Abstract
Background: Recent advances in the application of transcranial direct current stimulation (tDCS) in healthy populations have led to the exploration of the technique as an adjuvant method to traditional speech therapies in patients with post-stroke aphasia.
Aims: The purpose of the review is: (i) to review the features of tDCS that make it an attractive tool for research and potential future use in clinical contexts; (ii) to describe recent studies exploring the facilitation of language performance using tDCS in post-stroke aphasia; (iii) to explore methodological considerations of tDCS that may be key to understanding tDCS in treatment of aphasia post stroke; and (iv) to highlight several caveats and outstanding questions that need to be addressed in future work.
Main Contribution: This review aims to highlight our current understanding of the methodological and theoretical issues surrounding the use of tDCS as an adjuvant tool in the treatment of language difficulties after stroke.
Conclusions: Preliminary evidence shows that tDCS may be a useful tool to complement treatment of aphasia, particularly for speech production in chronic stroke patients. To build on this exciting work, further systematic research is needed to understand the mechanisms of tDCS-induced effects, its application to current models of aphasia recovery, and the complex interactions between different stimulation parameters and language rehabilitation techniques. The potential of tDCS is to optimise language rehabilitation techniques and promote long-term recovery of language. A stimulating future for aphasia rehabilitation!
Language-based therapeutic interventions for post-stoke aphasia are effective at alleviating some degree of language impairment (Kendall et al., Citation2008; Nickels, Citation2002; Vitali et al., Citation2007). The dose of speech and language therapy (SLT) delivered may have a critical effect on recovery (Breitenstein et al., Citation2009; Brindley, Copeland, Demain, & Martyn, Citation1989; Meinzer, Djunja, Barthel, Elbert, & Rockstroh, Citation2005; Meinzer et al., Citation2004; Poeck, Huber, & Willmes, Citation1989). The idea being that for beneficial therapeutic outcome “more is better”. Indeed, in studies that report positive therapy outcomes patients were provided with, on average, a total of 98.4 hours of therapy, while studies in which no improvement was observed, only 43.6 hours of therapy was delivered (Bhogal, Teasell, Foley, & Speechley, Citation2003). Although modest evidence exists for the efficacy of more intensive treatment and constraint induced language therapy (CILT) in individuals with chronic stroke-induced aphasia (Barthel, Meinzer, Djundja, & Rockstroh, Citation2008; Cherney, Patterson, Raymer, Frymark, & Schooling, Citation2008; Meinzer et al., Citation2004, Citation2005; Pulvermuller et al., Citation2001), extensive language therapy, whether delivered intensively or not, is difficult to administer with limited clinical resources not least because significantly more people withdraw from intensive SLT than conventional SLT (Kelly, Brady, & Enderby, Citation2010). Consequently one approach may be to consider adjuncts to therapy that may facilitate treatment, such as brain stimulation interventions. The facilitation of performance could manifest in (i) an increase to the total amount of learning achieved, such that a higher performance level is reached, or (ii) an increased rate at which learning is achieved, such that the same maximum performance is ultimately achieved as with standard therapy alone but over a different timescale, i.e., with less hours of behavioural therapy. It is not clear what form of stimulation, for example repetitive transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), or epidural electrical stimulation (EES) is most effective and clinically appropriate. However, tDCS is easier and less expensive, and theoretically could be even self-administered, while TMS requires special equipment and trained health professionals, and it carries a seizure risk; EES, on the other hand, is an invasive method that effectively limits the range of its application. In this paper we summarise results from tDCS studies that aim to elicit improvements in language performance and language learning in chronic post-stroke aphasic patients.
The translation of tDCS studies of language from healthy participants into aphasic patients has been limited. To date only four studies have reported data from post-stroke aphasic speakers (see ). Although the number of studies is small, there is preliminary and exciting evidence that tDCS may play a role in aphasia rehabilitation. However, before we start our review of these studies, in Section 1 we briefly recap some of the basic mechanisms of tDCS that are relevant to the treatment of post-stroke aphasic patients. For a more comprehensive work detailing the mechanism of tDCS, TMS, and an overview of both methods we direct readers to excellent papers by Nitsche et al. (Citation2008), Jahanshahi and Rothwell (Citation2000), and Miniussi et al. (Citation2008) respectively. For a review of the normative literature using tDCS to modulate language performance see Floël (2012). In Section 2 we review recent evidence for a facilitatory role of tDCS on naming performance in post-stroke aphasic patients, and in Section 3 we discuss several methodological parameters that, at present, remain unclear and require further consideration if tDCS is to be developed as a realistic therapeutic tool. We conclude in Section 4 by highlighting a number of caveats and questions that need to be addressed before tDCS may be clinically useful in aphasia rehabilitation. It is hoped that, by doing so, new insights and novel research approaches into the complex nature of aphasia and its treatment might lead to advances in the improvement of therapy outcomes in chronic post-stroke aphasic patients.
TABLE 1 tDCS and language performance in post-stroke aphasia
METHOD OF TDCS AND CONSIDERATIONS IN A DAMAGED BRAIN
In recent decades, tDCS has been re-evaluated and shown to reliably modulate human cortical function by inducing prolonged yet reversible shifts of cortical excitability. As such, it has been re-introduced as a non-invasive tool to guide neuroplasticity and modulate cortical function. tDCS relies on tonic stimulation of the cortex via the application of a direct, constant current flow of low intensity (1–2 mA) between two electrodes applied to the scalp over a relatively extended period of time (e.g., 5–20 minutes). This technique has been shown to elicit polarity-dependent changes in cortical excitability depending on the orientation of the stimulation electrodes (Nitsche & Paulus, Citation2000). In the motor cortex, anodal stimulation increases neuronal excitability whereas cathodal stimulation reduces excitability (Antal, Nitsche, Kruse, et al., 2004b; Kincses, Antal, Nitsche, Bartfai, & Paulus, Citation2004). Furthermore, emerging evidence suggests that the effects of tDCS can persist beyond the period of stimulation (Nitsche & Paulus, Citation2001; Nitsche, Schauenburg, et al., Citation2003), with changes in task performance reported as late as 6–12 months post-intervention (Cohen Kadosh, Soskic, Iuculano, Kanai, & Walsh, Citation2010; Dockery, Hueckel-Weng, Birbaumer, & Plewnia, Citation2009; Reis et al., Citation2009).
Although both TMS and tDCS are forms of non-invasive brain stimulation, it should be kept in mind that the effects of TMS and tDCS cannot be assumed to be the same and further research is needed to determine the mechanisms underlying the effects of each technique on the brain. The effects of stimulation on language function, its cortical network, and its behavioural consequences are very likely to differ (see Nitsche & Paulus, Citation2000, for discussion on motor cortical function). For example, unlike TMS, tDCS does not “stimulate” neurons; rather the effects are due to modification of ongoing activity. While TMS pulses depolarise axons and generate action potentials, tDCS effects are sub-threshold and occur via polarisation of the resting membrane potential. Anodal tDCS typically has an excitatory neuronal effect due to a shift towards neuronal depolarisation, while cathodal stimulation elicits a hyperpolarisation (Bindman, Lippold, & Redfearn, Citation1962; Nitsche & Paulus, Citation2000; Purpura & McMurtry, Citation1965). Indeed, recent evidence suggests that tDCS polarises the resting membrane potential through the modulation of sodium and calcium dependent channels and NMDA-receptor activity (Nitsche et al., Citation2004; see Stagg & Nitsche, Citation2011, for a comprehensive review of pharmacological intervention to explore the mechanism of tDCS), thereby increasing or decreasing the mean firing rate of the neuron and promoting mechanisms of long-term potentiation and long-term depression (LTP/LTD) (Liebetanz, Nitsche, Tergau, & Paulus, Citation2002; Nitsche, Fricke, et al., Citation2003). Furthermore, an increased secretion of brain-derived neurotrophic factor (BDNF), a protein essential for new learning, has been associated with anodal stimulation (Fritsch et al., Citation2010). If we are to induce long-term consolidation, or the re-learning of information—a goal of aphasia therapy—a neuromodulatory method that has features of LTP-like processes is attractive.
Another attractive feature of tDCS is the apparent lack of any significant side effects. Large-scale cohort testing from different research centres has reported the most common adverse sensation is a slight itching sensation under the electrode and seldom-occurring headache, fatigue, and nausea (see Nitsche et al., Citation2008, ; Poreisz, Boros, Antal, & Paulus, Citation2007). Although reports of skin burns associated with tDCS have been reported (Frank et al., Citation2011; Lagopoulos & Degabriele, Citation2008; Palm et al., Citation2008), these occurred in the context of extended stimulation periods (30 minutes), drying of electro-conductive gel, abraded skin surface, and the use of tap water to soak the sponge sheaths respectively. When conventional tDCS protocols were employed (i.e., 1–2 mA stimulation for up to 20 minutes), using optimised safety protocols, significant adverse effects, such as burning of the skin (Dundas, Thickbroom, & Mastaglia, Citation2007; Loo et al., Citation2011; Minhas et al., Citation2010) or heating of the electrodes and scalp surface (Datta, Elwassif, & Bikson, Citation2009; Nitsche & Paulus, Citation2000, Citation2001), were avoided. Under these protocol guidelines, tDCS may be considered safe (Nitsche, Schauenburg, et al., Citation2003). Furthermore, tDCS has not been reported to provoke seizures in non-acute neurological deficits, i.e., intervention protocols are well below the threshold of tissue damage (Liebetanz et al., Citation2009; Nitsche, Schauenburg, et al., Citation2003). Therefore, compared with TMS, tDCS may be a viable option for stimulation of perilesional cortex where the threshold to induce seizures is lower. In a clinical context this makes tDCS an appealing form of neurostimulation in chronic stroke populations (Priori, Hallett, & Rothwell, Citation2009). However, further investigation of tDCS hazards is certainly warranted in non-acute patients, both for existing protocols and those under development (Bikson, Datta, & Elwassif, Citation2009). In particular, the interaction of stimulation with different disease aetiologies and potential risk factors of seizure, such as lack of sleep (Nitsche et al., Citation2008)—as the propensity for seizure is much higher in acute and subacute patients. Therefore appropriate safety criteria are unknown, especially in the context of repeated protocols that may be required for rehabilitation.
