of cell death and diminishing the likelihood of significant heat-induced anti-cancer effects independent of the heating duration [Citation3, Citation24].
However, combination therapies, integrating hyperthermia with other modalities such as chemotherapy or radiotherapy, have demonstrated enhanced tumor-damaging effects even at lower temperature increments, as demonstrated by Oleson et al. [Citation25] showing that soft tissue sarcoma cells subjected to combined RF hyperthermia (RF-HT) show significant anti-cancer effects (marked by necrosis) when intra-tumoral temperatures of at least 40.6 °C are reached during treatment. Recently, it has been shown that even mild hyperthermia starting at intratumoral temperatures of 39 °C (up to 41 °C) proves beneficial to treatment outcomes when HT + RT combination therapy is applied. Mild hyperthermia seems to contribute efficiently to increasing tumor perfusion, thus enhancing radiosensitization by overcoming tumor-related hypoxia [Citation26, Citation27]. Whilst combination therapies seem to show beneficial effects at mild temperature increases, it becomes evident [Citation3, Citation24], however, that hyperthermia, if applied as a mono-therapeutic approach, requires much higher temperature elevations up to approx. > 42.5 °C to achieve significant cancer cell death.
Thus, to secure thermally insignificant conditions within the review for valid examination of non-temperature induced RF-EMF effects on cancer cells, the studies included herein (except Wust et al. [Citation5]) show a maximum temperature increase of 1.58 °C (from baseline value 37 °C) (see ), thus deeming exclusively thermal causes of the effects observed highly unlikely, as elaborated on above.
Table 1. Molecular effects of RF-EMF on cancer cells, sorted by frequency spectrum.
Frequency determines the depth of RF energy penetration, with higher frequencies resulting in more surface heating due to their tendency to penetrate less deeply [Citation28]. At the same time, intensity governs the energy delivered per unit area, leading to increased heating during RF exposure [Citation28]. In addition to considering frequency and intensity, accurate temperature calculation requires considering multiple factors, including intensity, frequency, applicator placement, exposure duration, tissue conductivity, -permittivity, and -density [Citation28]. Thus, due to the complexity of reliable thermal calculations, direct temperature monitoring or control, which is integral to ensuring non-thermal treatment conditions, as seen in , is essential. By fulfilling these requirements, the molecular mechanisms described in this review’s trials are considered non-temperature-induced.
This literature review thus aims to comprehensively examine non-temperature-induced molecular effects in cancer cells across the entire RF-EMF spectrum (3 kHz − 300 GHz). Additionally, it will assess the identified molecular effects of RF-EMF radiation regarding their potential as therapeutic targets in cancer treatment. It is our main goal to encourage further research in the field of RF-EMF cancer treatments and further the scientific discussion on the potential RF-EMF therapy might hold beyond its heating abilities.
2. Methods
Inclusion and Exclusion criteria: This literature review was was conducted following the PRISMA guidelines (https://www.prisma-statement.org). It solely incorporates preclinical trials reporting on the molecular effects of continuous wave RF-EMF radiation on human and animal cancer cells, tumor biopsies, and cancer animal models. Preclinical studies comparing the effects of RF-EMF on nonmalignant cells compared to malignant cells were also included. Most importantly, the trials herein must focus on cancer cells, cancerous tissues, or utilize animal models of cancer as their objects of investigation as this review aims to collect and analyze the potential biological effects of RF-EMFs on cancer specifically.
All studies reporting effects induced by frequencies outside the RF spectrum were excluded. Studies lacking temperature control (i.e., temperature measurement of the experimental populations exposed to RF or comparison to traditional hyperthermia as a control, i.e., using a water bath) or those trials applying RF-EMFs inducing significant temperature rise within the cells (Temp. > 40.5 °C) were excluded as well, as it is our goal to investigate the non-temperature induced effects of RF-EMF exclusively.
2.1. Search strategy
In the search for relevant sources reporting on RF-induced molecular effects on cancer cells, a systematic search-term-defined literature search was conducted across different databanks, specifically PubMed (MEDLINE) as well as Scopus (Elsevier). Additionally, further literature was identified by examining the sources from papers previously gathered and selected for inclusion. To ensure the inclusion of relevant publications labeled under slightly different keywords than the original search terms used, the MeSH (Medical Subjects Headings) system was utilized. The literature search was conducted on the 23rd of March 2024, as described in more detail in Supplementary Document 1.
2.2. Data extraction
All selected sources were thoroughly examined regarding their fulfillment of inclusion and exclusion criteria as described above (). To provide a systematic sorting system for the relevant data extracted from the included sources, tables with predefined data categories were developed, in which all extracted information was registered (). This allowed for better observation of relevant data trends and facilitated comparability and contextual organization.
Figure 1. Schematic depiction of the systematic literature selection. *Publications with titles already revealing a conflict with predetermined inclusion and exclusion criteria (i.e. non-RF-nature of EMFs applied (i.e. ELF-EMFs), or the examination of strictly non-cancerous tissues only, application of non-continuous waves, combination therapies, invasive RF-approaches etc.) were excluded before abstract and full text screening.
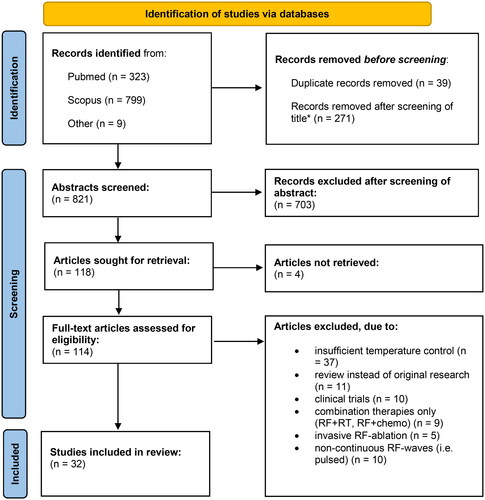
A risk of bias assessment was not performed as it was deemed unfitting for a preclinical review.
3. Results
Among the 32 preclinical studies incorporated in this review, a multitude of molecular effects within cancer cells induced by exposure to RF-EMF radiation were identified. provides an overview of the molecular effects observed within RF-EMF irradiated cancer cells and, if available, provides further information on the response of RF-EMF irradiated healthy tissue cells for comparing purposes. Specific information on the identified electro-mechanical effects and subsequent effects on cell viability, proliferation, and migration induced by varying time intervals, duration, and irradiation frequencies within the RF-EMF spectrum are also provided ().
The data extracted from the preclinical trials included in this review and summarized in allow for the observation of trends pointing to RF-EMF-specific non-temperature-induced effects that seem to be evoked within cancer cells upon irradiation. These effects will be described in the following.
3.1. Dosimetry & conditions of exposition
As stated previously, the circumstances surrounding exposure, including frequency, intensity, duration, and applicator design, as well as the probes subjected to RF exposure (such as (cancer) cell lines, tumor biopsies, or animal cancer models), exhibit notable variability. This variability precluded the possibility of conducting comprehensive statistical dosimetric comparisons. However, to equip the reader with a valuable tool for better comprehension of the specific dosimetric conditions, we allocated each preclinical trial referenced in the subsequent results section and listed within to a distinct dosimetry group. Each group was delineated based on preclinical trials employing analogous frequency, intensity, and SAR range. Within , dosimetry groups are arranged in ascending order of frequency, starting from the lowest frequency and ending with the highest frequency. Within each frequency group, further elaboration is provided on the applied intensities within the group (). This approach facilitates a rudimentary dosimetric comparison and enhances the interpretation of the ensuing effects described herein.
Group 1: The ‘TTF’ category, characterized by intermediate frequency and low intensity, encompasses trials applying a frequency range predominantly spanning 100-300 kHz, with two exceptions made by Karkabounas et al. [Citation29] who studied multi-frequency exposure at 10 kHz − 120 kHz, as well as 600 kHz exposure, performed Mamaghaniyeh et al. [Citation30]. Intensity applied by all Group 1 trials varies from 0.5-3 V/cm, for all but two trials (Smother et al. [Citation31] apply 1-6 V/cm; Chang et al. [Citation32] applying 4 V/cm).
Group 2: ‘Therabionic/AutEMDev’ approaches fall into the High Frequency, Low-Intensity treatments category. The incorporated trials mentioned herein focus on frequencies between 27.12 MHz and 147 MHz, with SAR values ranging from 0.01 to 0.4 W/kg. Notably, this group is characterized by consistent amplitude modulation with a specifically high modulation index of 80-85%.
Group 3: ‘Millimeter wave (MMW) + Microwave RF’ fall into the Extremely High Frequency, Low-Intensity category, frequencies span from 900 MHz to 105 GHz, with incident power densities (IPD) ranging from 0.001 to 0.2 mW/cm2 [Citation11, Citation33], as well as low SAR (0.0038 − 1 W/kg) [Citation10, Citation34] levels and field intensities 0.2898 V/cm [Citation9] indicated by those authors not providing the reader with specific IPD values.
Group 4: ‘Others’:
This category incorporates two treatment approaches with noteworthy results that however differ from the abovementioned groups in their treatment approaches, namely: 1) ‘Capacitive Hyperthermia’ (HT) applications, typified by intermediate frequency and high intensity, consistently operate at 13.56 MHz, with emitted power set at 10 Watt. 2) ‘Cold Atmospheric Plasma’ (CAP), a partially ionized gas composed of various reactive species emitting RF at multiple frequencies: 12.5 kHz, multiple microwave emission at GHz, and photon emission in the UV-VIS range and an emitted power of 10 V/cm.
Additionally, special mention is attributed to further studies employing multi-frequency exposure [Citation19, Citation29] or wide-band frequency sweeps [Citation11, Citation33], as they introduce unique complexities in dosimetric analysis, which will be elaborated upon in the discussion section.
