ABSTRACT
Background: Previous research indicates that combining behavioural therapy with transcranial direct current stimulation (tDCS) may be more effective than therapy alone in increasing naming ability in stroke survivors with chronic anomia. Anodal (excitatory) stimulation targeting left perilesional areas and/or cathodal (inhibitory) stimulation targeting right contralesional areas may particularly benefit non-fluent patients with localised damage to the left frontal lobe. However, studies have yet to systematically compare the effects of varying the laterality and polarity of tDCS within individual patients in order to determine optimal stimulation parameters.
Aims: The primary purpose of the current study was to determine which tDCS parameters would result in the greatest improvements in naming ability in an individual (JSc) with chronic Broca’s aphasia (9 years post-stroke) due to a left frontal lesion. A range of secondary outcome measures were also collected to explore the potential effects of therapy on JSc’s connected speech, emotional well-being, and communicative effectiveness.
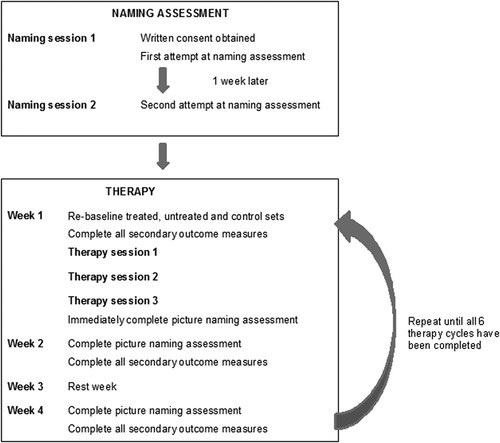
Methods & Procedures: Following baseline naming assessment, JSc completed six, 4-week long cycles of therapy, each involving a different stimulation condition: four active (perilesional anodal, perilesional cathodal, contralesional anodal, and contralesional cathodal) and two sham control conditions (perilesional and contralesional). In the first week of each cycle, he completed three, 20-min therapy sessions, during which he carried out a personalised picture name repetition therapy task at the same time as receiving tDCS. Naming ability was measured before, immediately after, 1 week after, and 3 weeks after each week of therapy. The secondary outcome measures were completed before, 1 week after, and 3 weeks after each week of therapy.
Outcomes & Results: Naming accuracy immediately after stimulation increased significantly more following perilesional anodal stimulation than following perilesional sham stimulation, and this effect remained significant at 3 weeks post-therapy. Treatment effects on the secondary outcome measures were less consistent.
Conclusions: The results agree with previous work demonstrating the importance of activation in left frontal perilesional regions for accurate picture naming in stroke survivors with non-fluent aphasia. We have shown that the effectiveness of a total of 1 h of behavioural anomia therapy can be significantly increased when combined with perilesional anodal tDCS and demonstrated the feasibility of a longitudinal, repeated measures design with multiple outcome measures. Greater understanding of the optimal tDCS parameters to enhance anomia therapy outcomes in individual patients with differing lesion and behavioural profiles may one day assist with the translation of therapy plus tDCS protocols into everyday clinical practice.
Introduction
Anomia, or word finding difficulty, is the most common symptom across all types of aphasia (Postman-Caucheteux et al., Citation2010) and often persists into the chronic stage, when more severe acute deficits have resolved (Pedersen, Vinter, & Skyhoj Olsen, Citation2004). Problems with spoken word production can adversely impact the daily functioning and quality of life of both stroke survivors and their communication partners (Hilari, Needle, & Harrison, Citation2012). Consequently, facilitating word finding is a frequent aim in language rehabilitation following a stroke. Impairment-based behavioural speech and language therapy aims to help a stroke survivor to “re-learn” words that they are unable to retrieve or produce (Nickels, Citation2002b). Therapy can lead to both improved object naming (Lambon Ralph, Snell, Fillingham, Conroy, & Sage, Citation2010) and increased word finding in everyday conversation (Best et al., Citation2011). However, individuals with chronic anomia may make slow progress to achieve relatively small gains, especially if treatment is not provided intensively (Barthel, Meinzer, Djundja, & Rockstroh, Citation2008). A growing body of evidence indicates that concurrent use of neurostimulation techniques has the potential to optimise both the effectiveness and efficiency of more traditional interventions (Holland & Crinion, Citation2012; Torres, Drebing, & Hamilton, Citation2013). The current study investigated the effects of combining transcranial direct current stimulation (tDCS), with behavioural therapy in an individual with chronic Broca’s aphasia. This aphasia sub-type is typically associated with damage to language areas in the left frontal lobe and, in addition to anomia, is characterised by non-fluent, effortful spontaneous speech and short, agrammatic utterances (Fridriksson, Hubbard, et al., Citation2012) .
tDCS is a non-invasive neurostimulation technique that uses a battery pack and two saline-soaked electrodes to deliver weak electrical currents (usually 1–2 mA) to the brain. The active electrode is positioned on the scalp over the region of interest in order to stimulate the underlying cortex. The reference electrode completes the circuit and is most frequently placed on the contralateral supra-orbit or shoulder (Fridriksson, Citation2011). Positive (anodal) stimulation is associated with increased neuronal activation, whilst negative (cathodal) stimulation is associated with decreased neuronal activation (Nitsche & Paulus, Citation2000). When administered in line with established safety protocols, tDCS is considered appropriate for use with both healthy individuals and stroke survivors (Nitsche et al., Citation2008, Citation2003; Poreisz, Boros, Antal, & Paulus, Citation2007; Rossi, Hallett, Rossini, & Pascual-Leone, Citation2009). Unlike transcranial magnetic stimulation (TMS), tDCS is not associated with an increased seizure risk, nor does it affect the movement of the articulatory musculature. Moreover, although occasional side effects such as headaches or localised tingling have been noted, many individuals undergoing low intensity (<1.5mA) tDCS do not experience any adverse effects as a result of stimulation, and those who do typically report only mild symptoms that fade within the first minute of stimulation (Flöel, Rösser, Michka, Knecht, & Breitenstein, Citation2008; Gandiga, Hummel, & Cohen, Citation2006; Kessler, Turkeltaub, Benson, & Hamilton, Citation2012). This latter observation means that individuals are often unable to distinguish sham conditions, in which stimulation is turned on for up to 1 min before being slowly turned off, from longer periods of active stimulation, thus allowing direct comparisons to be made between conditions in which participants receive therapy plus stimulation and those in which they receive therapy alone.
Our recent review indicates that combining tDCS with behavioural speech and language therapy can enhance picture naming ability in individuals with chronic anomia (>6 months post-stroke) (Sandars, Cloutman, & Woollams, Citation2016). Specifically, applying excitatory anodal stimulation to the left frontal lobe has been shown to significantly increase noun naming accuracy relative to sham treatment. For example, Baker, Rorden, and Fridriksson (Citation2010) administered 1 mA anodal stimulation to left frontal areas of 10 patients (four with Broca’s aphasia) for 20 min per day for 5 consecutive days at the same time as carrying out a computerised therapy task. For each participant, tDCS was applied directly over the region in which activation had shown the greatest association with correct oral picture naming during pre-therapy functional scans. Active stimulation significantly increased patients’ naming accuracy of treated nouns, both immediately and 1 week following stimulation. The naming of untreated items also increased numerically following anodal stimulation at both time points, although this trend failed to reach statistical significance. Closer inspection of Baker et al.’s (Citation2010) results reveals that four participants (two non-fluent, two fluent) responded more favourably to left anodal stimulation than the remaining six. All four of these participants had damage to the left frontal cortex, meaning that stimulation was applied very close to their lesion sites. Baker et al.’s results are supported by Vestito and colleagues (Citation2014), who paired noun and verb naming therapy with 20 min of 1.5 mA anodal tDCS for 5 days per week for 2 weeks. Stimulation targeted the crossing point between points T3 and F7 on the international 10–20 electrode positioning system; a site that was again close to damaged areas in the frontal lobes of each participant. Compared to sham, active stimulation resulted in significantly greater increases in the number of items correctly named from baseline for all three participants. This effect was maintained for 16 weeks following intervention. Finally, Meinzer and colleagues (Citation2016) combined an intensive language treatment protocol (2 ˟ 1.5 h sessions per day ˟ 4 days per week ˟ 2 weeks) with 1 mA anodal tDCS applied to the left motor cortex (M1) for the first 20 min of each therapy session. This particular frontal lobe stimulation site was selected due to identified functional links between the language production network and primary motor system (e.g., Pulvermüller & Fadiga, Citation2010), and because the majority of this region was structurally intact in all of their participants. In a between-participants design, 13 patients (3 with Broca’s aphasia) received active stimulation, whilst the remaining 13 (6 with Broca’s aphasia) received sham stimulation. Results showed significant increases in treated and untreated noun naming accuracy for both groups of participants. However, gains were greater, and more likely to be maintained at the 6-month follow-up, for those who had received active rather than sham stimulation.