Despite these caveats the technology is easy to use, relatively inexpensive, and portable, allowing treatment to be delivered not only in clinical settings but also in the patient's own home. tDCS is also well suited to online application; i.e., brain stimulation concurrent with behavioural intervention. For example, inferior frontal cortex (Broca's area) can be easily and comfortably targeted with tDCS. At the start of stimulation, most participants will perceive a slight itching sensation under the electrodes, which then quickly fades in most cases. In contrast, TMS and in particular rTMS, of the same region can inadvertently stimulate the trigeminal facial nerves causing facials twitches, or painful sensations making certain online tasks, such as spoken picture naming, very difficult. Therefore, for a treatment study that requires multiple sessions, TMS and repetitive TMS may be a less-suitable option. For research purposes it is also easier to conduct placebo (sham) stimulation-controlled studies with tDCS, because, with the exception of a slight itching sensation and mild sensory phenomena (including retinal phosphenes associated with current switching), participants rarely experience sensations related to the stimulation (Gandiga, Hummel, & Cohen, Citation2006). Therefore reliable effects of stimulation in the absence of any confounding “placebo effects” (i.e., the influence of participants' expectation of an effect on the observed results) may be achieved using tDCS.
Due to its appeal as a potential treatment tool, some manufacturers (NeuroConn, Germany, personal communication) are exploring mini-tDCS kits for patient's self-administration at home with stimulation dosages pre-programmed by their clinician. Some additional precautions should be considered for safe use of tDCS: (i) patients should have no metallic implants near the electrodes, and (ii) personnel conducting tDCS should be appropriately trained before applying the technique, as experience with the method is still limited and the risk profile of stimulation is not yet completely known. In the future, if successful, one could envisage a home treatment programme that the speech therapist would design involving concurrent tDCS with, e.g., a computer-delivered aphasia treatment programme.
tDCS AND REHABILITATION OF APHASIC STROKE PATIENTS
Two approaches have primarily been used in the application of cortical stimulation (tDCS and TMS) to rehabilitation of post-stroke aphasic patients. Both approaches to language recovery are based on a model of interhemispheric rivalry between the residual speech areas in the stroke-damaged left hemisphere and intact, non-stroke, right hemisphere (akin to models of motor recovery after stroke). In essence, the model proposes that speech deficits are due to (1) reduced output from the stroke-damaged left hemisphere and (2) excess inhibition of the left hemisphere from the intact, non-stroke, right hemisphere. Thus improvement may be possible either by increasing the output of the perilesional left hemisphere or decreasing the output from the intact, non-stroke, right hemisphere. In simple terms this could be achieved by using cortical stimulation to increase the excitability of the residual left hemisphere cortices or decrease the excitability of the intact right hemisphere (see Lindenberg, Renga, Zhu, Nair, & Schlaug, Citation2010; Vines, Cerruti, & Schlaug, Citation2008; both studies used bi-hemispheric stimulation to increase/decrease left and right cortical excitability). Despite the potential application of tDCS for language rehabilitation, to date just four studies in post-stroke aphasic patients have been reported (see ). All of these studies aimed to improve speech production deficits by modulating the left hemisphere. The first study focused on the effects of a single session of stimulation applied to left frontal cortex (Monti et al., Citation2008). More recent work has emphasised the long-term effects on speech function that occur after repeated stimulation of left frontal or temporal cortices (Baker, Rorden, & Fridriksson, Citation2010; Fiori et al., Citation2010; Fridriksson, Richardson, Baker, & Rorden, Citation2011). We discuss each approach in turn below.
Single-session studies
The rationale behind these studies is to test if a single application of tDCS induces immediate gain in the selected outcome measures. At the same time these studies provide information about the safety and comfort of the intervention protocol. tDCS paradigms involving healthy speakers have typically used single-exposure approaches. That is, on a given session of tDCS a participant is exposed to anodal, cathodal, or sham stimulation for a relatively short period of time (e.g., 20 minutes) and their performance on language tasks is monitored. Tasks have included verbal fluency (Iyer et al., Citation2005), picture naming (Fertonani, Rosini, Cotelli, Rossini, & Miniussi, Citation2010; Sparing, Dafotakis, Hesse, & Fink, Citation2007), proper name retrieval (Ross, McCoy, Wolk, Coslett, & Olson, Citation2010), nonword–picture matching (Fiori et al., Citation2010; Liuzzi et al., Citation2010) and a wide range of designs, tDCS protocols and sites of stimulation were used. The evidence to date suggests that anodal relative to sham stimulation applied to the left hemisphere language cortices can significantly enhance normal language performance as measured by reaction time or accuracy.
Ideally, when applied to post-stroke aphasic patients, the gain should not just be greater than the effect of placebo (sham), or a traditional intervention, e.g., anomia treatment, but should also be of clinical significance. Although a satisfactory definition of clinical significance is difficult, most investigators would be content with an improvement of around 10% in standard aphasia measures, especially in the chronic stage post-stroke. Monti and colleagues (Citation2008) used a single-session approach to assess change in post-stroke aphasic patients' picture-naming abilities. Eight chronic non-fluent aphasic patients took part in two sessions of tDCS separated by at least a week. In one session half of the patients received 2 mA anodal stimulation for 10 minutes applied to left fronto-temporal cortex immediately before a picture-naming task (i.e., offline). During the second session patients received sham stimulation applied to the same cortical region prior to picture naming. For the remaining patients, the intervention procedure was identical except that cathodal stimulation was delivered. The order of stimulation (anodal or cathodal) and sham was counterbalanced across sessions and patients. In contrast to the facilitatory results for anodal stimulation reported in the healthy participant literature, Monti and colleagues found an improvement in naming accuracy for cathodal stimulation in the order of 10.6%, with no significant effects after anodal and sham stimulation. This apparent paradoxical improvement in naming accuracy was argued to be due to a tDCS-induced depression of cortical inhibitory inter-neurons in the lesioned hemisphere, which led to a disinhibition of the cortex thereby improving the functioning of the damaged language cortices. Independent of the neural mechanisms, which remain elusive, this study indicated that it is possible to improve performance on a language task by means of a single dose of tDCS applied to the damaged left hemisphere. Subsequent tDCS and aphasia studies have aimed to investigate whether these intriguing naming improvements could be made to persist over an extended period of time.
Multiple-session studies
The hypothesis underlying multiple-session studies is that the short-lasting effects from a single session will accumulate with repeated sessions and eventually lead to a permanent improvement in function. Precisely how this approach might lead to a permanent improvement in language function in aphasic speakers after stroke is not clear. One possibility is that increasing the output from the stroke-damaged, left hemisphere will lead to more effective relearning of language. In other words, brain stimulation itself would not produce any lasting changes in language function; instead it would temporarily create a state that optimises relearning and rehabilitation. This natural process would lead to an improvement in language function and reduction in aphasic deficits. In healthy participants multiple sessions of left-sided anodal stimulation can improve overall performance when learning new information and associations relative to sham (e.g., de Vries et al., Citation2010; e.g., Fiori et al., Citation2010; Floël, Rosser, Miichka, Knecht, & Breitenstein, Citation2008). For language learning, a recent study applied anodal stimulation to left Broca's area online during the acquisition phase of an artificial grammar and demonstrated enhanced performance at detecting syntactic violations along with an increased use of rule-based decision making (de Vries et al., Citation2010). Similarly, anodal stimulation applied to left Wernicke's area during acquisition of an artificial lexicon increased the rate and overall success of language learning compared to sham stimulation after a single exposure to tDCS (Floël et al., Citation2008).
In relatively well-recovered aphasic patients, as in normal individuals, successful speech production has been correlated with brain activity in the stroke-damaged left hemisphere (Fridriksson, Bonilha, Baker, Moser, & Rorden, Citation2010). In this context, the interhemispheric model would predict that facilitating the remaining left perilesional cortex with excitatory stimulation could improve speech recovery (see later). Three tDCS studies (see ) adopted this approach using a repeated intervention protocol that spanned several days with anodal tDCS delivered during a given task. This approach has been influenced, at least in part, by (i) the facilitatory effects observed in single-exposure studies of healthy participants and (ii) the idea that the favourable effects of mass-practice seen in behaviour-only anomia treatments may also be valid in combined tDCS and behavioural training approaches. Preliminary results suggest that anodal tDCS applied to the lesioned left hemisphere can facilitate naming performance post-treatment.
Figure 1. Summary schematic representation of potential tDCS effects in language performance in relation to normal re-learning after single and repeated interventions protocols (related to ). Solid green line represents normal re-learning curve. Solid vertical red line indicates the potential percentage change in performance that may occur after a single intervention of tDCS. Dashed red line indicates potential performance change over 5 days from repeated tDCS delivered concurrently with treatment. Normal and tDCS-enhanced learning share an equivalent learning profile, however resulting gains post-tDCS may be modified by approximately 25%, with the effects of tDCS persisting for up to 3 weeks. To view this figure in colour, please see the online issue of the Journal.