The distribution of sources across treatment groups in our analysis reveals notable disparities, which could potentially introduce biases in our data interpretation. Group 1 (TTF) appears overrepresented with 20 sources, likely due to its low-intensity profile and widespread clinical acceptance and availability. This popularity has led to intensified research efforts, contributing to the abundance of literature available for this treatment modality. Conversely, Group 2 (Therabionic/AutEMDev) with only four sources as well as Group 3 (Millimeter wave + Microwave-RF) with 6 sources are notably underrepresented when compared to the abundance of preclinical trials available for Group 1 (TTF). Especially underrepresentation of Group 3 (millimeter wave-/microwave-RF) might additionally stem from its limited clinical applicability in oncology due to its comparably low penetration depth, leading to a smaller body of available preclinical literature on this treatment modality.
Furthermore, it is important to note that the preclinical trial conducted by Wust et al. [Citation5] exhibits significant temperature increases during exposure to capacitive HT, reaching up to 42 °C. Despite these temperature elevations, comparisons with control groups treated using traditional water baths at similar temperatures (42°) suggest that the effects identified within the experimental group that go beyond what was observed in or lacked in the control group altogether can be viewed as non-temperature-induced effects.
3.2. RF-EMF radiation decreases cancer cell proliferation, viability, and metastasis
Of the 32 preclinical trials analyzed, 26 directly reported reduced cancer cell proliferation, viability, or migration in vitro and in vivo in animal tumors upon RF-EMF treatment. Six trials [Citation30, Citation32, Citation35–38] do not directly address viability, proliferation, or migratory behavior of exposed cell or animal populations; however, they do report significant alterations in gene expression, cellular morphology, and ionic concentrations, suggesting potential anti-tumor effects. The absence of direct measurements on viability, proliferation, or migration in the abovementioned studies may be due to one of two causes. One: the authors did not observe any significant effect upon cancer cell exposure to RF-EMF which might be the cause why data in this regard does not appear in the abovementioned studies, as seems to be the case for the studies conducted by Jeong et al. [Citation38] and Chang et al. [Citation32]. Or two: the lack of data does not necessarily suggest a lack of significant impact on cancer cells but instead simply underscores a gap in the assessment of these parameters, as there is no mention to be found of exploration of these parameters for the remaining four publications [Citation30, Citation35–37]. Overall, the vast majority of preclinical trials thus demonstrated a significant reduction in proliferation, migration, or cell viability following RF-EMF application. The mechanisms underlying these effects are diverse and potent RF-EMF frequencies for inducing such effects span a wide range of the RF-EMF spectrum.
3.2.1. Cell proliferation
Several authors in this review report significantly reduced cell proliferation following RF-EMF treatment. Within the intermediate RF-EMF spectrum between 100-300 kHz applied through TTF or TTF-like applicators (generating an alternating electric field that is applied to the patient’s body via pairs of insulated wires), a reduction in cell proliferation within a multitude of different cancer cell lines in vitro as well as reduced tumor growth in animal models in vivo could be identified, when compared to untreated sham controls, demonstrated by the works of, i.e., Lei et al. [Citation39] (p < 0.001, human cervical adenocarcinoma, hepatocellular carcinoma cell lines), Kirson et al. [Citation40] (p < 0.05, human melanoma, glioma, lung, prostate, and breast cancer cell lines as well as mouse melanoma, rat glioma, and mouse adenocarcinoma cell lines), Karkabounas et al. [Citation29] (p < 0.05, Leiomyosarcoma cells and smooth muscle cells extracted from Wistar rats) and Kim et al. [Citation41] (reduced clonogenic efficiency p < 0.001, human glioblastoma cells. Furthermore, Smothers et al. [Citation31] highlight the dependence of anti-proliferative effect achievement on tumor-specific dosimetry (here: correct choice of intensity). Uniform TTFields exposure at 1.5 V/cm for 24 h significantly impedes the proliferative rate of triple-negative breast cancer (TNBC) cells, resulting in a notable decrease in cell count (p < 0.0001) [Citation31]. However, TNBC cells display rapid recovery after 48 h of TTFields treatment, reaching nearly full confluence by 72 h (p = 0.0015), thus also indicating the reversibility of the observed effects when treatments like TTF are not applied continuously. Notably, at 4.4 V/cm, TTFields demonstrate no discernible impact on either cell line (p = 0.408) [Citation31], thus indicating that application of higher intensities seems to not always necessarily result in more substantial effect achievement (here: reduction of cell count). Zimmerman et al. [Citation42] on the other hand focus on the importance of cancer-type specific modulation, indicating a notable reduction in proliferation among specific cancer cell lines, particularly HepG2 and Huh7, with respective decreases of 60% (p = 0.00993) and 14% (p = 0.018) after exposure to modulation frequencies tailored to hepatocellular carcinoma (HCC), as also supported by the findings of Jimenez et al. [Citation43], Sharma et al. [Citation44], and Dutta et al. [Citation35]. Furthermore, Zimmerman et al. [Citation42] found that THLE-2 cells, representing healthy liver cells, show no significant response to the same HCC-specific modulation frequencies (p = 0.6550) [Citation42]. Additionally, MCF-7 cells exhibited a 30% growth inhibition (GI) (p = 0.0230) when exposed to breast tumor-specific modulation frequencies, while MCF-10A cells, another healthy cell line, demonstrated negligible growth inhibition (GI) (p = 0.8579) under similar conditions [Citation42]. Notably, MCF-7 cells exposed to HCC-specific modulation frequencies exhibit only a 3.99% growth inhibition (GI) (p = 0.8815), thus further highlighting the importance of tailored amplitude modulation for eliciting significant effects. These findings, in conjunction with the results reported by Smothers et al. [Citation31], suggest that optimizing treatment through tumor type-specific settings, such as intensity and modulation frequencies, may enhance the efficacy of inhibiting cancer cell proliferation.
Furthermore, cell proliferation inhibitory effects were observed after high-frequency RF-EMF. Jimenez et al. [Citation43] observe significant proliferation inhibition after 27.12 MHz amplitude-modulated RF-EMF treatment with Therabionics (spoon-shaped applicator emitting AM-RF-EMF at 27.12 MHz with tumor-specific amplitude-modulation) for mice xenografts with implanted human hepatocellular carcinoma cells (reduced tumor size p = 0.019 after six weeks of treatment) and Sharma et al. [Citation44] for several human breast carcinoma cell lines (reduced cancer cell proliferation p < 0.05) respectively.
Beneduci et al. [Citation33] similarly report growth inhibitory effects after RF-EMF application of the microwave spectrum at 53-78GHz in MCF-7 human breast cancer cells (reduced cell growth by 60%). These findings suggest that a broad range of specific frequencies, rather than a singular narrow RF-EMF range, can induce significant anti-proliferative effects in cancer cells.
3.2.2. Cancer cell viability
Among the incorporated literature, 14 preclinical trials consistently report a substantial reduction in cancer cell viability following RF-EMF radiation. The molecular mechanisms inducing cancer cell death manifest through various fatal pathways. Silginer et al. [Citation45] observe significant levels of cancer cell autophagy (p < 0.05) after TTF exposure in human long-time glioma cell lines. Kim et al. [Citation46] report a reduction in viability (p < 0.05) in human glioblastoma (GBM) cell lines, while [Citation28] mitotic cell death is reported by Giladi et al. [Citation47] and Gera et al. [Citation48], further adding to the spectrum of responses. Apoptosis induction is demonstrated by Lee et al. [Citation49] in human liposarcoma cell lines (p < 0.01), Wust et al. [Citation5] in human colorectal cancer cell lines (p < 0.00005), Gökcen et al. [Citation9] in human colonic adenocarcinoma cells (p < 0.05), and Xu et al. [Citation50] in human GBM cell lines (p < 0.001). The varied time intervals and durations of RF-EMF radiation applied across different frequency ranges, as detailed in , highlight the variability in effective radiation duration for different treatment settings and approaches, ranging from a brief 5 min to several days of continuous radiation [Citation40, Citation51].
3.2.3. Cancer cell invasiveness, migration, and metastasis
Several authors report anti-migratory and metastasis-inhibitory effects within cancer cells post RF-EMF exposure. Kirson et al. [Citation52] confirm a reduction in metastasis in melanoma-inoculated mice and VX2 rabbit liver tumors upon TTF at 100 kHz (mice) and 200 kHz (rabbits). After continuous TTF treatment for seven consecutive days, tumor-bearing mice showed a significant decrease in the number and weight of lung metastasis compared to untreated controls (p < 0.01) [Citation52]. Kim et al. furthermore report the inhibition of epithelial-mesenchymal transition and reduced invasion of glioma cell lines treated with TTF (p < 0.001) [Citation41], while Lee et al. [Citation49] show reduced migration of liposarcoma cells after application of TTF (p < 0.001). Furthermore, Xiang et al. show an inhibition of cell migration by 80% (200 kHz TTF) p < 0.0001, with migration decreasing inversely as the intensity rises from 0.5-2 V/cm in glioblastoma cells U87-MG as well as Smothers et al. reporting significantly reduced migration (p = 0.0004) after 24h 1.5 V/cm 150 kHz (TTF) treatment. Sharma et al. [Citation44] present similar inhibitory effects on metastatic behavior after the application of high RF (27.12 MHz) on brain metastasis, which were significantly reduced after RF-EMF treatment (p < 0.05).
Karkabounas et al.’s study [Citation29] introduces divergent findings, reporting an unchanged metastatic potential in leiomyosarcoma cells after RF-EMF exposure in the intermediate frequency spectrum. This conclusion is based on their measurement of the ability for platelet aggregation, a crucial factor for successful metastasis. However, the absence of data on the metastatic state of in vivo animal models prompts the need for further confirmation of these results.
Overall, several authors presented strong evidence for the reduction of metastasis and invasiveness of cancer cells in vitro and in vivo following RF-EMF treatment [Citation31, Citation41, Citation44, Citation49, Citation52, Citation53] one preclinical trial reported an unchanged metastatic potential upon RF-EMF treatment [Citation29]. The remaining trials [Citation9, Citation19, Citation29, Citation33–35, Citation39, Citation40, Citation43, Citation45–48, Citation50, Citation51, Citation54–59] do not present any data on metastatic behavior in regards to RF-EMF exposure.