Baker et al.’s (Citation2010) and Vestito et al.’s (Citation2014) findings are consistent with neuroimaging studies indicating that better language recovery in the chronic stage is associated with increased activation in key left hemisphere language regions, such as the inferior frontal gyrus (IFG), which encompasses what is classically known as Broca’s area (Saur et al., Citation2005; Szaflarski, Allendorfer, Banks, Vannest, & Holland, Citation2013). When language areas are irrevocably damaged, compensatory activation in undamaged regions immediately surrounding the lesioned tissue (“perilesional” areas), has been consistently linked to language improvements in stroke survivors with chronic aphasia (Turkeltaub, Messing, Norise, & Hamilton, Citation2011). For instance, regions perilesional to Broca’s area, including BA32 (anterior cingulate gyrus) and BAs 10 and 11/47 (medial and middle frontal gyrus) have been shown to be more active in patients with better language recovery than in both those with poorer language outcomes and control participants (Fridriksson, Bonilha, Baker, Moser, & Rorden, Citation2010; Fridriksson, Richardson, Fillmore, & Cai, Citation2012). Similarly, facilitating activation in proximal, functionally connected frontal regions (M1) may assist linguistic recovery in individuals with left language network lesions (Meinzer et al., Citation2016).
fMRI studies have also revealed that activation in right hemisphere regions (including the right IFG and right superior temporal gyrus) in stroke survivors with chronic aphasia is higher than in healthy controls when completing a range of language tasks (Naeser et al., Citation2004; Perani et al., Citation2003), and that such activation is associated with semantic errors and omissions during picture naming (Postman-Caucheteux et al., Citation2010). Although controversial, one theory proposes that transcallosal disinhibition can arise following damage to the left hemisphere (Geranmayeh, Brownsett, & Wise, Citation2014; Karbe et al., Citation1998; Turkeltaub et al., Citation2012). According to this theory, a reduction in typical inhibition from the damaged left hemisphere during language tasks allows homologous areas in the right hemisphere to become overactive. These right hemisphere regions may, in turn, further suppress activation in existing left hemisphere regions (Martin et al., Citation2009). Hyperactivation in the right hemisphere may consequently prevent recruitment of perilesional areas in the left hemisphere, thus hindering recovery from aphasia (Hamilton, Chrysikou, & Coslett, Citation2011).
In line with the notion of transcallosal disinhibition, using cathodal tDCS to inhibit dysfunctional activation in the right hemisphere of individuals with chronic anomia may indirectly facilitate left lateralisation and enhance picture naming ability. Kang, Kim, Sohn, Cohen, and Paik (Citation2011) showed that administering 20 min of 2 mA cathodal stimulation to the right Broca’s homologue each weekday for a fortnight alongside tailored noun retrieval therapy resulted in a trend towards increased naming accuracy in a group of 10 patients with chronic anomia. More recently, Rosso et al. (Citation2014) reported significant increases in picture naming accuracy following a single 15 min session of 1 mA cathodal tDCS to the right IFG, despite the absence of a concurrent therapy task. However, this effect held true only for patients whose lesions incorporated Broca’s area. One possible explanation for this finding is that, in reducing right IFG activation, stimulation facilitated the functionally beneficial recruitment of perilesional left IFG regions in these individuals.
The majority of existing studies examining the effects of tDCS on picture naming ability in stroke survivors with chronic anomia have incorporated only one active electrode montage (Sandars et al., Citation2016). This means that it is impossible to compare the relative effects of, say, applying anodal stimulation to the left IFG with cathodal stimulation to the right IFG within the same individuals. A notable exception is a study carried by Shah-Basak and colleagues (Citation2015), in which 12 chronic non-fluent patients were given single, 20-min sessions of 2 mA tDCS in 4 active conditions and 1 sham condition. Using the 10–20 EEG positioning system, electrodes were applied to site F3 in the left frontal lobe (located superior to the IFG and anticipated to be perilesional for all participants) and its right homologue, F4. In the four active conditions, participants received anodal and cathodal stimulation to F3 and anodal and cathodal stimulation to F4, whilst attempting to name 20 item pictures. Naming ability was assessed immediately before and after stimulation session to determine which electrode montage led to the greatest improvements in naming accuracy prior to participants progressing to the second stage of the project. Results showed that, at group level, cathodal stimulation to left frontal areas was associated with the greatest improvements in naming accuracy.
On first glance, these findings appear at odds with those reported previously (Baker et al., Citation2010; Kang et al., Citation2011; Meinzer et al., Citation2016; Rosso et al., Citation2014; Vestito et al., Citation2014). However, there are several potential explanations for these discrepant results. Firstly, participants did not complete a therapy task at the same time as receiving each single stimulation session during the first stage of Shah-Basak et al.’s (Citation2015) study. Secondly, the hierarchical model proposes that patients with very extensive left hemisphere lesions that make left lateralisation impossible may necessarily rely on homologous regions within the right hemisphere in order to regain any language function, although this represents a less effective strategy than recruitment of left perilesional areas (Heiss & Thiel, Citation2006). Indeed, although left cathodal stimulation was most effective overall in enhancing naming ability in Shah-Basak et al.’s (Citation2015) study, individual level analysis indicates that the participants who benefitted the most from left cathodal stimulation were those whose lesions extended superiorly and medially into the left parietal and temporal lobes. In comparison, anodal stimulation applied to left frontal regions tended to be most effective for participants with smaller lesions confined to the left IFG and immediately surrounding tissue, in line with the work of Baker et al. (Citation2010) and Vestito et al. (Citation2014).
In summary, for stroke survivors with lesions localised to the left frontal lobe, combining behavioural speech and language therapy with anodal stimulation targeting perilesional tissue and/or cathodal stimulation targeting the contralesional right homologue currently appears to be most likely to result in the greatest improvements in picture naming accuracy. There exist, however, a number of limitations in the existing literature on this issue (Sandars et al., Citation2016). First and foremost, the relative effects of combining multiple different electrode montages with therapy have yet to be explored using a within-participants design. Secondly, although a growing body of evidence continues to highlight the importance of facilitating activation in perilesional regions, few studies have used detailed brain imaging techniques to identify such regions on a patient-by-patient basis. Thirdly, the majority of previous researchers have not investigated the potential for tDCS to improve generalisation of therapeutic effects from treated to untreated items. This is an important issue because treatment can only ever target a small proportion of the words that individuals have difficulty in retrieving, and such generalisation has been documented by others following behavioural anomia therapy (Best et al., Citation2013). Finally, it is important that statistically significant increases in picture naming accuracy translate into meaningful improvements in patients’ everyday communicative abilities (Best et al., Citation2011; Herbert, Hickin, Howard, Osborne, & Best, Citation2008). Significant positive effects of bilateral tDCS (simultaneous left frontal anodal and right frontal cathodal stimulation) on psychosocial and mood measures have been reported (Manenti et al., Citation2013), and Meinzer et al. (Citation2016) noted improvements in partner-rated perceptions of communicative effectiveness following anodal tDCS applied to the perilesional primary motor cortex. However, studies have yet to determine the effects of unilateral tDCS plus therapy on a broad range of measures designed to capture changes in functional communication, connected speech, and well-being.