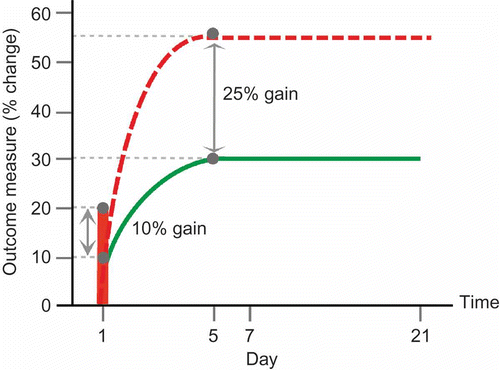
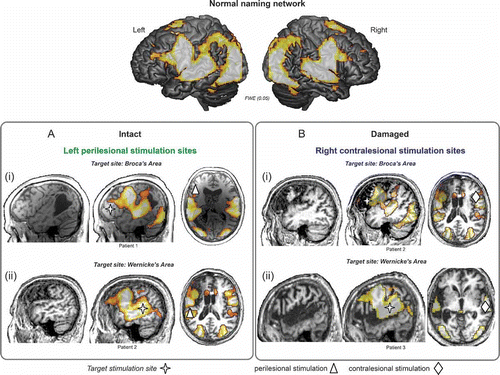
Figure 2. Illustration of potential target sites of stimulation to facilitate naming performance in relation to structural brain damage and the normal naming network in three different patients. The top panel illustrates the extensive bilateral fronto-temporal naming network found in an fMRI study of healthy older particpants (cf. Holland et al., Citation2011). Activation is overlaid onto a rendered cortical surface from SPM8. In the lower panels of the figure we overlay the normal pattern of brain activation onto three chronic aphasic stroke patients' individual structural MRI scans. The aim here is to illustrate from left to right for each patient: (1) their structural brain damage within the left hemisphere, (2) the normal naming network overlaid onto their individual brain scan to show the relationship between the lesion damage to the naming network and the target stimulation site (red cross), and (3) the stimulation sites feasible for each patient depending on the proposed target site. Panel A highlights, in two chronic aphasic stroke patients, that structurally intact regions of cortex within the lesioned left hemisphere may serve as potential candidate sites for anodal stimulation to facilitate treatment of anomia in (Ai) Broca's area and (Aii) Wernicke's area. Panel B highlights that when the lesion is has damaged relevant cortices in the left hemisphere perilesional stimulation may not be possible. Therefore, for the two patients illustrated, facilitation of the contralesional hemisphere may be the optimal approach to aid recovery: (Bi) right homologue to Broca's area and (Bii) Wernicke's area. Although not illustrated here, in cases where patients have suffered a large MCA infarct affecting the whole of the left hemisphere and resulted in extremely limited perilesional tissue, stimulation of the contralesional hemisphere would be the sole option. Furthermore, studies of aphasia recovery have successfully used anodal stimulation of the left perilesional hemisphere to elicit positive behavioural outcomes, however the effects of anodal or cathodal stimulation of the right contralesional hemisphere are, as yet unknown, and will be dependent on the theoretical hypotheses of the research. To view this figure in colour, please see the online issue of the Journal.
In the first treatment study 10 aphasic speakers were trained for five consecutive days using a spoken word-to-picture match task during which they received 20 minutes of anodal (1 mA) or sham stimulation of left frontal and precentral cortex (Baker et al., Citation2010). The patients' naming accuracy improved on average by 8.4% after anodal stimulation compared to sham. Furthermore, improvements in naming were retained for at least 1 week for items that had been treated. Consistent with the behavioural rehabilitation literature, the observed facilitatory effects did not generalise to untreated items matched for psycholinguistic complexity. In a second study by this group the same treatment paradigm was used in eight mild fluent aphasic patients with lesions restricted to left posterior cortical and subcortical regions. Anodal stimulation was applied to left posterior perilesional cortex (Fridriksson et al., Citation2011) and as accuracy for naming was high at baseline in these patients, the outcome measure was reaction time. Anodal stimulation resulted in faster naming responses compared to sham stimulation. This naming improvement in the order of 38.2% faster persisted for at least three weeks. Again, the facilitatory effect of anodal stimulation was restricted to the treated items only. In the third study, Fiori and colleagues (Citation2010) trained three non-fluent aphasic patients to name 80 pictures they could comprehend but not accurately produce. During naming practice 40 pictures were paired with 1 mA of anodal tDCS applied to left Wernicke's area and 40 pictures with sham stimulation of the same region. Anodal tDCS significantly improved naming accuracy by 20.5% on average compared to sham after 5 days of intervention.
DISCUSSION
Results from these first studies demonstrate, at the very least, that tDCS applied to the stroke-damaged left hemisphere in chronic aphasic patients is in principle (1) safe, there were no reported side effects reported in any of the studies, (2) well tolerated even when given over multiple sessions, and (3) can significantly modulate change in speech performance and relearning (see for schematic of mean percentage change in performance over time based on studies of language reviewed here). However, with such preliminary data a number of factors and questions emerge that may be key to understanding and developing tDCS as a truly adjuvant therapy tool in language recovery after aphasic stroke. The aim of this section is to discuss how to modify cortical excitability by tDCS with special emphasis on three methodological aspects. The first, Where to stimulate, considers the optimal site of stimulation in aphasic stroke patients and the role of the ipsilesional and contralesional hemispheres in supporting language rehabilitation. The second, When to stimulate, considers whether tDCS delivered before language rehabilitation (offline) or concurrently with the language task (online) may be most effective. The third, How long to stimulate, concerns the durations of tDCS intervention protocols. We discuss each in turn below. However, it is also important to emphasise that tDCS applied to aphasia research is in its early stages and therefore future studies might change some of the current concepts.
Where to stimulate
Identifying cortical regions to target with tDCS
The aim of current stimulation protocols is modulation of task-relevant regions of cortex. For tDCS to successfully modulate a component of an intact or impaired language system, the brain regions that support a given task must first be identified. Functional magnetic resonance imaging (fMRI) has been extensively used to explore which regions of the brain are associated with a variety of different language tasks. In the case of speech production meta-analyses revealed that a large, predominantly left-lateralised perisylvian network is involved. Depending on the task delivered during scanning, the extended speech production network is differentially engaged ( for review see Price, Citation2010; Vigneau et al., Citation2006) with frontal (de Zubicaray & McMahon, Citation2009; Fridriksson et al., Citation2009; Papoutsi et al., Citation2009), premotor, motor (Brown et al., Citation2009), or posterior temporal (Abel et al., Citation2009; Hocking, McMahon, & de Zubicaray, Citation2009) regions predominantly activated.
In an attempt to determine the key, or functionally critical, regions within these language networks identified by fMRI, researchers have used TMS and rTMS. For example, in healthy speakers TMS “virtual lesion” methods have been applied to determine the involvement of (i) frontal regions during action naming (Cappa et al., Citation2002) and repetition priming (Thiel et al., Citation2005); (ii) left anterior temporal regions during verb generation (Holland & Lambon Ralph, Citation2010), synonym judgement (Pobric, Jefferies, & Lambon Ralph, Citation2007); and left posterior temporal regions during object recognition (Stewart, Meyer, Frith, & Rothwell, Citation2001). Each study used results from functional neuroimaging to inform the stimulation site that when temporarily “lesioned” by the TMS protocols would determine its relevance for a chosen language task.
While the consensus is that the left hemisphere plays a dominant role in language processing, it is worth remembering that the right hemisphere is also significantly activated in a range of language tasks. A recent study of healthy participants used fMRI to guide rTMS to target the right posterior inferior frontal gyrus (IFG) during phonological decisions on words (Hartwigsen et al., Citation2010). They found that TMS applied over the left, right, or bilateral IFG disrupted phonological processing to an equivalent degree. This suggests that the left and right IFG do not have the capacity to rapidly compensate for disruption to the other during rTMS in healthy participants. However, in chronic post-stroke aphasia the interhemispheric relationship may be altered as a consequence of time and brain–language recovery after stroke. Understanding these patterns of variation may be key factors in developing more sophisticated models of language processing and recovery that will, in turn, inform suitable target sites for stimulation in aphasic patients.
Identifying viable cortex to stimulate in damaged brains
When translating stimulation protocols from healthy participants to post-stroke aphasic patients, the consideration is not only in terms of how the stimulation target site may be engaged by the language task, but also how the stroke damage impacts on the targeted cortical region's structure and function. In the four studies reviewed here, two used each patient's own fMRI brain activation patterns collected during overt picture naming to subsequently guide the placement of tDCS electrodes (Baker et al., Citation2010; Fridriksson et al., Citation2011) while the remaining two studies were guided by data from previous neuroimaging studies.