3.2.4. RF-EMF effects on healthy cells
To validate the selectivity of RF-EMF-induced cell damage, several authors compare the effects of RF-EMF radiation on cell viability and proliferation within cancer cells to those in healthy tissue cells [Citation11, Citation29, Citation31, Citation39, Citation40, Citation42, Citation43, Citation51]. Karkabounas et al. [Citation29] demonstrate a 98% reduction in viable leiomyosarcoma cells isolated from tumor-bearing Wistar rats after 48 h of intermediate-frequency, low-intensity RF-EMF (10KHz -120KHz) treatment (p < 0.0001), while smooth muscle cells isolated from the Wistar rat’s aorta show no significant reduction in cell viability after the same duration, frequency, and intensity of RF-EMF radiation (p < 0.4). Branter et al. [Citation51] observe a selective reduction in cell viability in rapidly proliferating brain tumor cells (GBM) (p < 0.0001) as well as in healthy neural stem cells (p < 0.0024) after intermediate-frequency RF-EMF (TTF). In contrast, healthy astrocytes’ cell viability, considered non-proliferating cells, remains unaffected by the same TTF treatment schedule [Citation51].
These findings are supported by Kirson et al. [Citation40] (BHK cells: changes in cell viability and proliferation, p = 0.97), Komoshvili et al. [Citation11] (no significant effect on cell mortality in healthy breast epithelial cells (MCF-10A) after exposure to wide-band millimeter waves for up to 16 min), Lei at al [Citation39]. (slight proliferation stimulation instead of arrest in fibroblasts after TTF exposure), and Jimenez at al [Citation43]. who did not measure any significant changes in cell viability or proliferation upon RF-EMF (Therabionics) radiation of healthy tissue cells. Zimmerman et al. [Citation42] similarly examined the impact of Therabionics RF on cell proliferation, reporting that healthy THLE-2 liver cells showed no significant proliferation changes (p = 0.6550). In contrast, HepG2 and Huh7 HCC cells exhibited reduced proliferation (60% reduction with p = 0.00993 for HepG2 and 14% reduction with p = 0.018 for Huh7) with HCC-specific modulation. Nonetheless, while THLE cell proliferation remained unaffected by Therabionics treatment, THLE-2 cells displayed a significant upregulation of PLP2 expression following exposure to HCC-specific modulation frequencies (p = 0.00055). Smothers et al. [Citation31] found that TTF induced significant cell death in triple-negative breast cancer (TNBC) cells at 1.5-3 V/cm (p < 0.0001), while healthy breast epithelial cells showed negligible effects. However, at 6 V/cm, healthy breast epithelial cells showed even higher cell death rates than TNBCs (p = 0.0017) [Citation31]. Branter et al. [Citation51] similarly displayed significantly reduced cell counts for several GBM cell lines at their respective tumor-specific optimal frequency (100, 200, 300, and 400 kHz), while non-dividing astrocytes remained unaffected. However, a significant reduction of human neural stem cell counts following TTFields treatment of 41% and 37% (p = 0.0018 and p = 0.0024; t-test) after TTF at 200 kHz and 400 kHz for 72h [Citation51], suggesting potential adverse effects on healthy rapidly dividing cells.
These results illustrate the complexity of RF-EMF interactions with healthy cells and underscore the need for further research to gain a more comprehensive understanding and establish safe and efficient exposure regimens.
3.3. Bio-Electrical effects
Several preclinical trials incorporated in this review report of electrical stimulation of cancer cells upon RF-EMF exposure. Said electrical stimulation seems to predominantly occur in the form of RF-stimulated ion movements, subsequently potentially triggering changes in protein expression patterns as will be elaborated on below. All potential bio-electric effects reported within the analyzed literature are presented in the following:
3.3.1. RF-EMF stimulates cancer cells electrically and induces potential cancer-damaging electrical changes
Four publications included in this review [Citation35, Citation43, Citation44, Citation57] confirm significant ion fluxes across cancer cell membranes. Neuhaus et al.’s [Citation57] findings identify the L-type calcium channel CACNA1C as a potential RF-stimulation target. They measure a substantial increase in free Ca2+ within GBM cells after 20 min of TTF stimulation (p < 0.01). Applying benidipine, a calcium channel blocker, abolishes the TTF-induced Ca2+ rise, confirming Ca2+ channel opening rather than general membrane damage. Neuhaus et al. [Citation57] also show a significant rise in free cytoplasmic Ca2+ with increasing intensity. Applying 2.5 V/cm induces significantly higher Ca2+ influxes than TTF at 0.25 V/cm (p < 0.05). Furthermore, a significant potassium efflux occurs in GBM cells upon TTF stimulation (p < 0.01), caused by the opening of BK K + channels [Citation57].
Neuhaus et al. [Citation57] also report the dissipation of the mitochondrial membrane potential in GBM cells after TTF stimulation (p < 0.01), potentially causing apoptosis. As BK K + channels (Big Conductance - Calcium-activated Potassium channels) respond to elevated Ca2+ levels and are not directly voltage-gated, they may activate through CACNA1C opening. BK K + channels, found in the mitochondrial membrane, regulate the mitochondrial membrane potential [Citation60], suggesting they might mediate the observed changes in mitochondrial potential by Neuhaus et al. [Citation57].
Jimenez et al. [Citation43] and Sharma et al. [Citation44] identify the T-type calcium channel CACNA1H as a potential RF-EMF target. Both report significant Ca2+ influx into human breast cancer cells (p < 0.01) and hepatocellular carcinoma cells (p = 0.0016), respectively. Sharma et al. [Citation44] link RF-EMF-induced calcium influx into breast cancer cells to subsequent anti-proliferative effects. RF-EMF radiation significantly reduced cancer cell proliferation via CACNA1H opening (p < 0.05) compared to cells receiving Ca2+ channel blockers (Ethosuximide). Sharma et al. [Citation44] present a model for the molecular mechanisms underlying the correlation between Ca2+ influx and the observed anti-proliferative effects. RF-EMF-induced calcium influx may modulate gene expression via intracellular calcium elevation, influencing Ca2+-sensitive cytosolic enzymes that modulate intrinsic pathways regulating proliferation, metastasis, and apoptosis. Sharma et al. detected a significant downregulation of HMGA2 and miR1246 expression levels (p < 0.05) in breast carcinoma cells after RF-EMF exposure compared to sham-treated controls. They elaborate that CAMKII, activated via Ca2+ accumulation, induces ß-catenin degradation. As ß-catenin usually positively influences HGMA2 and miR1246 expression, reduced levels due to Ca2+-induced ß-catenin degradation lead to anti-proliferative and metastasis inhibitory effects. As HGMA2 and miR1246 are known to control metastasis and cancer cell proliferation [Citation44], the authors thus deliver an explanatory approach for Ca2+-induced anti-proliferative and metastasis inhibitory effects following RF-EMF treatment.
Aberrant gene expression in cancer cells post-RF-EMF exposure is detected by several preclinical trials [Citation9, Citation34, Citation41, Citation46, Citation50, Citation51, Citation59]. Survivin and miR-29b, anti-apoptotic factors, are downregulated, while pro-apoptotic factors, including p53, p21/WAF1, p73, bax, and caspase-3, show upregulation after RF-EMF treatment. Specific intracellular signaling pathways are activated or repressed, negatively affecting cancer cell proliferation and viability.
Caraglia et al. [Citation10] suggest that MW-EMF exposure reduces proliferative genes through heat shock protein (HSP)-dependent mechanisms. They found that 3 h of MW exposure decreased HSP90 expression by 5-fold while upregulating HSP20 and HSP70 expression. Additionally, MW-EMFs induced 2-fold ubiquitination of Ras and Raf-1, leading to their reduced expression and activation of the proteasome-dependent degradation pathway [Citation10]. This indicates a potential mechanism for MW-EMF-induced apoptosis by inactivating the HSP90/multi-chaperone complex and degrading key proteins essential for cell proliferation and survival signaling [Citation10]. Interestingly, overexpression of HSP90 counteracted MW-EMF-induced apoptosis, highlighting its protective role [Citation10].
Whether the differential expression of genes involved in cell fatal cascades is directly activated by RF-EMF induced ion signaling as described by Sharma et al. [Citation44], caused by protein instability due to reduced concentration of chaperones as proposed by Caraglia et al. [Citation10] or rather is a result of otherwise induced RF-EMF related cell damage, which in turn might activate said cell fatal pathways is not fully understood yet and needs further research.