To address these outstanding issues, we designed a comprehensive, long-term intervention programme for stroke survivors with chronic anomia. This involved six, 4 week-long cycles of noun picture naming therapy, each paired with a different tDCS electrode montage (perilesional anodal, perilesional cathodal, perilesional sham, contralesional anodal, contralesional cathodal, and contralesional sham) with stimulation sites for each individual determined on the basis of high resolution structural MRI scans. Here, we report the results for a patient with chronic non-fluent aphasia associated with a relatively circumscribed left IFG lesion. The primary aim of the current study was to investigate which tDCS parameters would result in the greatest improvements in naming ability for this individual, by systematically manipulating laterality and polarity. We hypothesised that combining computerised anomia therapy with anodal stimulation applied to perilesional regions in the left IFG, and/or with cathodal stimulation applied to homologous regions in the right IFG, would be significantly more effective than combining therapy with anodal stimulation to the contralesional hemisphere, cathodal stimulation to the perilesional left hemisphere, or sham stimulation. Our design allowed us to consider the extent to which gains for each therapy cycle generalised to untreated items. A range of secondary outcome measures were also included to explore the impact of the intervention programme on JSc’s emotional well-being, his connected speech elicited from a picture description task, and both self- and carer-reported perceptions of his communicative effectiveness.
Method
Participant
JSc was an 81-year-old right-handed retired engineer with 12 years of formal education. He had a left middle cerebral artery infarction in November 2005, almost 9 years prior to recruitment to the current study. He lived with his wife and enjoyed completing sudoku and jigsaw puzzles, plus watching car restoration programmes on television. Socially, he and his wife were active members of a local stroke support group and, together, they enjoyed regular day trips by coach and longer breaks to visit their children and extended family. He was able to walk independently and drive short distances. JSc had no history of epilepsy and was not taking any medications known to affect the central nervous system, although he had long-standing mild tinnitus. He presented with frequent word-finding difficulties, telegrammatic speech, and mild oral apraxia, with good comprehension of simple everyday conversation.
JSc completed a comprehensive battery of background language and neuropsychological tests. These included a number of subtests from the PALPA (Kay, Lesser, & Coltheart, Citation1992), 64-item Cambridge Semantic Battery (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, Citation2000) and Comprehensive Aphasia Test (CAT, Swinburn, Porter, & Howard, Citation2005), plus the short form of the Boston Diagnostic Aphasia Examination (Goodglass, Kaplan, & Barresi, Citation2001), the latter of which resulted in a diagnostic classification of Broca’s aphasia. The results of these assessments are shown in . Formal testing indicated that JSc had significant difficulties with phonological input and output processing and performed poorly on tests of non-word auditory discrimination (PALPA 1), immediate non-word repetition (PALPA 8), and oral confrontation naming. His anomia was moderate-severe and he made frequent phonological and omission errors, and occasional semantic substitution errors, on both the 64-item naming test and Boston Naming Test (BNT, Kaplan, Goodglass, & Weintraub, Citation2001). Although JSc also had some receptive syntactic difficulties at sentence level, his single word comprehension was within normal limits, as evidenced by his performance on the spoken and written word-to-picture matching tasks. Other strengths included forward and backward digit span and performance on Raven’s Coloured Progressive Matrices (Raven, Citation1962), a test of non-verbal reasoning.
Table 1. JSc’s percentage scores on the battery of language and neuropsychological tests. Scores in bold indicate performance outside the normal range.
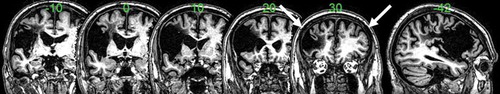
A high-resolution structural T1-weighted MRI scan was acquired on a 3.0 T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) using an 8-element SENSE head coil (). A T1-weighted inversion recovery sequence with 3D acquisition was employed with the following parameters: TR (repetition time) = 9.0 ms, TE (echo time) = 3.93 ms, flip angle = 8°, 150 contiguous slices, slice thickness = 1 mm, acquired voxel size 1.0 ˟ 1.0 ˟ 1.0 ˟ 1.0 ˟ 1.0 mm3, matrix size 256 ˟ 256, FOV = 256 mm, T1 (inversion time) = 1150 ms, SENSE acceleration factor 2.5, total scan acquisition time = 575 s.
As shown in , JSc’s lesion (volume = 147.67 voxels) involved both inferior and medial areas of the left frontal cortex (including Broca’s area), and the left insula.
Procedure
The design of the current study is illustrated in .
Naming assessment
Prior to commencing therapy, JSc completed a detailed naming assessment in his home. The stimuli were 408 black and white images taken from the International Picture Naming Project (Szekely et al., Citation2004), randomly divided into 8 blocks of 51 items matched on length in phonemes, number of syllables, frequency, and age of acquisition. The items were presented on a laptop computer using E-Prime (Psychology Software Tools Inc., Sharpsberg, Philadelphia), with the initial presentation of each image accompanied by a discreet beep sound to facilitate later measurement of naming speed. JSc was asked to try to produce the name of each item. No cues were provided, although general encouragement was given. Each image was shown for up to 10 s, after which time images automatically timed out if they had not yet been named correctly. JSc completed blocks 1–8 in order in the first assessment session and in the reverse order (i.e., from 8–1) in the second session. Both sessions were recorded using an Olympus VN-713PC digital voice recorder, placed to the side of the laptop computer. JSc’s first naming attempts were graded as correct or incorrect. Other verbalisations, including filler words/phrases (e.g., “er”, “come on, think”), were ignored. From a total of 405 items that were presented twice (three items were inadvertently skipped), JSc named 116 incorrectly on both occasions, 162 correctly on both occasions, and 127 items incorrectly on one occasion.
A total of 18, personalised, 20-item sets were created using JSc’s responses across both naming assessment sessions. All 116 items named incorrectly twice and 124 randomly selected items named incorrectly once were randomised and used to create 12, 20-item therapy sets (six treated, six untreated). Six sets contained 10 double incorrect and 10 single incorrect items, 1 set contained 11 double incorrect and 9 single incorrect items, and the remaining 5 sets contained 9 double incorrect and 11 single incorrect items. From the 162 items named correctly twice, 120 were randomly selected to create six, 20-item correct control sets.
All 12 treated and untreated therapy sets were matched, as far as possible, on a number of psycholinguistic variables (see Appendix A in Supplemental data). The values for each of these variables were taken from the IPNP. There were no significant differences between the 12 sets with regards to length in phonemes (F(11,228) = 0.173, p = 0.999), number of syllables (F(11,228) = 0.227, p = 0.996), frequency (F(11,228) = 0.718, p = 0.721), or name agreement (F(11,228) = 1.470, p = 0.144), nor were there any significant differences between the items named incorrectly once and those named incorrectly twice within the 12 sets in terms of length in phonemes (F(1,238) = 2.524, p = 0.113), number of syllables, (F(1,238) = 3.573, p = 0.060), and frequency (F(1,238) = 2.281, p = 0.132), although the difference between the single and double incorrect items in terms of name agreement was significant (F(1,238) = 10.189, p < 0.01). The single incorrect items had higher name agreement compared to the double incorrect items.
All six correct control sets were matched to each other on the same psycholinguistic variables (see Appendix B in Supplemental data). There were no significant differences between the control sets in terms of length in phonemes (F(5,114) = 0.090, p = 0.994), number of syllables (F(5,114) = 0.231, p = 0.948), frequency (F(5,114) = 0.452, p = 0.811), or name agreement (F(5,114) = 0.083, p = 0.995). There were significant differences between the six correct control sets and the 12 incorrect therapy sets with respect to length in phonemes (F(1,358) = 6.760, p < 0.05), number of syllables (F(1,358) = 5.245, p < 0.05), and frequency (F(1,358) = 4.492, p < 0.05). The items that JSc named correctly across both naming assessment sessions had fewer phonemes and syllables and were more frequent than the items that he named incorrectly during at least one naming assessment session.
The 12 incorrect sets were randomly allocated to be treated or untreated, and all sets were randomly allocated to the 6 therapy cycles. Each therapy cycle included one treated, one untreated, and one correct control set.