In each study the active electrode was placed directly over the cortex to be modulated, as the electrical field strength induced by tDCS decreases rapidly with distance from the electrode (Miranda, Lomarev, & Hallett, Citation2006). However, the placement of the active electrode on the scalp varied across the four studies reviewed here. They included primarily left frontal (Baker et al., Citation2010) and left temporal regions (Fiori et al., Citation2010; Fridriksson et al., Citation2011; Monti et al., Citation2008). Baker et al. (Citation2010) found that, across their group of patients with frontal or posterior lesions, those who showed the greatest improvement in naming accuracy after frontal anodal tDCS were those with perilesional areas closest to the electrode site. Indeed, in a more homogeneous group of patients, seven out of eight patients with posterior cortical or subcortical lesions showed faster naming responses after posterior anodal tDCS (Fridriksson et al., Citation2011), again highlighting the role of perilesional regions in supporting language processes. In the study by Fiori and colleagues (Citation2010) they noted that the lesion of one of their patients, M.T., completely overlapped with the electrode location, yet surprisingly this patient also showed an improvement in naming accuracy by the fifth day of treatment in the order of ∼7% relative to sham. These results suggest that the cortical effect of tDCS in this patient must be remote from the electrode site, but how this is mediated is unclear.
Using the model of interhemispheric rivalry, if speech production performance is to be successfully improved after partial left hemisphere stroke damage then anodal tDCS applied to structurally and/or functionally intact perilesional cortex should increase the residual left hemisphere's output and improve language function. Indeed, both Baker et al., (Citation2010) and Fridriksson et al., (Citation2011) have used this approach. Here functioning perilesional left hemisphere cortex was identified using fMRI pre-treatment to ensure that tDCS was delivered to both functionally and structurally viable cortical tissue. However, if suitable cortex in the stroke-damaged left hemisphere were not available due to an extensive lesion for example, an alternative approach to treatment would be necessary. Again, based on the model of interhemispheric rivalry, in such cases tDCS could be applied to the right hemisphere to enhance residual output from the stroke-damaged hemisphere by removing any potential interference, or reducing the excitability of the right hemisphere i.e., inhibitory stimulation of the contralesional, right hemisphere (see ).
Stimulation of the contralesional hemisphere as a method of suppressing the maladaptive strategies of the unaffected hemisphere and promoting activity of the damaged hemisphere has been explored in the context of motor recovery post-stroke. Results indicate that, like positive effects observed for anodal stimulation of the ipsilesional hemisphere, cathodal stimulation of the contralesional hemisphere also enhanced motor performance (Boggio et al., Citation2007; Fregni et al., Citation2005). Furthermore, bi-hemispheric stimulation of the left and right hemisphere with anodal and cathodal in healthy participants enhanced motor performance to a greater degree than unilateral or sham stimulation (Vines et al., Citation2008). This pattern of improved performance following “dual” stimulation was replicated in a group of 10 chronic stoke patients compared to a group of 10 patients who received sham stimulation (Lindenberg et al., Citation2010), suggesting preliminary support for this approach.
Surprisingly, such an electrode arrangement has not yet been used in tDCS studies of language recovery in post-stroke aphasic patients. While in the TMS literature stimulation of the contralesional hemisphere as a method of suppressing the maladaptive strategies of the right, non-lesioned hemisphere during language tasks and thereby promoting activity of the lesioned hemisphere has been the main approach to date (Hamilton, Chrysikou, & Coslett, Citation2011). Functional neuroimaging studies have shown chronic over-activation of the non-dominant language homologues (Blank, Bird, Turkheimer, & Wise, Citation2003; Leff et al., Citation2002; Naeser et al., Citation2004; Rosen et al., Citation2000), especially in severely aphasic patients (Crinion & Price, Citation2005). In the case of speech production it is hypothesised that suppressive stimulation over the right frontal cortices (homologue to Broca's area) would promote the recovery of speech function. Naeser et al., (Citation2005) found such an effect in four chronic patients of variable severity when given 10 daily sessions of 1 Hz rTMS over the right hemisphere homologue of Broca's area. The patients had an immediate improvement in picture naming output that, for three patients, persisted for up to 8 months.
In contrast, a later study by Winhuisen and colleagues (Citation2005) showed that verbal fluency could be significantly reduced after stimulation of the right inferior frontal gyrus (IFG). They studied 11 patients within 2 weeks of their stroke and applied 4 Hz rTMS over the right IFG for 10 seconds during a verbal fluency task. Pre-treatment positron emission tomography (PET) showed that eight patients showed activation of the right IFG during this task and, in five of these patients, fluency was reduced by stimulation of the right IFG. The conclusion from this study was that in some patients activity in the right IFG at least in this early time post stroke was contributing to speech rather than interfering with function of Broca's area on the left. These data highlight the issue of general applicability of a treatment approach using suppression of the non-stroke hemisphere; for a subset of these patients the right IFG was also essential for residual language function. Therefore the preliminary results from tDCS of the right hemisphere to promote motor recovery are encouraging (e.g., Lindenberg et al., Citation2010; Vines et al., Citation2008), but more research is needed to explore whether these issues of individual differences that apply to TMS effects of the motor cortex may also apply for tDCS of the language cortices.
Furthermore, Martin and colleagues (Citation2004), using a single session of 1 Hz rTMS under magnetic resonance imaging navigation, showed that the nature of the stimulation effect may depend on the exact site of stimulation: picture naming was transiently improved by rTMS only after suppression of the anterior portion of the IFG (Broca's area), whereas stimulation of the posterior parts of Broca's area had the opposite effect. If cognitive models predict subtle gradations within proximal cortical regions, an issue that is particularly problematic for tDCS is spatial specificity within a target stimulation site, which may preclude its use to explore such empirical questions. We discuss this issue in the following section.
tDCS focality
While TMS has been successfully used in exploring subtle spatial variation across different cortical regions (Devlin, Matthews, & Rushworth, Citation2003; Gough, Nobre, & Devlin, Citation2005; Nixon, Lazarova, Hodinott-Hill, Gough, & Passingham, Citation2004) tDCS is argued to lack such spatial specificity due to (a) using large electrodes and (b) the bipolar scalp electrode arrangement used in many studies. Electrodes are typically quite large, ranging from 25–35cm2, therefore tDCS may stimulate not only the intended cortex, but also adjacent cortices. This limits the use of tDCS for detailed anatomical mapping and differential stimulation of cortical regions in close proximity to one another. As such, the issue of focality remains an important consideration in brain stimulation studies and may preclude the use of tDCS in favour of (r)TMS to explore certain research questions. However, to increase the focality of tDCS, researchers are investigating different electrode geometries (Datta, Bansal, et al., Citation2009) and reducing the size of the electrodes, while maintaining the level of direct current delivered. For example, it has been shown that a smaller (3.5cm2) electrode and reduced current 0.1 mA induced equivalent effects on cortico-spinal activity (Nitsche, Doemkes, et al., Citation2007). However, the effects of smaller electrodes on tissue excitability due to changes in current orientation and edge effects, for example, are unclear and require further development and investigation highlighting that the solution is not a simple one (Nitsche et al., Citation2008).
Another consideration of tDCS is that, by definition, it relies on delivering current via electrodes placed on the scalp surface (transcranially). It has been suggested that much of the current delivered is shunted through the scalp, with only half of an injected current entering the cortex (Miranda et al., Citation2006). The electrical field strength induced by tDCS also decreases rapidly with distance from the electrode, which may preclude the use of tDCS to target deep cortical structures, such as the insula, in speech production tasks in healthy and impaired speakers.
When deciding where to stimulate with tDCS, there is also the question of where to place the reference electrode. All tDCS protocols require that there are two electrodes between which a direct, constant current must flow from the anode (positively charged) to the cathode (negatively charged). In paradigms that refer to anodal or cathodal stimulation effects the second “reference” electrode is located over an area deemed to be remote from cortical regions involved in the task. In tDCS studies of language processing to date, the location of the reference electrode has varied from contralateral supraorbital ridge (Fiori et al., Citation2010; Liuzzi et al., Citation2010), vertex (Sparing et al., Citation2007), the cheekbone (Ross et al., Citation2010), to an extracephalic site (Baker et al., Citation2010; Fertonani et al., Citation2010; Fridriksson et al., Citation2011; Monti et al., Citation2008). Issues arise concerning each montage of electrodes. If both electrodes are placed on the scalp (bi-cephalic montage), it has been argued that observed tDCS effects may be due to a combination of facilitation and/or inhibition across the brain, giving rise to a widespread change of cortical excitability (Lang et al., Citation2005). However, making inferences about the source of tDCS effects is difficult. Although this is an important consideration, evidence from the motor-tDCS literature suggests that neurobehavioural measures of excitability of the motor cortex (e.g., motor evoked potentials) are unaffected by a supraorbital reference electrode (Nitsche & Paulus, Citation2000). To further address such concerns, another approach has been to use an enlarged reference electrode relative to the active electrode. A large reference electrode renders the current too dispersed to be effective (current density = current strength divided by the electrode size)—therefore making the reference electrode stimulation theoretically neutral—allowing for the inference that the observed effects on brain and behaviour are solely due to modulation of the target cortex by the active electrode. Research in the motor literature indicates that tDCS effects were equivalent across the standard and enlarged electrode geometries (Nitsche, Doemkes, et al., Citation2007).
Avoiding any potential confounding effects of two electrodes with opposite polarities over the brain, an extracephalic reference was used in two of the tDCS studies in this review (e.g., Baker et al., Citation2010; Fridriksson et al., Citation2011). In motor studies this electrode arrangement has been suggested to allow for potential spinal influence on observed effects via cortico-spinal connections (e.g., Nitsche, Doemkes, et al., Citation2007). However, a recent study exploring autonomic responses to anodal, cathodal, and sham stimulation of the frontal midline with an extracephalic reference found no significant effects of stimulation with an extra-cephalic reference (Vandermeeren, Jamart, & Ossemann, Citation2010). It is important to note that at this stage it is not clear which is the “best” approach to use, but any differences in the arrangement of electrode may influence effects of stimulation (Miranda et al., Citation2006) and should therefore be clearly reported to allow for comparison across studies (cf. Nitsche et al., Citation2008).