3.3.2. RF-EMF-induced electro-mechanical stimulation causes aberrant cancer cell morphology and potential cancer cell damage through disturbance of cellular mechanical processes
The influence of RF-EMF on cellular dynamics extends beyond mere electrical effects, provoking an intricate interplay of electro-mechanical stimulation that subsequently shapes cancer cell morphology and may potentially cause damage by disrupting fundamental cellular mechanical processes within cancer cells. Thus, it can be observed throughout the literature that RF-EMFs seem to induce morphological changes by targeting various cellular structures, as described in the following:
3.3.2.1. Cytoskeletal organization and mitotic processes
Treatments like TTF are believed to hinder specific intracellular processes by electrically polarizing certain particles vital for the formation of, for instance, the spindle apparatus and cleavage furrow during mitosis, thus imposing anti-proliferative effects [Citation40]. This suggests that RF-EMF may target cancer cells in an electro-mechanical manner (electrical polarization (via RF-EMF) seemingly leading to a mechanical consequence, i.e., disturbance of spindle apparatus formation). This phenomenon becomes evident when analyzing data on mitotic and cytokinetic disturbance following RF-EMF exposure, as extensively examined and described by various authors in this review [Citation30, Citation34, Citation36, Citation38, Citation40, Citation42, Citation47, Citation48, Citation51, Citation53]. The literature herein suggests RF-EMF interferes with the specific arrangement of highly polarized, spatially oriented proteins in mitotic cells, such as tubulin and Septin 7. Kirson et al. for instance, propose that RF-EMFs polarize tubulin protein dimers, forcing their alignment with the electric field [Citation40]. This inhibits tubulin-(de-)polymerization, crucial for correct spindle apparatus formation, leading to increasing incidences of multi-spindled cells [Citation38], suppressing chromosome segregation, as well as proper chromosome alignment [Citation36], and ultimately inducing mitotic disturbance, and inhibiting cancer cell proliferation. Giladi et al. [Citation47] report increased depolymerized tubulin after intermediate-frequency RF-EMF exposure (p = 0.011). They observe increased cells with multiple nuclei after RF treatment (p < 0.001), consistent with Gera et al.’s findings of abnormal nuclei in RF-irradiated cells (p < 0.0003). Notably, Le et al. [Citation36] reported the presence of multinucleated cancer cells after TTF treatment, thus reinforcing the observation of aberrant nuclear morphology upon TTF exposure. Gera et al. [Citation48] report a significant decrease in correct Septin 7 localization within the cleavage furrow after RF-EMF exposure (p < 0.0003). This may lead to genomic instability, evidenced by increased cells with divergent chromosome counts, potentially followed by mitotic catastrophe and cell death [Citation47]. Disturbance of mitosis and cytokinesis provides an explanatory approach for selective cancer cell damage induced by electrical RF-EMF stimulation, attributed to the cancer cell’s highly proliferative state compared to resting healthy tissue cells.
Concomitantly with mitotic and cytokinetic disruption caused by TTF-induced disturbance of cytoskeletal mitotic organization, most of the preclinical trials mentioned above provide data on subsequent cell cycle disruption as a potential consequence of the abovementioned morphological phenomena. Le et al. [Citation36] demonstrated a significant increase in the duration of mitosis and cytokinesis in HeLa cells following TTFields treatment, with notable p-values (< 0.001). Similarly, Giladi et al. [Citation47] observed a prolongation of mitotic duration in cancer cells induced by TTFields, leading to mitotic cell death, with significant p-values (< 0.001)., supported by Kirson et al. [Citation40] showing a significant prolongation of mitosis upon TTFields treatment (p < 0.01). These observations highlight the molecular effects of TTFields on mitotic processes, suggesting a mechanism by which TTFields disrupt cancer cell division and proliferation.
Furthermore, Jeong et al. [Citation38] reported that TTFields induce cell cycle arrest at the G2/M phase transition in U373 cells, as evidenced by an increased percentage of cells in the G2/M phase. Branter et al. [Citation51] supported these findings, demonstrating G2/M-phase accumulation in pediatric brain tumor cell lines following TTFields treatment, with significant p-values indicating a predominantly cytostatic effect on cell cycle progression. Interestingly, Buttiglione et al. [Citation34] observed RF-induced apoptosis accompanied by cell cycle arrest and accumulation in the G2/M phase, coupled with a decrease in the proportion of cells in the G0-G1 phase. Notably, Buttiglione is the only author to observe cell cycle-relevant effects in a frequency range different from TTFields, utilizing 900 MHz high-frequency low-intensity fields.
3.3.2.2. Cancer cell morphology
Interestingly, several authors describe significant morphological changes upon RF-EMF exposure that might be indicative of anti-migratory, anti-proliferative, and cell-damaging effects [Citation11, Citation29, Citation32, Citation33, Citation36, Citation38, Citation42, Citation47]. RF-EMF-treated cancer cells exhibit increasing nuclear and cell membrane blebs [Citation53], smoothing cell membranes by losing pseudopodia and microvilli alongside highly condensed DNA (heterochromatin) and multinucleation. Komoshvili et al. [Citation11] observed distinct morphological changes in cellular shape and size that appear directly related to cancer cell progression into an altered (inactive or non-viable) state. The authors describe distinct morphological changes in H1299 lung cancer cells following exposure to millimeter waves (MMW). Under the 4-min MMW regime, H1299 cells exhibited increased nuclear size, irregular shape, and prominent nucleoli, indicative of apoptosis [Citation11]. Additionally, the study noted that following exposure to MMW, H1299 lung cancer cells exhibited morphological changes associated with senescence. These changes included an increase in cell and nuclei sizes, as well as flattened forms with kidney-like nuclei shapes. The temporal kinetics of senescence differed from apoptosis, with senescence peaking on Day 14 for 2 and 4-min exposures. This suggests that a subset of MMW-exposed H1299 cells underwent senescence as an alternative fate to apoptosis [Citation11]. Thus, it becomes evident that morphological changes may likewise indicate cell fate post-RF-treatment.
3.3.2.3. Genomic structures
Karanam et al. [Citation37] propose the concept of TTF-induced mechanical damage extending to the genomic level. However, instead of targeting the distribution of genetic material within the cytoplasm during mitosis, as Kirson et al. [Citation40] and others described, the authors herein report direct alterations of genomic structures intra-nuclearly. Exposure to Tumor Treating Fields (TTFields) leads to increased γ-H2AX foci and decreased expression of replication initiation and elongation genes (MCM10 and MCM6), resulting in replication stress and DNA damage [Citation37]. Moreover, TTField exposure induces significant reductions in DNA fiber length (p < 0.05) and increases R-loop formation (p < 0.05), thereby exacerbating genomic instability in cancer cells [Citation37]. These findings underscore the multifaceted impact of TTFields on cellular processes, including DNA replication and mitotic regulation, suggesting RF-induced cell death might occur through genomic instability.
3.3.2.4. Cellular membranes
Wust et al. [Citation5] propose that cancer cell membranes, with heightened membrane elasticity compared to healthy tissue counterparts, as observed by the authors, may serve as significant targets for RF-EMF-induced cancer cell damage. They show a significant increase in colorectal cancer cell membrane elasticity with increasing malignancy grade when compared to membranes of healthy fibroblasts and hepatocytes (p < 0.005) (healthy cells: E = 10-25 kPa; CRC cells: E = 2.3 kPa). RF-EMF treatment thus potentially induces significant membrane oscillations in phase with externally applied RF-EMF if the external source meets the cancer cells’ specific resonant frequencies, possibly leading to membrane rupture and cancer cell damage [Citation5].
Recent work by Lin et al. [Citation19] supports this hypothesis, observing marked cell membrane oscillations within cancer cells in phase with externally applied RF across various frequencies. Lin et al. hypothesize that external alternating-current RF-EMF electrically stimulates the ion cloud surrounding the cell membrane, creating significant oscillations if the externally applied radio frequencies match the natural frequency. If RF-EMF of matching frequency hits the cell membrane perpendicularly, transverse sound waves across the membrane interface are produced, leading to significant membrane oscillations. Lin et al. measure time-dependent height changes in cell membranes in response to three different frequency spectra: 1) 12.5 kHz by traditional paired electrode RF, 2) 8-18GHz emitted by a microwave horn antenna, 3) cold atmospheric plasma (CAP) discharge tube emitting three major wavelength groups: 12.5 kHz, multiple microwave emission at GHz, and photon emission in the UV-VIS range. Both the plate electrodes at 12.5 kHz and the multiple frequency emissions produced by the CAP discharge tube show marked correlations between RF-EMF and subsequent cell membrane oscillations, appearing in phase. Lin et al. emphasize the importance of meeting the natural frequency of the targetable cell membrane, which is tightly connected to the membrane’s resonance capacity. The authors measure the natural frequencies of three cancer cell lines and corresponding healthy cell lines, finding a significant difference in natural frequencies within most cell line pairs. While two cancer cell lines (U87 gliomas, A59 lung carcinoma epithelial cells) tended to resonate at higher frequencies within the kHz spectrum (ca. 4-6 kHz), their corresponding healthy tissue cells (normal astrocytes, normal lung tissue cells) exhibited natural frequencies well below 1 kHz. The third pair shows an opposite correlation, with the melanoma cell (B16F10) exhibiting a much lower natural resonance frequency than the corresponding healthy fibroblast [Citation19]. A clear distinction in membrane resonance of cancer cells vs. healthy cells is achieved for all three cell line pairs, yet a final tendency regarding the divergence of cancer cell membrane resonance from healthy cells resonance by increased or decreased resonance frequency cannot be determined yet. While all three RF-emission sources create a significant decrease in cell viability (plate electrodes: p = 0.04, MW-antenna p < 0.01, CAP discharge tube p < 0.0001), CAP discharge emission causes the most significant decrease in cell viability, despite traditional two-plate RF at 12.5 kHz reflecting the natural frequency of cancer cell membranes well [Citation19]. The authors propose that the multifrequency approach from the CAP discharge tube might be superior in its cell viability-reducing function, targeting the natural frequencies of several cellular structures and states, thereby potentiating RF-EMF effects within cancer cells.
Chang et al. [Citation32] similarly report significant membrane changes observed upon exposure of glioblastoma and astrocytoma cell lines to 200 kHz TTFields. The authors detected significantly increased Ethidium D uptake in U87-MG/eGFP-fLuc cells (p < 0.0001), indicating enhanced plasma membrane permeability. Moreover, TTField exposure notably augmented the uptake of 5-ALA (photodynamic substance) into these cells within 6 h (p = 0.047), maintaining the increase for up to 24 h (p = 0.011) as well as increased dextran FITC4 (fluorescent dextran) uptake (p < 0.01 for 20 kDa) [Citation32]. Furthermore, scanning electron microscopy (SEM) analysis demonstrated a substantial rise in the number and size of membrane holes induced by TTFields (p = 0.0002 and p = 0.0005, respectively), which were reversible after 24 h without exposure (p = 0.007 and p = 0.0007, respectively) [Citation32], again indicating the need for continuous TTF exposure as described earlier [Citation31]. The authors furthermore found that TTF exposure in their trial induces structural changes in cancer cell membranes, transitioning from densely matted extensions to shorter, bulbous structures. In contrast, the membrane morphology of normal human PCS-201 cells seemed to remain unaffected [Citation32], thus rendering interactions of TTF with membraneous or submembranous (cytoskeletal) elements as possibly responsible for this effect. Lastly, the increase in hole size and number might be of clinical use, as they seem to increase chemotherapeutic drug uptake significantly.