Computerised naming therapy
All therapy sessions were carried out in a designated treatment room in a large, general hospital in the North West of England. Microsoft Powerpoint slides were created for the 20 treated items to be included in each therapy cycle. JSc was shown these on a laptop computer. The slides included a colour Google image of each item (i.e., not the line drawings used in the assessments) and an audio video clip of a woman’s mouth saying the name of the item, which were presented side by side in the centre of the slide. All images depicted typical examples of single items, with no visible brand names or other text. There was an automatic 2-s delay after the slide appeared to allow JSc time to process the item image before the audio video clip began to play. After the audio video clip had finished playing, JSc was asked to try to repeat back the item name. One he had attempted to name the item, the next slide was revealed. Repetition targets the phonological processes of word production and has been shown to be an effective method of improving single word naming in individuals with aphasia (Conroy, Sage, & Lambon Ralph, Citation2009). Furthermore, previous studies have shown that the same language regions in the left frontal lobe are important for both speech production and perception (Fridriksson et al., Citation2008), and consequently, greater therapeutic gains may be achieved in individuals with word finding difficulties, as well as apraxia of speech, when pictures are accompanied by audio visual rather than audio only speech (Fridriksson et al., Citation2009; Whiteside et al., Citation2012).
JSc received computerised therapy three times per week for 20 min during the first week of each therapy cycle (i.e., a total of 60 min of treatment per cycle). Each item was repeated 10 times per therapy session. Previous studies have included more intensive protocols (e.g., five sessions, 100-min stimulation at 1 mA (Baker et al., Citation2010); five sessions, 100-min stimulation at 2 mA (Kang et al., Citation2011); 16 sessions, 320-min stimulation at 1 mA (Meinzer et al., Citation2016); 10 sessions, 200-min stimulation at 1.5 mA (Vestito et al., Citation2014)). We chose to use three sessions per cycle not only because it reduced JSc’s participant burden and facilitated the fully within-participant design, but also to investigate the potential for gains to be achieved following less intensive intervention.
Transcranial direct current stimulation
tDCS was applied whilst JSc received computerised naming therapy. JSc completed two, 4-week therapy runs, spread over approximately 8 months. Each run comprised three therapy cycles involving anodal, cathodal, or sham stimulation. The first run targeted perilesional site F5 (as per the international 10–20 electrode positioning system) in the left hemisphere and the second run targeted its right homologue, FC6 (Kindler et al., Citation2012; Marangolo et al., Citation2013). JSc completed the six therapy cycles in the following order: perilesional anodal, perilesional cathodal, perilesional sham, contralesional anodal, contralesional cathodal, and contralesional sham stimulation. Ideally, each cycle began the week immediately following week 4 of the preceding cycle (as per ), In practice, some adjustments were required in order to accommodate Christmas/New Year and JSc’s other commitments. The exact intervals were perilesional anodal /25 days /perilesional cathodal /28 days /perilesional sham /39 days /contralesional anodal /26 days /contralesional cathodal /26 days /contralesional sham. Hence there was a minimum 25-day wash out period between the offset of one type of stimulation and the onset of another.
In each therapy session, 1 mA tDCS was delivered for 20 min by a NeuroConn DC Stimulator Plus device via two saline-soaked electrodes (5 ˟ 7 cm). The active electrode was placed on the chosen location on the scalp and the second (reference) electrode was placed on the contralateral shoulder, in order to minimise the likelihood of inadvertently inducing simultaneous electrical currents in the contralateral hemisphere (Datta, Baker, Bikson, & Fridriksson, Citation2011). During sham sessions, the stimulation was turned on for 1-min to invoke the initial tingling sensation of tDCS before being gradually ramped down to nil over a further 30 s. All tDCS plus therapy sessions were carried out by the lead author. Although she was not blinded to the type of stimulation administered, the same protocol was strictly followed during every session. This included placing the tDCS display screen out of JSc’s sight in order to further minimise the risk of him distinguishing between stimulation conditions. While side of stimulation would necessarily have been apparent to JSc, the polarity of stimulation in any given session would not. In addition, previous studies (e.g., Flöel et al., Citation2008) suggest that the use of 1 mA stimulation should have rendered sham and active stimulation indistinguishable to JSc.
Outcome measures
Naming
The primary outcome measure was naming accuracy. This was measured before the start of the first therapy session in each cycle in order to re-establish baseline accuracy for all of the treated, untreated and correct control items within that cycle. Naming ability was assessed again immediately after the third therapy session, at 1 week post-therapy and at 3 weeks post-therapy. On each occasion, JSc was presented with black and white line drawings (the same images used in the initial naming assessment) of all 60 items used in the current therapy cycle on a laptop screen. As in the initial naming assessment sessions, JSc was asked to try to produce the name of each item without any cues. Items were shown for up to 10 s, after which time images automatically timed out if they had not yet been named correctly. Previous studies have indicated that anodal tDCS plus naming therapy may increase noun naming speed of individuals with fluent aphasia (Fridriksson, Richardson, Baker, & Rorden, Citation2011), although similar effects have not been reported following frontal stimulation in individuals with non-fluent aphasia (Fiori et al., Citation2011; Kang et al., Citation2011; Monti et al., Citation2008; Volpato et al., Citation2013). To investigate any effects of treatment on JSc’s naming speed, the time he took to correctly name the correct control items was measured at the same four time points. The time from initial item presentation (signified on the recording by the accompanying beep) to the onset of the first naming attempt was calculated manually for each item, in milliseconds, using Audacity 2.0.0 (available at http://audacity.sourceforge.net/).
Secondary outcome measures
A range of secondary outcome measures were collected prior to the first therapy session in each cycle, at 1 week post-therapy and at 3 weeks post-therapy. In order to assess the extent of generalisation of therapy to connected speech, JSc completed a picture description task (“Cookie theft”, Goodglass et al., Citation2001). His verbal responses on each occasion were transcribed and timed, and the number of silent pauses (of at least one second duration) per response recorded. The following measures were also calculated: (1) total number of words or “tokens” per sample, which indicated quantity of speech output, (2) mean length of utterance (MLU) in morphemes, which indicated grammatical complexity, and (3) type/token ratio (TTR, calculated by dividing the number of unique words per sample by the total number of tokens), which indicated lexical diversity (as per Borovsky, Saygin, Bates, & Dronkers, Citation2007). A bespoke 10-item mood questionnaire was adapted from a subscale of the Communication Disability Profile (Swinburn & Byng, Citation2006) (see Appendix C in Supplemental data). Each item asked JSc to consider how he had felt over the last week, and was scored from 0–4, with 4 representing the most positive emotional states (e.g., not at all angry, frequently able to do things). His response selection was supported using a visual analogue scale of face drawings depicting different emotions, taken directly from the Communication Disability Profile. Total scores at each administration were converted to percentages, with higher percentages indicating better outcomes. To examine any effects of therapy on JSc’s perceptions of his functional communication and quality of life, he completed the validated 20-item Communication Outcome After Stroke (COAST) scale (Long, Hesketh, Paszek, Booth, & Bowen, Citation2008). JSc’s wife completed the 20-item validated Carer version of the COAST (Long, Hesketh, & Bowen, Citation2009) to gain an additional perspective regarding his communication skills. Fifteen items on the Carer COAST ask carers to rate their partner’s functional communicative abilities, whilst the remaining five ask the carer to indicate the extent to which their own quality of life is affected by their partner’s communication difficulties. The total scores on both the COAST and Carer COAST were converted to percentages, with higher percentages indicating better outcomes.
Results
JSc completed all six therapy cycles and reported no adverse effects (such as scalp reddening, tingling, or headaches) during or after any of the therapy plus tDCS sessions. When debriefed following his participation in the study, JSc confirmed that he had not perceived any differences between any of the six stimulation conditions, and could not tell the difference between active and sham conditions, although he felt that all of the cycles involving stimulation to the right hemisphere had been more beneficial and more comfortable.