When to stimulate
As with TMS, an important empirical question is when to deliver tDCS in relation to a task of interest, i.e., online: concurrently with the task or offline: pre-task (at rest). The choice depends on the hypotheses about the neural mechanisms supporting the behavioural effects. To date, the neural mechanisms that support tDCS remain unclear (Nitsche, Fricke, et al., Citation2003; Nitsche, Liebetanz, et al., Citation2003; Nitsche & Paulus, Citation2000).
The offline approach uses tDCS to prime the system at rest in preparation for a task to be performed after stimulation. It has been successfully used in language studies of both healthy (Fertonani et al., Citation2010) and impaired speakers (Monti et al., Citation2008). In contrast to Fertonani and colleagues, who report an improvement in performance after offline anodal tDCS, Monti et al. found an improvement in patients' naming accuracy after offline cathodal stimulation of left fronto-temporal cortex. This apparent contradictory effect observed for cathodal rather than anodal stimulation was argued to be due to tDCS-induced depression of cortical inhibitory inter-neurons in the lesioned hemisphere. This in turn led to a disinhibition of the cortex, thereby improving the function of the damaged left hemisphere language areas. This paradoxical improvement has also been reported in a motion detection study (Antal, Nitsche, Kruse, et al., Citation2004). Further research using offline stimulation to precondition the cortex prior to delivery of a task is certainly needed in order to better understand these contrasting effects.
In contrast, several studies have used online tDCS delivered during a language task (Iyer et al., Citation2005; Ross et al., Citation2010; Sparing et al., Citation2007), and during language learning in healthy participants (de Vries et al., Citation2010; Fiori et al., Citation2010; Floël et al., Citation2008). Consistent with the approach of these latter studies the facilitatory effects of anodal stimulation on motor learning was found to be maximal when delivered during motor skill training. In contrast, tDCS applied at rest, i.e., not paired with training, had no beneficial effect on motor performance (Nitsche, Roth, et al., Citation2007; Nitsche, Schauenburg, et al., Citation2003).
At a cellular and molecular level, studies in basic science suggest that one possible neurobiological account of the mechanism underlying the facilitatory effects of online anodal stimulation is that during anodal tDCS the intracellular calcium concentrations, which are important for processes of LTP, are increased via improved trans-membrane calcium conduction (Canepari, Djurisic, & Zecevic, Citation2007). Furthermore, there is an increased secretion of brain-derived neurotrophic factor (BDNF), a protein crucial for new learning that occurs during anodal stimulation (Fritsch et al., Citation2010). Conversely, anodal modulation of synaptic strength prior to motor learning has been shown to compromise performance (Kuo et al., Citation2008), an effect that was further modulated by the presence of a partial NMDA-receptor agonist (d-cycloserine). Similarly, the enhancement of motor cortex excitability by preconditioning with anodal tDCS elicited decreased excitation in rTMS protocols that had previously had no effect (Siebner et al., Citation2004). The excitability status, or homeostatic plasticity, of the underlying cortex is important as it modulates the ease with which synaptic connections can be facilitated or suppressed (e.g., Antal, Terney, Poreisz, & Paulus, Citation2007).
Increased excitability within a language-engaged network (during practice or therapy) and the associated neurobiological facilitation of cellular and molecular mechanisms of anodal tDCS may together have the capacity for increased neuroplasticity within the damaged language system, which may increase the potential for better learning or language recovery. Specifically, within a task-engaged network the synchronous firing of post-synaptic targets will encourage synaptic strengthening (Kuo et al., Citation2008; Nitsche, Roth, et al., Citation2007). Therefore, it could be argued that in the context of re-learning language and aphasia therapy tDCS delivered during, and not before, a task or learning paradigm could optimise outcome and long-term language performance. Although preconditioning—or offline stimulation—of the cortex with tDCS has been shown to enhance subsequent neural effects of TMS (e.g., Lang et al., Citation2004; Siebner et al., Citation2004), the comparison of offline and online tDCS stimulation protocols, their effect on behavioural performance and relationship to the underlying neural mechanism of tDCS requires further systematic research.
How long to stimulate
The efficacy of tDCS from current density and stimulation duration has been systematically explored in the primary motor cortex. Increasing current density or stimulation duration, holding the other parameter constant, results in longer-lasting and stronger effects (Nitsche, Nitsche, et al., Citation2003; Nitsche & Paulus, Citation2000, Citation2001). However, this might not be a linear relationship in each case. Larger current densities also increase the depth of the electrical field modulating deeper cortical regions not affected by lower intensity protocols and where the effects of tDCS might be different compared with more superficial cortical structures (Creutzfeldt, Fromm, & Kapp, Citation1962). Moreover, large current densities might be painful. Current densities delivered during language studies have varied between 0.029 and 0.08 mA/cm2 in most published studies (see Floël, 2012, and ). These limits will probably continue to expand with experience (Nitsche et al., Citation2008).
Similarly, one might consider that repeated delivery of tDCS paired with repeated activation of a task-engaged network should be incrementally beneficial. However, stimulation durations, which are likely to result in excitability changes lasting more than 1 hour, should be applied with caution, because changes lasting that long could be consolidated and stabilised leading to unintended or adverse effects: i.e., beyond a certain period of time, the enhancing effects of anodal tDCS may switch and become inhibitory (Fricke et al., Citation2010). The same concerns apply for repeated application of tDCS to the same brain region without an appropriate interval between sessions (Monte-Silva et al., Citation2010). When repetitive tDCS is performed to prolong and stabilise long-lasting after-effects, participants are generally stimulated once a day (Boggio et al., Citation2007; Fregni et al., Citation2006). Although repeated interventions in the order of days appeared to give facilitatory effects on language performance in aphasic speakers (see ), and language learning in healthy speakers (de Vries et al., Citation2010; Floël et al., Citation2008), it remains unclear whether this protocol is optimally suited to maximise the electrophysiological effects of tDCS.
At present, 20 minutes of anodal tDCS given within a session appears to be facilitatory, with the effects lasting at least as long as the duration of stimulation. However, beyond 20 minutes of stimulation the effects may not be as expected. For repeated application of tDCS, we suggest a sufficiently long intersession interval between tDCS interventions to avoid unintended carry-over effects. The duration of this interval depends on the stimulation procedure. If the aim is to induce more long-term and stable changes in cortical function, repeated daily tDCS sessions may be adequate. However, further studies are certainly needed to explore the optimal intersession interval for stabilising effects. So contrary to the general “more is better” approach for aphasia behavioural therapeutic interventions, this adage may not be the case with tDCS.
CAVEATS
Despite significant limitations to our understanding of the mechanism of tDCS, the preliminary work with chronic aphasic stroke patients reviewed here indicate that it is possible to improve language task performance by means of noninvasive electrical stimulation applied to the damaged brain. This raises the exciting hypothesis that currently available tDCS has the potential to modulate language rehabilitation. Several caveats should be kept in mind.
First, studies implemented so far have mostly focused on the ability of tDCS to elicit improvements in speech production by stimulation of left-hemisphere perilesional regions. Yet the accompanying neural mechanisms underlying these changes have not been investigated. The paths of tDCS-induced effects remain unclear and behavioural changes may result through specific enhancement of activity in the targeted cortical area or via distant effects on other interconnected cortical areas. Therefore more sophisticated head models incorporating detailed anatomical information from structural MRI or DTI may be necessary to constrain tDCS effects. At the very least in aphasic stroke patients a detailed anatomical MRI scan is necessary to be confident that the active electrode is placed over structurally intact cortex.
Second, detailed theoretical and anatomical models of language processing that guide the application of tDCS to particular regions of cortex are also necessary. fMRI and TMS studies of language processing have highlighted that the right hemisphere may well have a contributing role to play in aphasia recovery emphasising that therapeutic interventions, such as tDCS, that stress targeting the left or right-hemisphere currently should be based on supporting functional neuroimaging of patients and language tasks. Studies combining tDCS with other brain imaging and neurophysiologic mapping methods, e.g., fMRI or electroencephalography (EEG), promise to provide invaluable insights on the correlation between modification of language and its underlying neurophysiological mechanisms.
Third, most basic neuroscience research in the field has focused on studying the effects of stimulation over the motor cortex, where anodal stimulation has a facilitatory effect and cathodal an inhibitory effect. However, some studies have reported that anodal stimulation of the frontal cortex failed to induce significant improvements in behaviour (Antal, Nitsche, Kincses, et al., Citation2004; Nitsche, Schauenburg, et al., Citation2003). Similarly, cathodal tDCS does not always inhibit language performance in healthy participants (e.g., Fertonani et al., Citation2010; Iyer et al., Citation2005; Sparing et al., Citation2007). However, studies indicating a lack of effect of stimulation in a particular cortical region are not proof that that area is not involved. The most parsimonious interpretation of these findings is that more elaborate, hypothesis-driven behavioural paradigms or stimulation parameters may be necessary to study the functional role of the cortical regions involved in language processing. Alternatively, it is possible that the “threshold” for facilitating motor learning by stimulation of motor cortex is lower than by stimulation of other cortical areas for language processing, an issue that needs to be investigated in future experiments.