Conclusively, these findings suggest the ability of RF-EMF treatment to disrupt various cellular structures, including the plasma membrane, spindle apparatus, and DNA replication machinery, leading to anti-proliferative effects.
4. Discussion
Analysis of the literature above provides a first overview of the manifold non-temperature-induced molecular effects observable upon RF-EMF radiation on cancer cells and suggests that specifically ion channels and cell mechanics seem to be targeted via RF-EMF treatment.
While most of the analyzed pre-clinical trials offer evidence of cancer-damaging treatment outcomes (i.e., proliferation inhibition, metastasis reduction, cancer cell death) in addition to the described molecular effects, not all of the trials offer evidence that the molecular effect described upon RF stimulation are directly linked and causal to the subsequently observed cancer cell damage. This means that whilst in some cases the reported molecular effect might indeed be responsible for the observed cancer cell death, in other cases this correlation was not evidenced by the authors. This has to be kept in mind in order to understand that not all of the above-described molecular effects have been evidenced to directly influence cancer cells negatively.
Nevertheless, notably, none of the studies reviewed, observed any cancer cell proliferation or migratory stimulation of cancer cells upon RF-EMF treatment. This is especially noteworthy as the observed RF-EMF effects on cancer cells thus seem to contrast with the reported positively stimulatory effects of EMF on healthy tissue cells. There, RF-EMF treatment is utilized to reduce inflammation and increase cell proliferation, applied therapeutically for i.e., wound healing, bone growth, and neurological stimulation (transcranial magnetic and electrical stimulation for depression, Parkinson’s, Alzheimer’s) [Citation21, Citation61–63].
However, careful dosimetry is crucial for safe and effective RF therapy. Preclinical trials analyzed here offer insights into optimal intensity and frequencies for therapeutic application. Silginer et al. [Citation45], Xiang et al. [Citation53], Branter et al. [Citation51], Karkabounas et al. [Citation29], Kim et al. [Citation46], Jeong et al. [Citation38]and Kirson et al. [Citation40] all report on the importance of optimal frequency choice for TTF application in each different cancer cell line. They report each cancer cell line to exhibit a specific frequency optimum at which its anticancer effects are highest. Applying frequencies outside this optimum leads to either reduced magnitude or a complete lack of significant cancer-damaging effects. Zimmerman et al. [Citation42], Jimenez et al. [Citation43], Sharma et al. [Citation44] and Dutta et al. [Citation35]highlight a significant increase in treatment efficacy when applying cancer-type-specific modulation frequencies at a comparably high modulation index of (80-85%) with carrier frequencies within the low-moderate MHz spectrum. Similarly, these authors report reduced magnitude or lack of significant cancer-damaging effects when other random modulation frequencies are applied instead of resonant cancer-type specific amplitude modulation frequencies. Similar, if not more severe effects could be observed concerning the choice of applied electric field intensities. While some authors report a rise in treatment efficiency (rates of cancer cell death) with increasing treatment intensity, i.e., Silginer et al. [Citation45], Kirson et al. [Citation40], Neuhaus et al. [Citation57], two authors within this review, Smothers et al. [Citation31] Branter et al. [Citation51] observed a significant reduction in breast epithelial cell count when significantly rising treatment intensity from the commonly applied TTF 1-3 V/cm to 6 V/cm) [Citation31] as well as a reduction in highly proliferating neural stem cells when exposed to an in vitro applicator modeling the commonly applied TTF Optune device (1-3 V/cm) [Citation51] While the large majority of the trials included in this review show no adverse effects within healthy cells upon RF-EMF exposure, the findings of Branter et al. [Citation51] and Smothers et al. [Citation31] nevertheless underscore the importance of carefully titrating RF intensity to avoid unintended consequences on healthy cells yet achieving significant cancer cell damage and highlight the need for further examination of RF-EMF effects on healthy cells within therapeutic ranges.
Thus, precise dosimetry is essential to avoid adverse health effects, as highlighted by the International Commission on Non-Ionizing Radiation Protection (ICNIRP) [Citation64]. The ICNIRP emphasizes the importance of limiting RF exposure to prevent whole-body heat stress and excessive localized heating to a maximum specific absorption rate (SAR) level of 2 W/kg for the general public [Citation64]. Notably, all preclinical trials included in this review, indicating SAR values, applied SARs ≤ 1 W/kg, thus well below the recommended SAR threshold of 2 W/kg. The rest of the trials indicated field intensity instead of SAR values. They reported the use of EMFs marked by very low intensities ≤ 3 V/cm (except for Lin et al. [Citation19] and Smothers et al. [Citation31] as elaborated on above) thus securing the potential for significant anti-cancer effects at seemingly safe exposure levels. The evaluation of RF electromagnetic fields (EMF) as a potential carcinogenic factor, as evidenced by the International Agency for Research on Cancer (IRAC) [Citation65] classification that was established based on the outcomes of the INTERPHONE study as well as the ‘Pooled analysis of case-control studies on malignant brain tumors and the use of mobile and cordless phones’ conducted by Hardell et al. [Citation66], suggests careful consideration of RF-EMF application nevertheless [Citation64]. While the IRAC classifies RF-EMF as ‘possibly carcinogenic to humans’ (Group 2B) based on what the IRAC labels as ‘limited evidence’, other studies [Citation67] including the large-scale prospective cohort COSMOS study [Citation68], on the other hand, suggest that long-term mobile phone use and thus the exposure to RF-EMF frequencies in the mobile phone range is not associated with an increased risk of developing glioma, meningioma, or acoustic neuroma. Furthermore, the ICNIRP emphasizes that RF exposure below the thermal threshold is unlikely to be associated with adverse health effects. This is consistent with the findings of preclinical studies included in the review, which apply low intensities of RF therapy well below the recommended SAR levels.
This being said, nevertheless, the literature suggests that RF-EMF holds significant therapeutic potential for inducing substantial damage selective to cancer cells. However, as elaborated above, correct dosimetry of the applied EMFs is vitally important and must be secured to facilitate efficient and safe treatment outcomes.
The distinctive response of cancer cells toward RF-EMF stimulation, in terms of proliferative and migratory inhibition as well as enhancement of cancer cell death, seems to not be reproducible in non-rapidly dividing healthy cells [Citation11, Citation29, Citation39, Citation40, Citation43, Citation51]. This opens up the question of where this seemingly selective targeting of cancer cells during RF-EMF exposure might be rooted. One explanatory approach might be that cancer cells may be more susceptible to RF-EMF-induced damage due to their unique bio-electrical and mechanical characteristics. Cancer cells showcase elevated base depolarization in the electrochemical potential across their plasma membranes compared to normal cells [Citation69, Citation70]. This deviation influences the behavior of ion channels, particularly voltage-gated ion channels (VGICs), which exhibit high sensitivity to changes in membrane potential [Citation71]. Additionally, cancer cells often upregulate VGICs, which can present as several-fold significant increases in channel density and number [Citation14–16, Citation72–74], thus offering a possible potentiation of the RF-EMF effect on ion movement due to a significantly higher number of possible targets (here ion channels) as proposed by Wust et al. [Citation6].
Furthermore, cancer cells also possess distinct mechanical properties, notably altered plasma membrane elasticity compared to healthy cells [Citation5, Citation12, Citation13, Citation17, Citation18, Citation75, Citation76]. This affects their natural resonance frequency, clearly differentiating the resonance spectrum of cancer cells from the resonance spectra found in healthy tissue cells [Citation19] which provides a valid explanation for the selective induction of damaging cell membrane oscillations in cancer cells via RF-EMF radiation as described by Lin et al. [Citation19]. The variations in observations regarding cancer cell membrane elasticity can be attributed to the complex and heterogeneous nature of cancer [Citation77]. Multiple factors contribute to the mechanical properties of cancer cell membranes, including alterations in lipid composition, changes in cytoskeletal proteins, and modifications in membrane-associated receptors [Citation77]. Additionally, the specific type and stage of cancer, as well as the genetic makeup of individual tumors, can influence membrane elasticity differently [Citation77]. Moreover, differences in experimental techniques and methodologies across studies can also contribute to discrepancies in findings [Citation78]. Therefore, the observed increases or decreases in cancer cell membrane elasticity may reflect the diversity of cancer biology and underscore the need for further research to elucidate the underlying mechanisms.
The specific cell lines examined by Lin et al. are generally considered to exhibit higher elasticities and softer properties than their healthy tissue correlates [Citation79–81]. Thus, one would expect the natural resonance frequencies of cancer cell membranes examined by Lin et al. to be significantly lower than the resonance frequencies observed in healthy tissue cells exhibiting a higher Young’s modulus and thus possessing higher levels of stiffness [Citation5, Citation12, Citation13, Citation17]. There is an ongoing discussion about the specific resonance frequencies of cancer cells. While some authors like Jaganathan et al. [Citation82] (modeling the resonance behavior of healthy breast tissue cells vs. breast cancer cells (MCF-7) as spherical bodies) find the resonance frequency of healthy cells to be significantly higher than the resonance frequencies measured for breast cancer cells (p < 0.05), other authors like Lin et al. [Citation19] or Heyden et al. [Citation83] measure higher natural resonance frequencies for cancer cell’s plasma as well as nuclear membranes compared to healthy cells of similar origin. Thus, the search for the correct cancer resonance frequencies is ongoing and a topic of current scientific discussion, although it can be expected that different cancer types will exhibit somewhat different resonance frequencies, which will have to be determined individually, as mechanical and morphological properties, despite showing clear trends that allow the distinguishment between healthy and cancerous cells, still show slight variations among different cancer cell lines.