Naming accuracy
Treated items
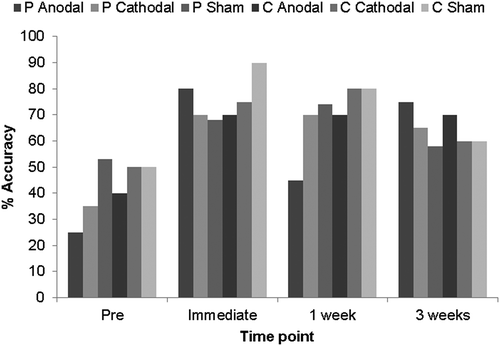
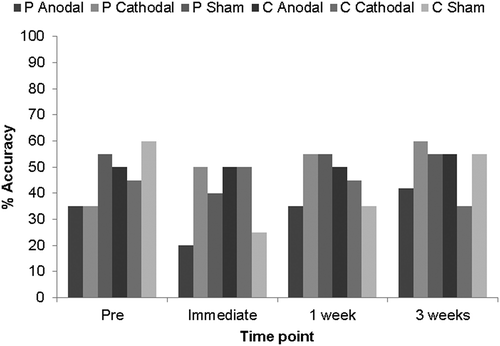
As shown in , naming accuracy for the treated items increased numerically from baseline in all stimulation conditions, indicating a strong overall beneficial effect of this brief, targeted therapeutic intervention. McNemar tests were used to determine the statistical significance of changes from baseline within the six stimulation conditions at each time point. Perilesional anodal stimulation resulted in the greatest increases immediately post-therapy (55%) and 3 weeks post-therapy (50%). These increases were significant at both time points (immediate χ2 = 6.67, p = 0.007; 3 weeks χ2 = 6.75, p = 0.006). Increases in naming accuracy were also significant in the perilesional cathodal condition at all three time points (immediate χ2 = 5.143, p = 0.016; 1 week χ2 = 7.11, p = 0.004; 3 weeks χ2 = 6.13, p = 0.008), and in the contralesional sham condition immediately post-therapy (χ2 = 4.90, p = 0.021). A breakdown of error types to treated items for each condition at each time point is provided in Appendix D of the Supplemental data.
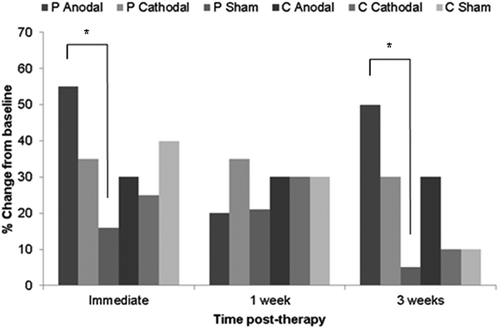
To assess the impact of stimulation, the change from baseline in the number of items correctly named after therapy for each perilesional and contralesional active stimulation condition was compared to their respective sham conditions at each time point using chi-square tests. As shown in , the effect of perilesional anodal stimulation was significantly greater than that for perilesional sham, both immediately post-therapy (χ2 = 4.57, p = 0.032), and 3 weeks later (χ2 = 6.99, p = 0.008). The effect of perilesional cathodal stimulation was not significantly greater than that for perilesional sham, at any time point (χ2 = 3.01, p = 0.08).
Untreated items
shows the percentage naming accuracy for untreated items across all conditions at all time points. Naming accuracy increased numerically at all time points following perilesional cathodal stimulation, immediately post-therapy following contralesional cathodal stimulation and at 3 weeks post-therapy following perilesional anodal and contralesional anodal stimulation. In the remaining conditions/at the remaining time points, naming accuracy remained the same or decreased following therapy. McNemar tests were used to determine the significance of any changes in accuracy from baseline after intervention. None of the post-therapy increases or decreases in naming accuracy for the untreated items were significant.
Speed of naming
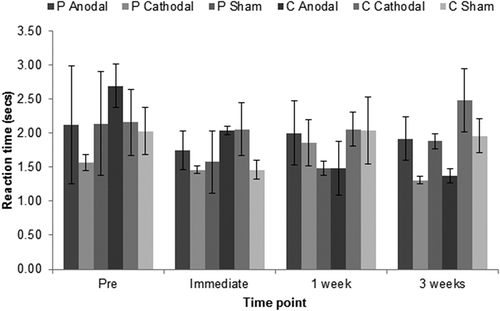
For the 20 double correct control items in each therapy cycle, the mean time JSc took to name items correctly at baseline, immediately post-therapy, 1 week post-therapy, and 3 weeks post-therapy are shown in . Wilcoxon Signed Ranks tests showed that there were no significant changes from baseline in the length of time taken to correctly name the control items following therapy in any of the six conditions, at any of the follow-up points.
Secondary outcome measures
Picture description task
The total response length (in seconds), number of pauses, total number of tokens, total number of morphemes, MLU in morphemes, and TTR (expressed as a percentage) were calculated for all the responses JSc gave when asked to describe the “Cookie theft” image before therapy, 1 week post-therapy, and 3 weeks post-therapy in each of the 6 stimulation conditions. These values are shown in .
Table 2. Total response length (s), number of pauses, number of tokens, number of morphemes, MLU, and TTR for the picture description task.
indicates that JSc’s scores on all 6 measures were highly variable across the 18 assessment sessions. Within each of the stimulation conditions, there were no consistent patterns of improvement or reduction in performance on any of the measures from baseline to 1 week or 3 weeks post-therapy. However, between the conditions, three trends emerged over time from the first cycle (perilesional anodal) to the last (contralesional sham), namely, the total length of JSc’s responses and the number of pauses he made tended to decrease over time, whilst his MLU increased, consistent with a cumulative improvement over the course of repeated cycles of behavioural therapy. There were no obvious trends between stimulation conditions with regards to JSc’s total number of tokens, total number of morphemes, and TTR.
Mood questionnaire, COAST, and carer COAST
shows the total percentage scores on the 10-item bespoke mood questionnaire, COAST, and Carer COAST before therapy, 1 week post-therapy and 3 weeks post-therapy, in each of the 6 stimulation conditions.
Table 3. Total percentage scores on the mood questionnaire, COAST, and Carer COAST.
JSc’s scores on the mood questionnaire varied both within and between stimulation conditions (total range = 42.50–87.50%). Wilcoxon Signed Ranks tests showed that the decreases in percentage score from the start of the perilesional anodal therapy cycle to 1 week post-therapy (z = −2.13, p = 0.033) and 3 weeks post-therapy (z = −2.24, p = 0.025) were both significant, as were the decreases in percentage score from the start of the contralesional cathodal therapy cycle to 1 week post-therapy (z = −2.45, p = 0.014) and 3 weeks post-therapy (z = −2.43, p = 0.015). The pre-therapy percentage scores on the mood questionnaire were, however, higher in the perilesional anodal and contralesional cathodal stimulation conditions than in the other four cycles. For the contralesional anodal condition, scores on the mood questionnaire were significantly greater at 1 week post-therapy (z = 2.07, p = 0.038) and 3 weeks post-therapy (z = 2.46, p = 0.014).
As with the mood questionnaire, JSc’s total percentage scores on the COAST varied both within and between stimulation conditions (total range = 36.50–65.00%). Wilcoxon Signed ranks tests showed that the decrease in percentage score from the start of the perilesional anodal therapy cycle to 1 week post-therapy was significant (z = −2.97, p = 0.003), and persisted at the 3 week post-therapy mark (z = −2.07, p = 0.038), although the pre-therapy percentage score in this cycle was numerically higher than in all the other conditions other than the contralesional sham condition. In contrast, the increased scores from baseline to 1 week post-therapy in the perilesional sham condition (z = 2.33, p = 0.020), the contralesional cathodal condition (z = 2.65, p = 0.008) and the contralesional sham condition were also significant (z = 2.89, p = 0.004). Overall, there was a trend for JSc to score more highly on the COAST as he continued to participate in the study: his average percentage score across the three measurement points within the final stimulation condition (contralesional sham, 56.25%) was significantly greater than his average percentage score across the three measurement points within the first stimulation condition (perilesional anodal, 43.75%) (z = 3.76, p = 0.000).
JSc’s wife’s scores on the Carer COAST showed less variability within and between cycles (range = 55.00–73.75%) than his scores on the COAST. Wilcoxon Signed Ranks tests confirmed that there were no significant differences in baseline scores across the six stimulation conditions nor from baseline to either 1 week or 3 weeks post-therapy within any of the stimulation conditions, although in the perilesional anodal condition, the increase in scores from 1 week post-therapy to 3 weeks post-therapy was significant (z = 2.51, p = 0.012).