Finally, studies to date have focused on relatively short-term improvements in language performance (longest follow-up data available in aphasic patients was 3 weeks post training; Fridriksson et al., Citation2010). It is not known whether these intriguing task-specific improvements may be persistent or have any impact on real-life functional communication abilities. More experiments are required to assess the effects of repeated applications of tDCS in association with multiple training sessions, their interaction with specific language tasks and different intensive training regimes, such as constraint-induced aphasia treatment (CIAT) and the extent to which these performance improvements are retained in the long term. Therefore these factors should be considered when designing a study.
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, despite the infancy of the field and the very few studies to date, it does seem possible to improve aphasic stroke patients' performance on speech production tasks by means of tDCS. The lack of any reported adverse effects suggests that the procedure is safe, even in chronic stroke patients who have a higher reported incidence of seizures than healthy participants. Future work will have to address many so-far unanswered questions including:
(1) What are the optimal combinations of stimulation parameters to get maximal behavioural effects? For tDCS these parameters involve the size of electrodes, amplitude, duration of current, online versus offline, and the time-interval between stimulation periods. A systematic exploration of how these parameters interact and influence tDCS-induced effects in normal language function is certainly important. How these effects then translate to damaged brains is an additional level of complexity that may also vary with time post-stroke. Furthermore, whether established tDCS protocols may be usefully combined with other forms of therapeutic intervention: for example pharmacological interventions also needs investigation. Pharmacotherapy for recovery from aphasia when delivered in combination with behavioural training (Berthier et al., Citation2009) may have maximal potential when also combined with tDCS. While drugs may have a more global effect on brain function, tDCS can have a more regionally specific effect (Holland et al., Citation2011). Therefore, when administered in combination with language rehabilitation, these approaches may have a synergistic effect. For example, tDCS of frontal cortex during speech production in combination with pharmacological modification of the dopaminergic system may enhance, or prolong, therapeutic outcome compared to each intervention alone (e.g., Antal & Paulus, Citation2011).
(2) When applying tDCS to facilitate aphasia recovery is it more effective to stimulate the stroke-damaged or the non-stroke hemisphere? Although the model of interhemispheric rivalry has been used to support the idea that excitatory tDCS should be applied to the stroke hemisphere to optimise recovery, the corollary argument that inhibition of the right hemisphere with tDCS is beneficial requires further research. As suggested by some TMS studies of interference with language function, it may well be that in some aphasic patients the right hemisphere is contributing significantly to their language recovery, rather than suppressing remaining function of the stroke hemisphere. In this instance these patients may benefit from anodal tDCS to the right hemisphere: one approach may not be suitable for all. Further work is certainly needed to understand the mechanism of tDCS. Nevertheless, at this stage there is an encouraging possibility that tDCS of perilesional cortices when combined with behavioural rehabilitation may contribute to improved speech outcome in post-stroke aphasic patients. These preliminary foundations suggest that tDCS as a complementary tool for rehabilitation of language function in chronic stroke aphasic patients is an exciting opportunity and an important new avenue of research.
REFERENCES
- Abel , S. , Dressel , K. , Bitzer , R. , Kummerer , D. , Mader , I. Weiller , C. 2009 . The separation of processing stages in a lexical interference fMRI-paradigm . NeuroImage , 44 ( 3 ) : 1113 – 1124 .
- Antal , A. , Nitsche , M. A. , Kincses , T. Z. , Kruse , W. , Hoffmann , K. P. and Paulus , W. 2004 . Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans . European Journal of Neuroscience , 19 ( 10 ) : 2888 – 2892 .
- Antal , A. , Nitsche , M. A. , Kruse , W. , Kincses , T. Z. , Hoffmann , K. P. and Paulus , W. 2004 . Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans . Journal of Cognitive Neuroscience , 16 ( 4 ) : 521 – 527 .
- Antal , A. and Paulus , W. 2011 . A case of refractory orofacial pain treated by transcranial direct current stimulation applied over hand motor area in combination with NMDA agonist drug intake . Brain Stimulation , 4 ( 2 ) : 117 – 121 .
- Antal , A. , Terney , D. , Poreisz , C. and Paulus , W. 2007 . Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex . European Journal of Neuroscience , 26 ( 9 ) : 2687 – 2691 .
- Baker , J. M. , Rorden , C. and Fridriksson , J. 2010 . Using transcranial direct-current stimulation to treat stroke patients with aphasia . Stroke , 41 ( 6 ) : 1229 – 1236 .
- Barthel , G. , Meinzer , M. , Djundja , D. and Rockstroh , B. 2008 . Intensive language therapy in chronic aphasia: Which aspects contribute most? . Aphasiology , 22 ( 4 ) : 408 – 421 .
- Berthier , M. L. , Green , C. , Lara , J. P. , Higueras , C. , Barbancho , M. A. Davila , G. 2009 . Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia . Annals of Neurology , 65 ( 5 ) : 577 – 585 .
- Bhogal , S. K. , Teasell , R. W. , Foley , N. C. and Speechley , M. R. 2003 . Rehabilitation of aphasia: More is better . Topics in Stroke Rehabilitation , 10 ( 2 ) : 66 – 76 .
- Bikson , M. , Datta , A. and Elwassif , M. 2009 . Establishing safety limits for transcranial direct current stimulation . Clinical Neurophysiology , 120 ( 6 ) : 1033 – 1034 .
- Bindman , L. J. , Lippold , O. C. and Redfearn , J. W. 1962 . Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents . Nature , 196 : 584 – 585 .
- Blank , S. C. , Bird , H. , Turkheimer , F. and Wise , R. J. 2003 . Speech production after stroke: The role of the right pars opercularis . Annals of Neurology , 54 ( 3 ) : 310 – 320 .
- Boggio , P. S. , Nunes , A. , Rigonatti , S. P. , Nitsche , M. A. , Pascual-Leone , A. and Fregni , F. 2007 . Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients . Restorative Neurology and Neuroscience , 25 ( 2 ) : 123 – 129 .
- Breitenstein , C. , Kramer , K. , Meinzer , M. , Baumgartner , A. , Floël , A. and Knecht , S. 2009 . Intense language training for aphasia. Contribution of cognitive factors . Nervenarzt , 80 ( 2 ) : 149 – 150 .
- Brindley , P. , Copeland , M. , Demain , C. and Martyn , P. 1989 . Comparison of the speech of ten chronic Broca's aphasics following intensive and nonintensive periods of therapy . Aphasiology , 3 : 695 – 707 .
- Brown , S. , Laird , A. R. , Pfordresher , P. Q. , Thelen , S. M. , Turkeltaub , P. and Liotti , M. 2009 . The somatotopy of speech: Phonation and articulation in the human motor cortex . Brain and Cognition , 70 ( 1 ) : 31 – 41 .
- Canepari , M. , Djurisic , M. and Zecevic , D. 2007 . Dendritic signals from rat hippocampal CA1 pyramidal neurons during coincident pre- and post-synaptic activity: A combined voltage- and calcium-imaging study . Journal of Physiology , 580 : 463 – 484 .
- Cappa , S. F. , Sandrini , M. , Rossini , P. M. , Sosta , K. and Miniussi , C. 2002 . The role of the left frontal lobe in action naming – rTMS evidence . Neurology , 59 ( 5 ) : 720 – 723 .
- Cherney , L. R. , Patterson , J. P. , Raymer , A. , Frymark , T. and Schooling , T. 2008 . Evidence-based systematic review: Effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia . Journal of Speech, Language and Hearing Research , 51 ( 5 ) : 1282 – 1299 .
- Cohen Kadosh , R. , Soskic , S. , Iuculano , T. , Kanai , R. and Walsh , V. 2010 . Modulating neuronal activity produces specific and long-lasting changes in numerical competence . Current Biology , 20 : 1 – 5 .
- Creutzfeldt , O. D. , Fromm , G. H. and Kapp , H. 1962 . Influence of transcortical d-c currents on cortical neuronal activity . Experimental Neurology , 5 : 436 – 452 .
- Crinion , J. and Price , C. J. 2005 . Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke . Brain , 128 ( Pt 12 ) : 2858 – 2871 .
- Datta , A. , Bansal , V. , Diaz , J. , Patel , J. , Reato , D. and Bikson , M. 2009 . Gyri -precise head model of transcranial DC stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad . Brain Stimulation , 2 ( 4 ) : 201 – 207 .
- Datta , A. , Elwassif , M. and Bikson , M. 2009 . Bio-heat transfer model of transcranial DC stimulation: comparison of conventional pad versus ring electrode . Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society , 2009 : 670 – 673 .
- de Vries , M. H. , Barth , A. C. R. , Maiworm , S. , Knecht , S. , Zwitserlood , P. and Floël , A. 2010 . Electrical stimulation of Broca's area enhances implicit learning of an artificial grammar . Journal of Cognitive Neuroscience , 22 ( 11 ) : 2427 – 2436 .
- de Zubicaray , G. I. and McMahon , K. L. 2009 . Auditory context effects in picture naming investigated with event-related fMRI . Cognitive Affective and Behavioral Neuroscience , 9 ( 3 ) : 260 – 269 .
- Devlin , J. T. , Matthews , P. M. and Rushworth , M. F. 2003 . Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study . Journal of Neuroscience , 15 ( 1 ) : 71 – 84 .
- Dockery , C. A. , Hueckel-Weng , R. , Birbaumer , N. and Plewnia , C. 2009 . Enhancement of planning ability by transcranial direct current stimulation . Journal of Neuroscience , 29 ( 22 ) : 7271 – 7277 .