Assuming a linear elastic model, one would expect cancer cells with heightened membrane elasticity to exhibit lower natural frequencies than healthy cells with more rigid membranes. We point out that cells form a viscoelastic system exhibiting a broader range of behaviors. Adair [Citation84], for instance, considers the occurrence of membrane oscillations upon RF-EMF radiation unlikely as, due to the embedment of all membranes in a viscous surrounding, the ‘viscous impedance’ would significantly dampen and ultimately prevent any oscillating behavior theoretically occurring in isolated membranes. Fröhlich [Citation85] on the other hand, proposes that under particular circumstances, when the imposed RF-EMF precisely matches the natural resonance frequency of a biological membrane, oscillation behavior of the membrane may indeed occur. Thus, it becomes evident, when measuring membrane resonance, other factors besides membrane elasticity influence resonance behavior as well, such as the cancer cell’s environment (i.e., extracellular matrix) as well as the cytosol (which is a non–Newtonian fluid) and their mechanical attachment to and grouping with neighboring cells as well as intracellularly located shape-determining features, such as the cytoskeleton.
Cancer cells are known to exhibit a highly rigid extracellular matrix and a morphologically altered cytoskeleton [Citation76, Citation86], which might influence cancer cell’s resonance behavior significantly. For instance, the actin skeleton in breast cancer cells is characterized by a thinned-out, disorganized actin layer localized intracellularly directly underneath the cell membrane, which is usually prominently built and well organized in parallel bundles in healthy cells, supporting their membrane resilience [Citation76]. Due to this cytoskeletal change, some cancerous cell membranes seem less supported by their underlying actin skeleton, possibly leading to their heightened elastic properties and proneness to RF-EMF-induced membrane damage such as membrane blebbing [Citation19, Citation48].
RF-EMF treatment induces various morphological alterations in cancer cells, including membrane smoothing, aberrant nuclei structures, chromosome counts, blebbing of intracellular vesicles, and altered intracellular morphology [Citation29, Citation33]. This so-called ‘membrane-smoothing’ might be of particular importance as said cell protuberances are vitally important for the migratory activity of all cells, including cancer cells [Citation33, Citation87]. Thus, RF-EMF-induced eradication of cell protuberances might contribute to reduced migration in the treated cancer cells, as described above (section 3.2). Whether the protuberances on cancer cells disappear upon RF-EMF treatment due to direct mechanical damage induced by RF-EMF or whether the formation of protuberances might be inhibited by, i.e., forced polarization of the actin filaments along the EMF and thus inhibition of their correct polymerization, a process vital for the formation of protuberances in the first place (in the same manner tubulin is forcibly polarized during mitosis during TTF treatment [Citation40], is unclear and needs further investigation.
Besides the abovementioned RF-EMF-induced electro-mechanical effects in cancer cells, another observation that currently still lacks understanding is the fact that the simultaneous application of multiple frequencies, as described by Lin et al. [Citation19] seems to deliver superior anti-cancer results compared to traditional two-plate electrode emitting a single MHz-RF or single GHz-RF. The absence of a singular optimal frequency for targeting cancer cells via RF-EMF is suggested by the fact that multiple specific frequencies seem to be able to induce potent anti-cancer effects in the same cell line. For instance, MCF-7 breast cancer cells respond to both 150 kHz TFF RF [Citation47] and GHz spectrum RF (53.57-78.33) [Citation33]. This pattern extends across various cancer cell lines in this review, highlighting the effectiveness of multiple frequencies across the RF-EMF spectrum for inducing significant anti-cancer effects. An explanation for this observation might be that different frequencies target different specific cellular components, such as microtubules, vesicles, DNA structures, and mitochondrial membranes, as each cellular component is known to exhibit a distinct elastic modulus due to morphological and compositional differences at which resonance can be achieved [Citation19]. These cell component-specific elastic moduli and natural frequencies, in turn, might explain the broad array of cancer-type-specific RF-EMF frequencies yielding significant anti-cancer effects [Citation5, Citation88, Citation89].
It has to be mentioned that the reviewed preclinical trials significantly vary in treatment parameters, such as frequency ranges, intensities, exposure times, and cancer cell lines, which might present as the biggest weakness of this work. Since examining the non-temperature-induced effects of RF-EMF therapy on cancer cells presents a novel research field, the number of sources available covering this specific topic is somewhat scarce. This moved us to include papers covering a wide variety of different radio frequencies and applicator programming, as it is our goal to provide a broad overview of the majority of the so far discovered non-temperature induced effects upon RF-EMF radiation to provide new impulses for future research serving to improve RF-EMF therapy for cancer patients. Thus, it can be said that while the treatment parameters of the analyzed RF-EMF therapies within the preclinical works differ to a certain degree, limiting their comparability, all molecular effects observed upon RF-EMF radiation presented as significant non-temperature induced effects that were tumor-damaging and reoccurring within the papers cited.
Determining optimal dosimetry for the observed bio-electrical effects of RF-EMF treatment remains an ongoing challenge. However, early trends are detectable, indicating specific effects tied to distinct stimulatory frequency ranges. Notably, the TTF low intensity, intermediate frequency range of approximately 120-300 kHz (Group 1, ) emerges as a critical zone for mitotic inhibition, leading to cell cycle disturbance and inhibition, particularly at the G2/M phase. This inhibition appears linked to the stimulation of polar particles such as tubulin proteins or septin 7, integral to the formation of intermediate filaments that constitute the spindle apparatus during mitosis and cleavage furrow formation during cytokinesis [Citation40, Citation45, Citation47, Citation48, Citation51].
Moreover, electrical RF-EMF stimulation resulting in ion channel opening, especially VGICs, predominantly occurred in the low MHz (27.12 MHz − 147 MHz), low-intensity range (Group 2, ) with three instances documented in the literature [Citation35, Citation43, Citation44]. Interestingly, a similar effect is observed within the TTF range at 200 kHz [Citation57], indicating a versatile impact on cellular calcium fluxes. It is important to note that calcium channel opening in the MHz spectrum, explicitly involving the CACNA1H channel, occurred upon amplitude modulation. Studies by Jimenez et al. Sharma et al. and Dutta et al. utilized cancer-specific amplitude modulation frequencies, ranging from low Hz to low kHz frequencies, implying a sensitivity of calcium channel opening to low-intermediate RF-EMF ranges [Citation35, Citation43, Citation44, Citation90]. Within the low-intensity GHz treated (Group 3, ) cancer cells, four publications report significant alterations of RNA and protein expression levels upon RF treatment. This might indicate a possible direct effect of extremely high-frequency low-intensity RF-EMF on the stability of gene products such as mRNA and proteins, i.e., described by Caraglia et al. [Citation10] (HSP induced destabilization of Ras and Raf-1 protein folding).
These findings are mostly congruent with the findings of Xiang et al. [Citation53] who report electric field lines to predominantly surround cells when confronted with low frequencies (20 kHz), indicating minimal penetration into the cell cytoplasm, due to the capacitive properties of the membrane providing a conductive pathway for displacement currents, leading to a reduction in its resistance. However, as frequencies increase to intermediate (200 kHz) and higher radio frequencies (2 MHz), electric field lines penetrate the cell cytoplasm, increasing intracellular electric field strength. Additionally, the authors observed a consistent increase in the electric field intensity at both the plasma and nuclear membrane, with the frequency rising from 200 kHz to 2 MHz. These findings underscore the distribution of effect sites for the varying frequency spectrum observed throughout most preclinical trials presented herein. At intermediate frequencies (TTF-range) described by the authors, the cell membrane is perturbed, and field energy within the cytoplasm increases starkly, thus offering a potential explanation for the effects observed regarding changes in cytoskeletal organization and mitotic processes upon intermediate frequency RF radiation. In contrast, within increasing and higher frequency ranges, the observed RF effects seem to shift more to the genetic cascades, significantly influencing mRNA and protein levels, as higher frequencies allow for RF to significantly stimulate and possibly perturb the nuclear membrane as described by Xiang et al. [Citation53]. This correlation is not applicable continuously to all trials incorporated in a specific group that was allocated a frequency-dependent effect but may offer first insights into a possible dose-effect relationship or RF-EMF and their impact observed within cancer cells.
While observing initial trends in dose-effect relationships, it becomes evident that optimization of applicator parameters (intensity, frequency, modulation, and duration) must likely be tailored individually to diverse cancer types. Thus, further research is urgently needed to determine the proper dosimetry adjusted to each cancer type for achieving the desired anti-cancer bio-electrical effect and securing safe treatment conditions, ultimately enhancing RF-EMF treatment efficiency for maximal success in cancer patients.
5. Conclusion
The existent literature points toward a yet untapped therapeutic potential of RF-EMF treatment, which might aid in damaging cancer cells through bio-electrical and electro-mechanical molecular mechanisms while minimizing adverse effects on healthy tissue cells if appropriate and careful dosimetry is applied. Further research is imperative to determine optimal cancer-type-specific RF-EMF frequencies, field intensities, and exposure intervals, correlating them with underlying molecular mechanisms to maximize the therapeutic potential of RF-EMF therapy, thus ultimately providing cancer patients with the best possible treatment outcomes.
Acknowledgment
We would like to thank and dedicate this study to Peter Wust, who sadly died in 2022. Peter not only mastered research on hyperthermia but also tirelessly worked to study novel anticancer effects of electromagnetic fields. His profound insights, invaluable contributions, and his friendship have played a pivotal role in advancing knowledge in this field.
Data sharing
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Disclosure statement
PG received research funding from Oncotherm Kft, Budapest, Hungary; PG received research funding from Dr. Sennewald Medizintechnik GmbH, München, Germany; PG received consultancy fees from Dr. Sennewald Medizintechnik GmbH, München, Germany and from Sensius, Rotterdam, The Netherlands; he has no conflict of interest regarding the present work. There are no further conflicts of interest to disclose.