Discussion
The purpose of the current study was to systematically investigate the effects of varying the laterality and polarity of tDCS on the noun naming ability of an individual stroke survivor with chronic anomia in the context of Broca’s aphasia due to a left frontal lesion. On the basis of previous research, we anticipated that combining behavioural therapy with left anodal perilesional stimulation, and/or with right cathodal contralesional stimulation, would lead to the greatest therapeutic gains. This hypothesis was partially confirmed by our results. Pairing a computer-based repetition therapy task with anodal stimulation applied to perilesional areas in JSc’s left frontal lobe led to a significant increase in his immediate confrontation naming accuracy of treated items over and above the behavioural therapy gains following left sham stimulation. The significant benefit of perilesional anodal over perilesional sham stimulation was also evident at the final follow up, three weeks post-therapy. The finding that anodal stimulation to left frontal perilesional areas significantly increased noun naming accuracy relative to sham stimulation in this participant with Broca’s aphasia agrees with previous findings that have used a group study approach to explore left hemisphere anodal stimulation (Baker et al., Citation2010; Meinzer et al., Citation2016; Vestito et al., Citation2014), although it has been shown that tDCS can modulate larger functional networks beyond the site of stimulation (Peña-Gómez et al., Citation2012). Our results are in keeping with neuroimaging research highlighting the important role of left frontal perilesional areas in language recovery for patients in the chronic stage post-stroke, both spontaneously and following therapy (Fridriksson, Richardson et al., Citation2012; Marcotte et al., Citation2012; Meinzer et al., Citation2008). In contrast, cathodal stimulation applied to the right Broca’s homologue did not significantly enhance JSc’s naming accuracy, and therefore our results are not consistent with those reported by Kang et al. (Citation2011) and Rosso et al. (Citation2014), or more generally with the transcallosal disinhibition hypothesis (Geranmayeh et al., Citation2014; Karbe et al., Citation1998).
It is unclear why significant post-therapy improvements in naming accuracy were present immediately and 3 weeks following perilesional anodal stimulation but not evident at the interim 1 week follow-up. Anecdotally, JSc reported during the 1 week post-therapy follow-up session that he had been “getting more words all week”, but this perception was not reflected in his naming assessment scores. A potential explanation is that different neural mechanisms are responsible for immediate learning versus longer-term retention following tDCS plus therapy (Stagg & Nitsche, Citation2011). During stimulation, tDCS is thought to temporarily alter neuronal excitability via temporary changes in membrane polarity, whilst persisting effects are believed to be the result of lasting changes in synaptic strength via the process of long term potentiation (Nitsche et al., Citation2003). It is possible that a period of consolidation may be required to solidify immediate transient learning, during which time naming is unstable (Meinzer et al., Citation2016). Although this hypothesis would be difficult to confirm, repeating the same intervention protocol with additional individuals may help to clarify whether this unexpected finding is unique to JSc or common to the wider population of patients under similar stimulation conditions.
A limitation of the current study is the possibility that the significantly larger effects of perilesional anodal stimulation on naming ability are related in some way to the fact that JSc completed this condition first. Yet it is difficult to see how simple order and practice effects could have produced this result, for four related reasons. Firstly, different words were targeted in each cycle, and we found no significant gains for untreated items, hence there is we would not expect to see a cumulative benefit to performance across therapeutic cycles. Secondly, although baseline accuracy did tend to increase across conditions for treated items, this was not universal and none of these differences were significant (χ2s< 1.778, ps≥0.180). Thirdly, we assessed therapeutic gains relative to an immediate pre-intervention baseline, in order to control for any cumulative effect of therapy. Lastly, while it could be argued that increases in baseline might have led to smaller effects for later conditions, this would only be the case if performance for the treated items had reached ceiling, and as can be seen in , there were no ceiling effects in any condition. Indeed, although the largest immediate therapy effect was observed for the first perilesional anodal condition, significant effects were also present for the second perilesional cathodal condition and, importantly, the final contralesional sham condition. Nevertheless, it is still possible that JSc, who had not received any speech and language therapy for a number of years prior to his involvement in the current study, was particularly receptive to the first therapy cycle, with his response to subsequent cycles somewhat diminished due to factors such as motivation. It is also possible that the enhancement of therapy effects by neurostimulation is maximal on the first administration, irrespective of the particular montage used, in which case order rather than location of stimulation would be responsible for strength of the perilesional anodal benefits. Unfortunately, the risk of confounding order effects is unavoidable within single case research. Future research in which the order of the different stimulation conditions is counterbalanced across participants is needed to tease apart these alternative explanations for the strong effect of perilesional anodal stimulation observed in this case. Such research is required if we are to generalise the superiority of perilesional anodal stimulation to other cases of stroke aphasia.
With respect to the untreated items, our results show that naming accuracy increased numerically immediately following therapy in the perilesional cathodal and contralesional cathodal conditions, 1 week post-therapy in the perilesional cathodal condition and 3 weeks post-therapy in the perilesional anodal, perilesional cathodal, and contralesional anodal conditions, although these increases were not significant. It is possible that increases in the number of untreated items named correctly after therapy reflect some generalisation of treatment effects, in accordance with Best et al. (Citation2013). However, another possibility is that simply asking JSc to attempt to name the same items repeatedly provided retrieval practice and primed his subsequent naming attempts (Nickels, Citation2002a). If this kind of “repetition priming” had occurred, one would expect any gains in JSc’s naming accuracy to grow over time within each therapy cycle. This was indeed the case in the perilesional cathodal condition, but not in the other conditions. Future studies need to consider the impact of repetition priming when assessing therapeutic generalisation.
We also investigated the potential effects of tDCS plus therapy on JSc’s speed of correct naming for the double correct control items, but found no significant reductions in response time. The most likely reason for this is that these items were untreated; indeed, therapy plus anodal tDCS only resulted in significant decreases in naming speed of treated items for the fluent patients in Fridriksson et al.’s (Citation2011) study. Alternatively, speed of correct noun naming may be better facilitated for both fluent and non-fluent patients via stimulation to different cortical regions, such as Wernicke’s area in the temporal lobe (Fiori et al., Citation2011; Fridriksson et al., Citation2011). Adapting the design of the current study in the future to include treatment for double correct items could help to clarify whether or not tDCS plus naming therapy can increase naming speed as well as accuracy in individuals with chronic non-fluent aphasia.
An additional aim of the current study was to explore the effects of tDCS plus behavioural therapy for anomia on a range of secondary outcome measures designed to capture any changes in JSc’s connected speech output, mood, and perceptions of his communicative effectiveness. To date, only one study has examined the effects of unilateral tDCS on one such measure: partner-reported everyday communication skills (Meinzer et al., Citation2016). Consequently, the majority of our results cannot be directly compared with previous findings, but provide some interesting foundations for future investigation. With regards to the picture description task, there were no consistent patterns of improvement on any of the included measures within each of the stimulation conditions, indicating that the quantity, grammatical complexity, and lexical diversity of JSc’s elicited connected speech did not change as a result of any particular form of stimulation. This finding was disappointing but not entirely unexpected, given that generalisation from single words to connected speech is notoriously difficult to achieve, especially if the required vocabulary for detailed picture description is not directly targeted in therapy (e.g., Conroy et al., Citation2009). Over the course of JSc’s involvement in the study, however, the length of his utterances and the number of pauses he made tended to decrease, whilst his MLU increased. MLU is calculated by dividing the total number of morphemes by the total number of utterances, providing a measure of grammatical complexity (Borovsky et al., Citation2007). The total number of morphemes JSc produced each time he described the Cookie Theft picture was relatively stable. Consequently, in JSc’s case, increasing MLU over time reflects a reduction in the number of utterances (as a function of fewer pauses) rather than increasing grammatical complexity per se. Overall, his picture description became faster and less hesitant (i.e., more fluent) with repeated attempts. This finding is likely the result of accumulated retrieval practice for the same lexical items over the course of JSc’s involvement in the study, and is consistent with script training studies for people with non-fluent aphasia that have directly aimed to improve the production of particular narratives by providing multiple production opportunities (e.g., Lee, Kaye, & Cherney, Citation2009).