- Dundas , J. E. , Thickbroom , G. W. and Mastaglia , F. L. 2007 . Perception of comfort during transcranial DC stimulation: Effect of NaCl solution concentration applied to sponge electrodes . Clinical Neurophysiology , 118 ( 5 ) : 1166 – 1170 .
- Fertonani , A. , Rosini , S. , Cotelli , M. , Rossini , P. M. and Miniussi , C. 2010 . Naming facilitation induced by transcranial direct current stimulation . Behavioral Brain Research , 208 ( 2 ) : 311 – 318 .
- Fiori , V. , Coccia , M. , Marinelli , C. V. , Vecchi , V. , Bonifazi , S. Ceravolo , M. G. 2010 . Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects . Journal of Cognitive Neuroscience , 23 ( 9 ) : 2309 – 2323 .
- Floël , A. , Rosser , N. , Miichka , O. , Knecht , S. and Breitenstein , C. 2008 . Noninvasive brain stimulation improves language learning . Journal of Cognitive Neuroscience , 20 ( 8 ) : 1415 – 1422 .
- Frank , E. , Eichhammer , P. , Burger , J. , Zowe , M. , Landgrebe , M. Hajak , G. 2011 . Transcranial magnetic stimulation for the treatment of depression: Feasibility and results under naturalistic conditions: A retrospective analysis . European Archives of Psychiatry and Clinical Neuroscience , 261 ( 4 ) : 261 – 266 .
- Fregni , F. , Boggio , P. S. , Lima , M. C. , Ferreira , M. J. , Wagner , T. Rigonatti , S. P. 2006 . A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury . Pain , 122 ( 1–2 ) : 197 – 209 .
- Fregni , F. , Boggio , P. S. , Mansur , C. G. , Wagner , T. , Ferreira , M. J. L. Lima , M. C. 2005 . Transcranial direct current stimulation of the unaffected hemisphere in stroke patients . Neuroreport , 16 ( 14 ) : 1551 – 1555 .
- Fricke , K. , Seeber , A. A. , Thirugnanasambandam , N. , Paulus , W. , Nitsche , M. A. and Rothwell , J. C. 2010 . Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation (tDCS) of the human motor cortex . Journal of Neurophysiology , 105 ( 3 ) : 1141 – 1149 .
- Fridriksson , J. , Bonilha , L. , Baker , J. M. , Moser , D. and Rorden , C. 2010 . Activity in preserved left hemisphere regions predicts anomia severity in aphasia . Cerebral Cortex , 20 ( 5 ) : 1013 – 1019 .
- Fridriksson , J. , Moser , D. , Ryalls , J. , Bonilha , L. , Rorden , C. and Baylis , G. 2009 . Modulation of frontal lobe speech areas associated with the production and perception of speech movements . Journal of Speech Language and Hearing Research , 52 ( 3 ) : 812 – 819 .
- Fridriksson , J. , Richardson , J. D. , Baker , J. M. and Rorden , C. 2011 . Transcranial direct current stimulation improves naming reaction time in fluent aphasia: A double-blind, sham-controlled study . Stroke , 42 ( 3 ) : 819 – 821 .
- Fritsch , B. , Reis , J. , Martinowich , K. , Schambra , H. M. , Ji , Y. Cohen , L. G. 2010 . Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning . Neuron , 66 ( 2 ) : 198 – 204 .
- Gandiga , P. C. , Hummel , F. C. and Cohen , L. G. 2006 . Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation . Clinical Neurophysiology , 117 ( 4 ) : 845 – 850 .
- Gough , P. M. , Nobre , A. C. and Devlin , J. T. 2005 . Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation . Journal of Neuroscience , 25 ( 35 ) : 8010 – 8016 .
- Hamilton , R. H. , Chrysikou , E. G. and Coslett , B. 2011 . Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation . Brain and Language , 118 ( 1–2 ) : 40 – 50 .
- Hartwigsen , G. , Price , C. J. , Baumgaertner , A. , Geiss , G. , Koehnke , M. Ulmer , S. 2010 . The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: Evidence from dual-site TMS . Neuropsychologia , 48 ( 10 ) : 3155 – 3163 .
- Hocking , J. , McMahon , K. L. and de Zubicaray , G. I. 2009 . Semantic context and visual feature effects in object naming: An fMRI study using arterial spin labeling . Journal of Cognitive Neuroscience , 21 ( 8 ) : 1571 – 1583 .
- Holland , R. and Lambon Ralph , M. A. 2010 . The anterior temporal lobe semantic hub is a part of the language neural network: Selective disruption of irregular past tense verbs by rTMS . Cerebral Cortex , 20 ( 12 ) : 2771 – 2775 .
- Holland , R. , Leff , A. P. , Josephs , O. , Galea , J. M. , Desikan , M. Price , C. J. 2011 . Speech facilitation by left inferior frontal cortex stimulation . Current Biology , 21 ( 16 ) : 1403 – 1407 .
- Iyer , M. B. , Mattu , U. , Grafman , J. , Lomarev , M. , Sato , S. and Wassermann , E. M. 2005 . Safety and cognitive effect of frontal DC brain polarization in healthy individuals . Neurology , 64 ( 5 ) : 872 – 875 .
- Jahanshahi , M. and Rothwell , J. 2000 . Transcranial magnetic stimulation studies of cognition: An emerging field . Experimental Brain Research , 131 ( 1 ) : 1 – 9 .
- Kelly , H. , Brady , M. C. and Enderby , P. 2010 . “ Speech and language therapy for aphasia following stroke ” . In Cochrane Database of Systematic Reviews Vol. 5 , CD000425
- Kendall , D. L. , Rosenbek , J. C. , Heilman , K. M. , Conway , T. , Klenberg , K. Gonzalez Rothi , L. J. 2008 . Phoneme-based rehabilitation of anomia in aphasia . Brain and Language , 105 ( 1 ) : 1 – 17 .
- Kincses , T. Z. , Antal , A. , Nitsche , M. A. , Bartfai , O. and Paulus , W. 2004 . Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human . Neuropsychologia , 42 ( 1 ) : 113 – 117 .
- Kuo , M. F. , Unger , M. , Liebetanz , D. , Lang , N. , Tergau , F. Paulus , W. 2008 . Limited impact of homeostatic plasticity on motor learning in humans . Neuropsychologia , 46 ( 8 ) : 2122 – 2128 .
- Lagopoulos , J. and Degabriele , R. 2008 . Feeling the heat: The electrode-skin interface during DCS . Acta Neuropsychiatry , 20 : 98 – 100 .
- Lang , N. , Siebner , H. R. , Ernst , D. , Nitsche , M. A. , Paulus , W. Lemon , R. N. 2004 . Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects . Biological Psychiatry , 56 ( 9 ) : 634 – 639 .
- Lang , N. , Siebner , H. R. , Ward , N. S. , Lee , L. , Nitsche , M. A. Paulus , W. 2005 . How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? . European Journal of Neuroscience , 22 ( 2 ) : 495 – 504 .
- Leff , A. , Crinion , J. , Scott , S. , Turkheimer , F. , Howard , D. and Wise , R. 2002 . A physiological change in the homotopic cortex following left posterior temporal lobe infarction . Annals of Neurology , 51 ( 5 ) : 553 – 558 .
- Liebetanz , D. , Koch , R. , Mayenfels , S. , Konig , F. , Paulus , W. and Nitsche , M. A. 2009 . Safety limits of cathodal transcranial direct current stimulation in rats . Clinical Neurophysiology , 120 ( 6 ) : 1161 – 1167 .
- Liebetanz , D. , Nitsche , M. A. , Tergau , F. and Paulus , W. 2002 . Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability . Brain , 125 : 2238 – 2247 .
- Lindenberg , R. , Renga , V. , Zhu , L. L. , Nair , D. and Schlaug , G. 2010 . Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients . Neurology , 75 ( 24 ) : 2176 – 2184 .
- Liuzzi , G. , Freundlieb , N. , Ridder , V. , Hoppe , J. , Heise , K. Zimerman , M. 2010 . The involvement of the left motor cortex in learning of a novel action word lexicon . Current Biology , 20 ( 19 ) : 1745 – 1751 .
- Loo , C. K. , Martin , D. M. , Alonzo , A. , Gandevia , S. , Mitchell , P. B. and Sachdev , P. 2011 . Avoiding skin burns with transcranial direct current stimulation: Preliminary considerations . International Journal of Neuropsychopharmacology , 14 ( 3 ) : 425 – 426 .
- Martin , P. I. , Naeser , M. A. , Theoret , H. , Tormos , J. M. , Nicholas , M. Kurland , J. 2004 . Transcranial magnetic stimulation as a complementary treatment for aphasia . Seminars in Speech and Language , 25 ( 2 ) : 181 – 191 .
- Meinzer , M. , Djundja , D. , Barthel , G. , Elbert , T. and Rockstroh , B. 2005 . Long-term stability of improved language functions in chronic aphasia after constraint-induced aphasia therapy . Stroke , 36 ( 7 ) : 1462 – 1466 .
- Meinzer , M. , Elbert , T. , Wienbruch , C. , Djundja , D. , Barthel , G. and Rockstroh , B. 2004 . Intensive language training enhances brain plasticity in chronic aphasia . BMC Biology , 2 : 20
- Minhas , P. , Bansal , V. , Patel , J. , Ho , J. S. , Diaz , J. Datta , A. 2010 . Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS . Journal of Neuroscience Methods , 190 ( 2 ) : 188 – 197 .
- Miniussi , C. , Cappa , S. F. , Cohen , L. G. , Floël , A. , Fregni , F. Nitsche , M. A. 2008 . Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabititation . Brain Stimulation , 1 ( 4 ) : 326 – 336 .