Additional information
Funding
References
- Datta NR, Ordóñez SG, Gaipl US, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41(9):742–753. doi: 10.1016/j.ctrv.2015.05.009.
- Wust P, Hildebrandt B, Sreenivasa G, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497. doi: 10.1016/s1470-2045(02)00818-5.
- Wust P. Biological effects of hyperthermia. In: Thermotherapy in oncology. 1st ed. Bremen: UNI-MED Verlag AG; 2016. p. 9–44.
- Issels R, Kampmann E, Kanaar R, et al. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. International J Hyperthermia. 2016;32(1):89–95. doi: 10.3109/02656736.2015.1119317.
- Wust P, Veltsista PD, Oberacker E, et al. Radiofrequency electromagnetic fields cause non-temperature-induced physical and biological effects in cancer cells. Cancers (Basel). 2022;14(21):5349. doi: 10.3390/cancers14215349.
- Wust P, Kortüm B, Strauss U, et al. Non-thermal effects of radiofrequency electromagnetic fields. Sci Rep. 2020;10(1):13488. doi: 10.1038/s41598-020-69561-3.
- Gentilal N, Salvador R, Miranda PC. ; 2020A Thermal Study of Tumor-Treating Fields for Glioblastoma Therapy. In: brain and Human Body Modeling 2020: computational Human Models Presented at EMBC 2019 and the BRAIN Initiative.
- Naveh A, Bomzon Z. A proof of concept study for simulating heat transfer in patients treated with tumor-treating fields. International Journal of Radiation Oncology*Biology*Physics. 2019;105(1):E128. doi: 10.1016/j.ijrobp.2019.06.2253.
- Gökçen S, Kurt B, Küçükbağrıaçık Y, et al. Effects of radiofrequency radiation on apoptotic and antiapoptotic factors in colorectal cancer cells. Electromagn Biol Med. 2022;41(3):325–334. doi: 10.1080/15368378.2022.2095643.
- Caraglia M, Marra M, Mancinelli F, et al. Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. J Cell Physiol. 2005;204(2):539–548. doi: 10.1002/jcp.20327.
- Komoshvili K, Israel K, Levitan J, et al. W-band millimeter waves targeted mortality of H1299 human lung cancer cells without affecting non-tumorigenic MCF-10A human epithelial cells in vitro. Appl Sci. 2020;10(14):4813. Available from: www.mdpi.com/journal/applsci doi: 10.3390/app10144813.
- Jonas O, Mierke CT, Käs JA. Invasive cancer cell lines exhibit biomechanical properties that are distinct from their noninvasive counterparts. Soft Matter. 2011;7(24):11488. doi: 10.1039/c1sm05532a.
- Armistead FJ, Gala De Pablo J, Gadêlha H, et al. Physical biomarkers of disease progression: on-chip monitoring of changes in mechanobiology of colorectal cancer cells. Sci Rep. 2020;10(1):3254. doi: 10.1038/s41598-020-59952-x.
- Wang XT, Nagaba Y, Cross HS, et al. The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am J Pathol. 2000;157(5):1549–1562. doi: 10.1016/S0002-9440(10)64792-X.
- Zhang Y, Cruickshanks N, Yuan F, et al. Targetable T-type calcium channels drive glioblastoma. Cancer Res. 2017;77(13):3479–3490. doi: 10.1158/0008-5472.CAN-16-2347.
- Fourbon Y, Guéguinou M, Félix R, et al. Ca2+ protein alpha 1D of CaV1.3 regulates intracellular calcium concentration and migration of colon cancer cells through a non-canonical activity. Sci Rep. 2017;7(1):14199. doi: 10.1038/s41598-017-14230-1.
- Sauer F, Fritsch A, Grosser S, et al. Whole tissue and single cell mechanics are correlated in human brain tumors. Soft Matter. 2021;17(47):10744–10752. doi: 10.1039/d1sm01291f.
- Alibert C, Pereira D, Lardier N, et al. Multiscale rheology of glioma cells. Biomaterials. 2021;275:120903. doi: 10.1016/j.biomaterials.2021.120903.
- Lin L, McCraw MR, Uluutku B, et al. Cell membrane oscillations under radiofrequency electromagnetic modulation. Langmuir. 2023;39(9):3320–3331. doi: 10.1021/acs.langmuir.2c03181.
- Schofield Z, Meloni GN, Tran P, et al. Bioelectrical understanding and engineering of cell biology. J R Soc Interface. 2020;17(166):20200013. doi: 10.1098/rsif.2020.0013.
- George LF, Bates EA. Mechanisms underlying influence of bioelectricity in development. Front Cell Dev Biol. 2022;10:772230. doi: 10.3389/fcell.2022.772230.
- Robinson AJ, Jain A, Sherman HG, et al. Toward hijacking bioelectricity in cancer to develop new bioelectronic medicine. Adv Therap. 2021;4(3). doi: 10.1002/adtp.202000248.
- Lee SK, Jeakins GS, Tukiainen A, et al. Next-generation bioelectric medicine: harnessing the therapeutic potential of neural implants. Bioelectricity. 2020;2(4):321–327. doi: 10.1089/bioe.2020.0044.
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia. 1994;10(4):457–483. doi: 10.3109/02656739409009351.
- Oleson JR, Dewhirst MW, Harrelson JM, et al. Tumor temperature distributions predict hyperthermia effect. Int J Radiat Oncol Biol Phys. 1989;16(3):559–570. doi: 10.1016/0360-3016(89)90472-0.
- Dewhirst MW, Oleson JR, Kirkpatrick J, et al. Accurate three-dimensional thermal dosimetry and assessment of physiologic response are essential for optimizing thermoradiotherapy. Cancers (Basel). 2022;14(7):1701. doi: 10.3390/cancers14071701.
- Winslow TB, Eranki A, Ullas S, et al. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia. 2015 Aug 18;31(6):693–701. doi: 10.3109/02656736.2015.1037800.
- Lai H, Levitt BB. The roles of intensity, exposure duration, and modulation on the biological effects of radiofrequency radiation and exposure guidelines. Electromagn Biol Med. 2022;41(2):230–255. [cited 2024 Apr 10]. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=iebm20 doi: 10.1080/15368378.2022.2065683.
- Karkabounas S, Havelas K, Kostoula OK, et al. Effects of low intensity static electromagnetic radiofrequency fields on leiomyosarcoma and smooth muscle cell lines. Hell J Nucl Med. 2006;9(3):167–172.
- Mamaghaniyeh R, Zandieh A, Goliaei B, Seyed., et al. Effects of exposure to alternating low-intensity, intermediate-frequency electric fields on the differentiation of human leukemic cell line U937; 2023. Available from: https://onlinelibrary.wiley.com/doi/10.1002/bem.22487. doi: 10.1002/bem.22487.
- Smothers AR, Henderson JR, O’Connell JJ, et al. Discover oncology efficacy and selectivity of tumor-treating field therapy for triple-negative breast cancer cells via in-house delivery device. Discov Oncol. 2023;14(1):34. Available from: doi: 10.1007/s12672-023-00647-w.
- Chang E, Patel CB, Pohling C, et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018;4(1):113. Available from: doi: 10.1038/s41420-018-0130-x.
- Beneduci A, Chidichimo G, Tripepi S, et al. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005;25(2A):1009–1013.
- Buttiglione M, Roca L, Montemurno E, et al. Radiofrequency radiation (900 MHz) induces Egr-1 gene expression and affects cell-cycle control in human neuroblastoma cells. J Cell Physiol. 2007;213(3):759–767. doi: 10.1002/jcp.21146.
- Dutta SK, Ghosh B, Blackman CF. Radiofrequency radiation-induced calcium ion efflux enhancement from human and other neuroblastoma cells in culture. Bioelectromagnetics. 1989;10(2):197–202. doi: 10.1002/bem.2250100208.
- Le HT, Staelens M, Lazzari D, et al. ; 2022Real-Time Monitoring of the Effect of Tumour-Treating Fields on Cell Division Using Live-Cell Imaging; Available from: doi: 10.3390/cells11172712.
- Karanam NK, Ding L, Aroumougame A, et al. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: implications for cancer therapy. Transl Res. 2020;217:33–46. [cited 2024 Apr 10]. Available from: https://pubmed.ncbi.nlm.nih.gov/31707040/ doi: 10.1016/j.trsl.2019.10.003.
- Jeong H, Sung J, Oh SI, et al. Inhibition of brain tumor cell proliferation by alternating electric fields. Appl Phys Lett [Internet]. 2014;105(20):203703. [cited 2024 Apr 10]. Available from: /aip/apl/article/105/20/203703/27580/Inhibition-of-brain-tumor-cell-proliferation-by
- Lei KF, Hsieh SC, Goh A, et al. Proliferation arrest, selectivity, and chemosensitivity enhancement of cancer cells treated by a low-intensity alternating electric field. Biomed Microdevices. 2018;20(4):90. doi: 10.1007/s10544-018-0339-8.
- Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295. doi: 10.1158/0008-5472.can-04-0083.
- Kim EH, Song HS, Yoo SH, et al. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7(40):65125–65136. doi: 10.18632/oncotarget.11372.
- Zimmerman JW, Pennison MJ, Brezovich I, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer. 2012;106(2):307–313. doi: 10.1038/bjc.2011.523.
- Jimenez H, Wang M, Zimmerman JW, et al. Tumour-specific amplitude-modulated radiofrequency electromagnetic fields induce differentiation of hepatocellular carcinoma via targeting Cav3.2 T-type voltage-gated calcium channels and Ca2+ influx. EBioMedicine. 2019;44:209–224. doi: 10.1016/j.ebiom.2019.05.034.
- Sharma S, Wu S-Y, Jimenez H, et al. Ca2+ and CACNA1H mediate targeted suppression of breast cancer brain metastasis by AM RF EMF. EBioMedicine. 2019;44:194–208. doi: 10.1016/j.ebiom.2019.05.038.