One may have predicted that JSc’s mood would increase following therapy as naming ability improved, or alternatively, that it would decrease due to frustration and heightened awareness of his confrontation naming impairment. In fact, JSc’s scores on the mood questionnaire varied significantly both within and between stimulation conditions, indicating that his emotional state fluctuated throughout the duration of his participation in the study, and there were no consistent pre- to post-therapy patterns. The stimulation conditions in which JSc’s emotion scores significantly decreased following therapy (perilesional anodal and contralesional cathodal) had the highest baseline scores. Consequently, as naming ability for treated items increased in the perilesional anodal condition, JSc’s emotional state actually worsened. Conversely, his mood scores significantly increased only in the contralesional anodal condition, which had the lowest baseline, thus allowing greater room for improvement. The most plausible explanation for these findings as a whole is that JSc’s mood simply differed from week to week as is the case for many individuals, for reasons unrelated to the present study.
JSc’s perceptions of his own communicative competence (as measured by the COAST) also fluctuated within and between stimulation conditions, with similar patterns of increases following the lowest baselines and vice versa to those seen for the mood questionnaire. However, there was a general tendency for his perceptions to become more positive over time throughout his participation in the study. It is possible that this trend reflects a cumulative effect of therapy. Alternatively, it may be related to JSc’s belief that the three later cycles involving right hemisphere stimulation had, as a whole, been more effective than the earlier ones targeting the left hemisphere, despite this perception not being borne out in the naming data. For the Carer COAST, there was some variability in JSc’s wife’s ratings over the first month of completing the questionnaire, which yielded a significant increase between the 1- and 3-week follow-up that did map on to a naming benefit for treated items. This finding is in line with Meinzer et al. (Citation2016), who found that partner-reported improvements in everyday communication were significantly greater following left anodal than sham stimulation.
Conclusions
Our results indicate that, for this particular individual with chronic non-fluent aphasia, combining anodal tDCS delivered to perilesional regions in the left frontal lobe with speech and language therapy was significantly more effective in increasing his naming accuracy than therapy alone. These observations not only confirm previous findings but also demonstrate that correct naming can be significantly increased and maintained for 3 weeks via a very limited amount of input, in a patient almost a decade post-stroke. Our motivation for supplementing computerised anomia therapy with tDCS was to increase the efficiency as well as the effectiveness of behavioural treatment alone. In the current study, JSc received a total of just 1 h of stimulation, whereas in previous studies involving left anodal frontal stimulation, similarly significant results were obtained following 100 min of stimulation across five session over a week (Baker et al., Citation2010), and after 200 min of stimulation across 10 sessions (Vestito et al., Citation2014) and 320 min of stimulation across 16 sessions (Meinzer et al., Citation2016) over a fortnight. The gain in naming treated items achieved in the current study compared to previous ones suggests that may be possible to decrease the therapeutic dosage without compromising effectiveness. Fewer sessions would not only reduce patient burden but also be more practically viable in clinical settings. We have also shown that it is feasible to complete a relatively long-term, multiple outcome measure tDCS plus behavioural therapy programme that systematically varies the laterality and polarity of stimulation with stroke survivors in the chronic stage, something which the majority of previous studies have not attempted. It is not possible to make any generalisations about the wider population of stroke survivors from the results obtained from one individual, especially given variability in the optimal stimulation montage seen across individuals (Shah-Basak et al., Citation2015). Nevertheless, benefits for left frontal anodal stimulation are consistently seen amongst those with damage in this area (Baker et al., Citation2010; Shah-Basak et al., Citation2015; Vestito et al., Citation2014), and it seems reasonable to expect that the anodal perilesional stimulation benefits we observed should also be found in other individuals with similar behavioural and lesion profiles to JSc. Our own future work will continue to try to determine the optimal tDCS parameters to enhance the language recovery, well-being, and quality of life of greater numbers of individuals with differing lesion and behavioural profiles. Establishing these parameters may facilitate the adoption of tDCS into mainstream clinical practice.
Supplementary_data.docx
Download MS Word (124 KB)Acknowledgements
This research was funded by a Stroke Association Junior Research Training Fellowship awarded to the first author (Award number TSA JRTF 2013/01).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental meterial
The supplemental data for this article can be accessed here.
Additional information
Funding
References
- Baker, J. M., Rorden, C., & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41, 1229–1236. doi:10.1161/strokeaha.109.576785
- Barthel, G., Meinzer, M., Djundja, D., & Rockstroh, B. (2008). Intensive language therapy in chronic aphasia: Which aspects contribute most? Aphasiology, 22, 408–421. doi:10.1080/02687030701415880
- Best, W., Grassly, J., Greenwood, A., Herbert, R., Hickin, J., & Howard, D. (2011). A controlled study of changes in conversation following aphasia therapy for anomia. Disability and Rehabilitation, 33, 229–242. doi:10.3109/09638288.2010.534230
- Best, W., Greenwood, A., Grassly, J., Herbert, R., Hickin, J., & Howard, D. (2013). Aphasia rehabilitation: Does generalisation from anomia therapy occur and is it predictable? A case series study. Cortex, 49, 2345–2357. doi:10.1016/j.cortex.2013.01.005
- Borovsky, A., Saygin, A. P., Bates, E., & Dronkers, N. (2007). Lesion correlates of conversational speech production deficits. Neuropsychologia, 45, 2525–2533. doi:10.1016/j.neuropsychologia.2007.03.023
- Bozeat, S., Lambon Ralph, M. A., Patterson, K., Garrard, P., & Hodges, J. R. (2000). Nonverbal semantic impairment in semantic dementia. Neuropsychologia, 38, 1207–1215. doi:10.1016/S0028-3932(00)00034-8
- Conroy, P., Sage, K., & Lambon Ralph, M. (2009). Improved vocabulary production after naming therapy in aphasia: Can gains in picture naming generalise to connected speech? TLCD, 44, 1036–1062. doi:10.3109/13682820802585975
- Datta, A., Baker, J. M., Bikson, M., & Fridriksson, J. (2011). Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation, 4, 169–174. doi:10.1016/j.brs.2010.11.001
- Fiori, V., Coccia, M., Marinelli, C. V., Vecchi, V., Bonifazi, S., Ceravolo, M. G., … Marangolo, P. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23, 2309–2323. doi:10.1162/jocn.2010.21579
- Flöel, A., Rösser, N., Michka, O., Knecht, S., & Breitenstein, C. (2008). Noninvasive brain stimulation improves language learning. Journal of Cognitive Neuroscience, 20, 1415–1422. doi:10.1162/jocn.2008.20098
- Fridriksson, J. (2011). Measuring and inducing brain plasticity in chronic aphasia. Journal of Communication Disorders. doi:10.1016/j.jcomdis.2011.04.009
- Fridriksson, J., Baker, J. M., Whiteside, J., Eoute, D., Moser, D., Vesselinov, R., & Rorden, C. (2009). Treating visual speech perception to improve speech production in nonfluent aphasia. Stroke, 40, 853–858. doi:10.1161/strokeaha.108.532499
- Fridriksson, J., Bonilha, L., Baker, J. M., Moser, D., & Rorden, C. (2010). Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebral Cortex, 20, 1013–1019. doi:10.1093/cercor/bhp160
- Fridriksson, J., Hubbard, H. I., Hudspeth, S. G., Holland, A. L., Bonilha, L., Fromm, D., & Rorden, C. (2012). Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain, 135, 3815–3829. doi:10.1093/brain/aws301
- Fridriksson, J., Moss, J., Davis, B., Baylis, G. C., Bonilha, L., & Rorden, C. (2008). Motor speech perception modulates the cortical language areas. Neuroimage, 41, 605–613. doi:10.1016/j.neuroimage.2008.02.046
- Fridriksson, J., Richardson, J. D., Baker, J. M., & Rorden, C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia: A double-blind, sham-controlled study. Stroke, 42, 819–821. doi:10.1161/strokeaha.110.600288
- Fridriksson, J., Richardson, J. D., Fillmore, P., & Cai, B. (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60, 854–863. doi:10.1016/j.neuroimage.2011.12.057
- Gandiga, P. C., Hummel, F. C., & Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology, 117, 845–850. doi:10.1016/j.clinph.2005.12.003
- Geranmayeh, F., Brownsett, S. L., & Wise, R. J. (2014). Task-induced brain activity in aphasic stroke patients: What is driving recovery? Brain, 137, 2632–2648. doi:10.1093/brain/awu163
- Goodglass, H., Kaplan, E., & Barresi, B. (2001). Boston diagnostic aphasia examination. Baltimore, MD: Lippincott Williams & Wilkins.