- Miranda , P. C. , Lomarev , M. and Hallett , M. 2006 . Modeling the current distribution during transcranial direct current stimulation . Clinical Neurophysiology , 117 ( 7 ) : 1623 – 1629 .
- Monte-Silva , K. , Kuo , M. F. , Liebetanz , D. , Paulus , W. and Nitsche , M. A. 2010 . Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) . Journal of Neurophysiology , 103 ( 4 ) : 1735 – 1740 .
- Monti , A. , Cogiamanian , F. , Marceglia , S. , Ferrucci , R. , Mameli , F. Mrakic-Sposta , S. 2008 . Improved naming after transcranial direct current stimulation in aphasia . Journal of Neurology Neurosurgery and Psychiatry , 79 ( 4 ) : 451 – 453 .
- Naeser , M. A. , Martin , P. I. , Baker , E. H. , Hodge , S. M. , Sczerzenie , S. E. Nicholas , M. 2004 . Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method . NeuroImage , 22 ( 1 ) : 29 – 41 .
- Naeser , M. A. , Martin , P. I. , Nicholas , M. , Baker , E. H. , Seekins , H. Kobayashi , M. 2005 . Improved picture naming in chronic aphasia after TMS to part of right Broca's area: An open-protocol study . Brain and Language , 93 ( 1 ) : 95 – 105 .
- Nickels , L. 2002 . Therapy for naming disorders: Revisiting, revising, and reviewing . Aphasiology , 16 : 935 – 979 .
- Nitsche , M. A. , Cohen , L. G. , Wassermann , E. M. , Priori , A. , Lang , N. Antal , A. 2008 . Transcranial direct current stimulation: State of the art 2008 . Brain Stimulation , 1 ( 3 ) : 206 – 223 .
- Nitsche , M. A. , Doemkes , S. , Karakose , T. , Antal , A. , Liebetanz , D. Lang , N. 2007 . Shaping the effects of transcranial direct current stimulation of the human motor cortex . Journal of Neurophysiology , 97 ( 4 ) : 3109 – 3117 .
- Nitsche , M. A. , Fricke , K. , Henschke , U. , Schlitterlau , A. , Liebetanz , D. Lang , N. 2003 . Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans . Journal of Physiology , 553 : 293 – 301 .
- Nitsche , M. A. , Grundey , J. , Liebetanz , D. , Lang , N. , Tergau , F. and Paulus , W. 2004 . Catecholaminergic consolidation of motor cortical neuroplasticity in humans . Cerebral Cortex , 14 ( 11 ) : 1240 – 1245 .
- Nitsche , M. A. , Liebetanz , D. , Antal , A. , Lang , N. , Tergau , F. and Paulus , W. 2003 . Modulation of cortical excitability by weak direct current stimulation – technical, safety and functional aspects . Supplements to Clinical Neurophysiology , 56 : 255 – 276 .
- Nitsche , M. A. , Nitsche , M. S. , Klein , C. C. , Tergau , F. , Rothwell , J. C. and Paulus , W. 2003 . Level of action of cathodal DC polarisation induced inhibition of the human motor cortex . Clinical Neurophysiology , 114 ( 4 ) : 600 – 604 .
- Nitsche , M. A. and Paulus , W. 2000 . Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation . Journal of Physiology , 527 ( Pt 3 ) : 633 – 639 .
- Nitsche , M. A. and Paulus , W. 2001 . Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans . Neurology , 57 ( 10 ) : 1899 – 1901 .
- Nitsche , M. A. , Roth , A. , Kuo , M. F. , Fischer , A. K. , Liebetanz , D. Lang , N. 2007 . Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex . Journal of Neuroscience , 27 ( 14 ) : 3807 – 3812 .
- Nitsche , M. A. , Schauenburg , A. , Lang , N. , Liebetanz , D. , Exner , C. Paulus , W. 2003 . Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human . Journal of Cognitive Neuroscience , 15 ( 4 ) : 619 – 626 .
- Nixon , P. , Lazarova , J. , Hodinott-Hill , I. , Gough , P. and Passingham , R. 2004 . The inferior frontal gyrus and phonological processing: An investigation using rTMS . Journal of Cognitive Neuroscience , 16 ( 2 ) : 289 – 300 .
- Palm , U. , Keeser , D. , Schiller , C. , Fintescu , Z. , Nitsche , M. Reisinger , E. 2008 . Skin lesions after treatment with transcranial direct current stimulation (tDCS) . Brain Stimulation , 1 ( 4 ) : 386 – 387 .
- Papoutsi , M. , de Zwart , J. A. , Jansma , J. M. , Pickering , M. J. , Bednar , J. A. and Horwitz , B. 2009 . From phonemes to articulatory codes: An fMRI study of the role of Broca's area in speech production . Cerebral Cortex , 19 ( 9 ) : 2156 – 2165 .
- Pobric , G. , Jefferies , E. and Lambon Ralph , M. A. 2007 . Anterior temporal lobes: Mimicking semantic dementia by using rTMS in normal participants . Proceedings of the National Academy of Sciences , 104 ( 50 ) : 20137 – 20141 .
- Poeck , K. , Huber , W. and Willmes , K. 1989 . Outcome of intensive language treatment in aphasia . Journal of Speech and Hearing Disorders , 54 : 471 – 479 .
- Poreisz , C. , Boros , K. , Antal , A. and Paulus , W. 2007 . Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients . Brain Research Bulletin , 72 ( 4–6 ) : 208 – 214 .
- Price , C. J. 2010 . The anatomy of language: A review of 100 fMRI studies published in 2009 . Annals of the New York Academy of Sciences , 1191 : 62 – 88 .
- Priori , A. , Hallett , M. and Rothwell , J. C. 2009 . Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? . Brain Stimulation , 2 ( 4 ) : 241 – 245 .
- Pulvermuller , F. , Neininger , B. , Elbert , T. , Mohr , B. , Rockstroh , B. Koebbel , P. 2001 . Constraint-induced therapy of chronic aphasia after stroke . Stroke , 32 ( 7 ) : 1621 – 1626 .
- Purpura , D. P. and McMurtry , J. G. 1965 . Intracellular activities and evoked potential changes during polarization of motor cortex . Journal of Neurophysiology , 28 : 166 – 185 .
- Reis , J. , Schambra , H. M. , Cohen , L. G. , Buch , E. R. , Fritsch , B. Zarahn , E. 2009 . Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation . Proceedings of the National Academy of Sciences of the United States of America , 106 ( 5 ) : 1590 – 1595 .
- Rosen , H. J. , Petersen , S. E. , Linenweber , M. R. , Snyder , A. Z. , White , D. A. Chapman , L. 2000 . Neural correlates of recovery from aphasia after damage to left inferior frontal cortex . Neurology , 55 ( 12 ) : 1883 – 1894 .
- Ross , L. A. , McCoy , D. , Wolk , D. A. , Coslett , H. B. and Olson , I. R. 2010 . Improved proper name recall by electrical stimulation of the anterior temporal lobes . Neuropsychologia , 48 ( 12 ) : 3671 – 3674 .
- Siebner , H. R. , Lang , N. , Rizzo , V. , Nitsche , M. A. , Paulus , W. Lemon , R. N. 2004 . Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex . Journal of Neuroscience , 24 ( 13 ) : 3379 – 3385 .
- Sparing , R. , Dafotakis , M. , Hesse , M. D. and Fink , G. R. 2007 . Enhancing language performance with transcranial direct current stimulation in healthy humans: Implications for rehabilitation and recovery of function after stroke . Journal of Neurology , 254 : 65 – 65 .
- Stagg , C. J. and Nitsche , M. A. 2011 . Physiological basis of transcranial direct current stimulation . Neuroscientist , 17 ( 1 ) : 37 – 53 .
- Stewart , L. , Meyer , B. , Frith , U. and Rothwell , J. C. 2001 . Left posterior BA37 is involved in object recognition: A TMS study . Neuropsychologia , 39 : 1 – 6 .
- Thiel , A. , Haupt , W. F. , Habedank , B. , Winhuisen , L. , Herholz , K. Kessler , J. 2005 . Neuroimaging-guided rTMS of the left inferior frontal gyrus interferes with repetition priming . NeuroImage , 25 ( 3 ) : 815 – 823 .
- Vandermeeren , Y. , Jamart , J. and Ossemann , M. 2010 . Effect of tDCS with an extracephalic reference electrode on cardio-respiratory and autonomic functions . BMC Neuroscience , 11 : 38
- Vigneau , M. , Beaucousin , V. , Herve , P. Y. , Duffau , H. , Crivello , F. Houde , O. 2006 . Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing . NeuroImage , 30 ( 4 ) : 1414 – 1432 .
- Vines , B. W. , Cerruti , C. and Schlaug , G. 2008 . Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation . BMC Neuroscience , 9 : 103
- Vitali , P. , Abutalebi , J. , Tettamanti , M. , Danna , M. , Ansaldo , A. I. Perani , D. 2007 . Training-induced brain remapping in chronic aphasia: A pilot study . Neurorehabilitation and Neural Repair , 21 ( 2 ) : 152 – 160 .
- Winhuisen , L. , Thiel , A. , Schumacher , B. , Kessler , J. , Rudolf , J. Haupt , W. F. 2005 . Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: A combined repetitive transcranial magnetic stimulation and positron emission tomography study . Stroke , 36 ( 8 ) : 1759 – 1763 .