- Silginer M, Weller M, Stupp R, et al. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017;8(4):e2753–e2753. doi: 10.1038/cddis.2017.171.
- Kim EH, Jo Y, Sai S, et al. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene. 2019;38(39):6630–6646. doi: 10.1038/s41388-019-0882-7.
- Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2015;5(1):18046. doi: 10.1038/srep18046.
- Gera N, Yang A, Holtzman TS, et al. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One. 2015;10(5):e0125269. doi: 10.1371/journal.pone.0125269.
- Lee WS, Jang Y, Cho A, et al. Effectiveness of tumor-treating fields to reduce the proliferation and migration of liposarcoma cell lines. Exp Ther Med. 2023;26(2):363. doi: 10.3892/etm.2023.12062.
- Xu S, Luo C, Chen D, et al. Whole transcriptome and proteome analyses identify potential targets and mechanisms underlying tumor treating fields against glioblastoma. Cell Death Dis. 2022;13(9):797. doi: 10.1038/s41419-022-05127-7.
- Branter J, Estevez-Cebrero M, Diksin M, et al. Genome-wide expression and anti-proliferative effects of electric field therapy on pediatric and adult brain tumors. Int J Mol Sci. 2022;23(4):1982. doi: 10.3390/ijms23041982.
- Kirson ED, Giladi M, Gurvich Z, et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis. 2009;26(7):633–640. doi: 10.1007/s10585-009-9262-y.
- Xiang X-W, Liu H-T, Tao X-N, et al. Glioblastoma behavior study under different frequency electromagnetic field. iScience. 2023;26(12):108575. doi: 10.1016/j.isci.2023.108575.
- Logani MK, Szabo I, Makar V, et al. Effect of millimeter wave irradiation on tumor metastasis. Bioelectromagnetics. 2006;27(4):258–264. doi: 10.1002/bem.20208.
- Jo Y, Oh G, Gi Y, et al. Tumor treating fields (TTF) treatment enhances radiation-induced apoptosis in pancreatic cancer cells. Int J Radiat Biol. 2020;96(12):1528–1533. doi: 10.1080/09553002.2020.1838658.
- Jo Y, Kim EH, Sai S, et al. Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int J Mol Sci. 2018;19(11):3684. doi: 10.3390/ijms19113684.
- Neuhaus E, Zirjacks L, Ganser K, et al. Alternating electric fields (TTFields) activate Ca v 1.2 channels in human glioblastoma cells. Cancers (Basel). 2019;11(1):110. doi: 10.3390/cancers11010110.
- Giladi M, Munster M, Schneiderman RS, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12(1):206. doi: 10.1186/s13014-017-0941-6.
- Marinelli F, La Sala D, Cicciotti G, et al. Exposure to 900 MHz electromagnetic field induces an unbalance between pro-apoptotic and pro-survival signals in T-lymphoblastoid leukemia CCRF-CEM cells. J Cell Physiol. 2004;198(2):324–332. doi: 10.1002/jcp.10425.
- Wrzosek A, Augustynek B, Żochowska M, et al. Mitochondrial potassium channels as druggable targets. Biomolecules. 2020;10(8):1200. doi: 10.3390/biom10081200.
- Ross CL, Syed I, Smith TL, et al. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med. 2017;36(1):74–87. doi: 10.3109/15368378.2016.1160408.
- Costantini E, Sinjari B, D’Angelo C, et al. Human gingival fibroblasts exposed to extremely low-frequency electromagnetic fields: in vitro model of wound-healing improvement. Int J Mol Sci. 2019;20(9):2108. doi: 10.3390/ijms20092108.
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–156. doi: 10.1016/s1474-4422(03)00321-1.
- Ziegelberger G, Croft R, Feychting M, et al. Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Physics. 2020;118:483–524. doi: 10.1097/HP.0000000000001210.
- Baan R, Grosse Y, Lauby-Secretan B, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [cited 2024 Apr 10]. Available from: http://www.thelancet.com/article/S1470204511701474/fulltext doi: 10.1016/s1470-2045(11)70147-4.
- Hardell L, Carlberg M, Mild KH. Pooled analysis of case-control studies on malignant brain tumours and the use of mobile and cordless phones including living and deceased subjects. Int J Oncol. 201138(5):1465–1474 ; [cited 2024 Apr 10]. Available from https://pubmed.ncbi.nlm.nih.gov/21331446/ doi: 10.3892/ijo.2011.947.
- Liu Y-X, Li G-Q, Fu X-P, et al. Exposure to 3G mobile phone signals does not affect the biological features of brain tumor cells. BMC Public Health [Internet]. 2015;15(1):764. [cited 2024 Apr 10]. Available from: https://pubmed.ncbi.nlm.nih.gov/26253141/ doi: 10.1186/s12889-015-1996-7.
- Feychting M, Schüz J, Toledano MB, et al. Mobile phone use and brain tumour risk – COSMOS, a prospective cohort study. Environ Int. 2024;185:108552. doi: 10.1016/j.envint.2024.108552.
- Bautista W, Lipschitz J, McKay A, et al. Cancer stem cells are depolarized relative to normal stem cells derived from human livers. Ann Hepatol. 2017;16(2):297–303. doi: 10.5604/16652681.1231590.
- Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185. doi: 10.3389/fphys.2013.00185.
- Rao VR, Perez-Neut M, Kaja S, et al. Voltage-gated ion channels in cancer cell proliferation. Cancers (Basel). 2015;7(2):849–875. doi: 10.3390/cancers7020813.
- Huber SM. Oncochannels. Cell Calcium. 2013;53(4):241–255. doi: 10.1016/j.ceca.2013.01.001.
- Liu X, Chang Y, Reinhart PH, et al. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22(5):1840–1849. doi: 10.1523/jneurosci.22-05-01840.2002.
- Ge L, Hoa NT, Cornforth AN, et al. Glioma big potassium channel expression in human cancers and possible T cell epitopes for their immunotherapy. J Immunol. 2012;189(5):2625–2634. doi: 10.4049/jimmunol.1102965.
- Cross SE, Jin YS, Rao J, et al. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2(12):780–783. doi: 10.1038/nnano.2007.388.
- Li QS, Lee GYH, Ong CN, et al. AFM indentation study of breast cancer cells. Biochem Biophys Res Commun. 2008;374(4):609–613. doi: 10.1016/j.bbrc.2008.07.078.
- Xin Y, Li K, Huang M, et al. Biophysics in tumor growth and progression: from single mechano-sensitive molecules to mechanomedicine. Oncogene 2023 42:47 [Internet]. 2023 Oct 20;42(47):3457–3490 [cited 2024 Apr 12]. Available from: https://www.nature.com/articles/s41388-023-02844-x doi: 10.1038/s41388-023-02844-x.
- Wu P-H, Aroush DR-B, Asnacios A, et al. A comparison of methods to assess cell mechanical properties. Nat Methods. 2018;15(7):491–498 [cited 2024 Apr 11]. Available from: https://pubmed.ncbi.nlm.nih.gov/29915189/ doi: 10.1038/s41592-018-0015-1.
- Pontes B, Ayala Y, Fonseca A, et al. Membrane elastic properties and cell function. PLoS One [Internet]. 2013;8(7):e67708. Available from: www.plosone.org doi: 10.1371/journal.pone.0067708.
- Watanabe T, Kuramochi H, Takahashi A, et al. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (2)-epigallocatechin gallate-treated cells. J Cancer Res Clin Oncol. 2012;138(5):859–866. [cited 2024 Apr 13]. Available from: https://link.springer.com/article/10.1007/s00432-012-1159-5 doi: 10.1007/s00432-012-1159-5.
- Kwon S, Yang W, Moon D, et al. Comparison of cancer cell elasticity by cell type. J Cancer. 2020;11(18):5403–5412. [cited 2024 Apr 13]. Available from: /pmc/articles/PMC7391204/ doi: 10.7150/jca.45897.
- Jaganathan SK, Subramanian AP, Vellayappan MV, et al. Natural frequency of cancer cells as a starting point in cancer treatment. Curr Sci. 2016;110(9):1828–1832. doi: 10.18520/cs/v110/i9/1828-1832.
- Heyden S, Ortiz M. Oncotripsy: targeting cancer cells selectively via resonant harmonic excitation. J Mech Phys Solids. 2016;92:164–175. doi: 10.1016/J.JMPS.2016.04.016.
- Adair RK. Vibrational resonances in biological systems at microwave frequencies. Biophys J. 2002;82(3):1147–1152. doi: 10.1016/S0006-3495(02)75473-8.
- Fröhlich H. Evidence for Bose condensation-like excitation of coherent modes in biological systems. Phys Lett A. 1975;51(1):21–22. doi: 10.1016/0375-9601(75)90300-X.
- Runel G, Lopez-Ramirez N, Chlasta J, et al. Biomechanical properties of cancer cells. Cells. 2021;10(4):887. doi: 10.3390/cells10040887.
- Arjonen A, Kaukonen R, Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adh Migr. 2011;5(5):421–430. doi: 10.4161/cam.5.5.17723.
- Dazzoni R, Grélard A, Morvan E, et al. The unprecedented membrane deformation of the human nuclear envelope, in a magnetic field, indicates formation of nuclear membrane invaginations. Sci Rep. 2020;10(1):5147. doi: 10.1038/s41598-020-61746-0.
- Mai TL, Derreumaux P, Nguyen PH. Structure and elasticity of mitochondrial membranes: a molecular dynamics simulation study. J Phys Chem B. 2023127(50):10778–10791 doi: 10.1021/acs.jpcb.3c05112.
- Barbault A, Costa FP, Bottger B, et al. Amplitude-modulated electromagnetic fields for the treatment of cancer: discovery of tumor-specific frequencies and assessment of a novel therapeutic approach. J Exp Clin Cancer Res. 2009;28(1):51. doi: 10.1186/1756-9966-28-51.