- Hamilton, R. H., Chrysikou, E. G., & Coslett, B. (2011). Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language, 118, 40–50. doi:10.1016/j.bandl.2011.02.005
- Heiss, W. D., & Thiel, A. (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain and Language, 98, 118–123. doi:10.1016/j.bandl.2006.02.002
- Herbert, R., Hickin, J., Howard, D., Osborne, F., & Best, W. (2008). Do picture‐naming tests provide a valid assessment of lexical retrieval in conversation in aphasia? Aphasiology, 22, 184–203. doi:10.1080/02687030701262613
- Hilari, K., Needle, J. J., & Harrison, K. L. (2012). What are the important factors in health-related quality of life for people with aphasia? A systematic review. Archives of Physical Medicine and Rehabilitation, 93, S86–S95.e84. doi:10.1016/j.apmr.2011.05.028
- Holland, R., & Crinion, J. (2012). Can tDCS enhance treatment of aphasia after stroke? Aphasiology, 26, 1169–1191. doi:10.1080/02687038.2011.616925
- Kang, E. K., Kim, Y. K., Sohn, H. M., Cohen, L. G., & Paik, N. (2011). Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Medicine, Clinical Neurology and Exercise & Occupational Therapy, 29, 141–152.
- Kaplan, E. F., Goodglass, H., & Weintraub, S. (2001). The Boston Naming Test (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
- Karbe, H., Thiel, A., Weber-Luxenburger, G., Herholz, K., Kessler, J., & Heiss, W. D. (1998). Brain plasticity in poststroke aphasia: What is the contribution of the right hemisphere? Brain Lang, 64, 215–230. doi:10.1006/brln.1998.1961
- Kay, J., Lesser, R., & Coltheart, M. (1992). PALPA: Psycholinguistic assessments of language processing in aphasia. Hove, East Sussex: Psychology Press.
- Kessler, S. K., Turkeltaub, P. E., Benson, J. G., & Hamilton, R. H. (2012). Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimulation, 5, 155–162. doi:10.1016/j.brs.2011.02.007
- Kindler, J., Schumacher, R., Cazzoli, D., Gutbrod, K., Koenig, M., Nyffeler, T., … Müri, R. M. (2012). Theta burst stimulation over the right broca's homologue induces improvement of naming in aphasic patients. Stroke, 43(8), 2175–2179. doi: 10.1161/STROKEAHA.111.647503
- Lambon Ralph, M. A., Snell, C., Fillingham, J. K., Conroy, P., & Sage, K. (2010). Predicting the outcome of anomia therapy for people with aphasia post CVA: Both language and cognitive status are key predictors. Neuropsychological Rehabilitation, 20, 289–305. doi:10.1080/09602010903237875
- Lee, J. B., Kaye, R. C., & Cherney, L. R. (2009). Conversational script performance in adults with non-fluent aphasia: Treatment intensity and aphasia severity. Aphasiology, 23(7-8), 885–897. doi: 10.1080/02687030802669534
- Long, A., Hesketh, A., & Bowen, A. (2009). Communication outcome after stroke: A new measure of the carer’s perspective. Clinical Rehabilitation, 23, 846–856. doi:10.1177/0269215509336055
- Long, A., Hesketh, A., Paszek, G., Booth, M., & Bowen, A. (2008). Development of a reliable self-report outcome measure for pragmatic trials of communication therapy following stroke: The Communication Outcome after Stroke (COAST) scale. Clinical Rehabilitation, 22, 1083–1094. doi:10.1177/0269215508090091
- Manenti, R., Petesi, M., Brambilla, M., Rosini, S., Miozzo, A., Padovani, A., … Cotelli, M. (2013). Efficacy of semantic–phonological treatment combined with tDCS for verb retrieval in a patient with aphasia. Neurocase, 21, 109–119. doi:10.1080/13554794.2013.873062
- Marangolo, P., Fiori, V., Calpagnano, M. A., Campana, S., Razzano, C., Caltagirone, C., & Marini, A. (2013). tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience, Article 539. doi:10.3389/fnhum.2013.00539
- Marcotte, K., Adrover-Roig, D., Damien, B., de Préaumont, M., Généreux, S., Hubert, M., & Ansaldo, A. I. (2012). Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia, 50, 1776–1786. doi:10.1016/j.neuropsychologia.2012.04.001
- Martin, P. I., Naeser, M. A., Ho, M., Doron, K. W., Kurland, J., Kaplan, J., … Pascual-Leone, A. (2009). Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain and Language, 111, 20–35. doi:10.1016/j.bandl.2009.07.007
- Meinzer, M., Darkow, R., Lindenberg, R., & Flöel, A. (2016). Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain, 139, 1152–1163. doi:10.1093/brain/aww002
- Meinzer, M., Flaisch, T., Breitenstein, C., Wienbruch, C., Elbert, T., & Rockstroh, B. (2008). Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage, 39, 2038–2046. doi:10.1016/j.neuroimage.2007.10.008
- Monti, A., Cogiamanian, F., Marceglia, S., Ferrucci, R., Mameli, F., Mrakic-Sposta, S., … Priori, A. (2008). Improved naming after transcranial direct current stimulation in aphasia. Journal of Neurology, Neurosurgery & Psychiatry, 79, 451–453. doi:10.1136/jnnp.2007.135277
- Naeser, M. A., Martin, P. I., Baker, E. H., Hodge, S. M., Sczerzenie, S. E., Nicholas, M., … Yurgelun-Todd, D. (2004). Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. NeuroImage, 22, 29–41. doi:10.1016/j.neuroimage.2003.11.016
- Nickels, L. (2002a). Improving word finding: Practice makes (closer to) perfect? Aphasiology, 16, 1047–1060. doi:10.1080/02687040143000618
- Nickels, L. (2002b). Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology, 16, 935–979. doi:10.1080/02687030244000563
- Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., … Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1, 206–223. doi:10.1016/j.brs.2008.06.004
- Nitsche, M. A., Fricke, K., Henschke, U., Schlitterlau, A., Liebetanz, D., Lang, N., … Paulus, W. (2003). Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. The Journal of Physiology, 553, 293–301. doi:10.1113/jphysiol.2003.049916
- Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527, 633–639. doi:10.1111/j.1469-7793.2000.t01-1-00633.x
- Pedersen, P., Vinter, K., & Skyhoj Olsen, T. (2004). Aphasia after stroke: Type, severity and prognosis. Cerebrovascular Disease, 17, 35–43. doi:10.1159/000073896
- Peña-Gómez, C., Sala-Lonch, R., Junqué, C., Clemente, I. C., Vidal, D., Bargalló, N., … Bartrés-Faz, D. (2012). Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimulation, 5, 252–263. doi:10.1016/j.brs.2011.08.006
- Perani, D., Cappa, S. F., Tettamanti, M., Rosa, M., Scifo, P., Miozzo, A., … Fazio, F. (2003). A fMRI study of word retrieval in aphasia. Brain and Language, 85, 357–368. doi:10.1016/s0093-934x(02)00561-8
- Poreisz, C., Boros, K., Antal, A., & Paulus, W. (2007). Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bulletin, 72, 208–214. doi:10.1016/j.brainresbull.2007.01.004
- Postman-Caucheteux, W. A., Birn, R. M., Pursley, R. H., Butman, J. A., Solomon, J. M., Picchioni, D., … Braun, A. R. (2010). Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience, 22, 1299–1318. doi:10.1162/jocn.2009.21261
- Pulvermüller, F., & Fadiga, L. (2010). Active perception: Sensorimotor circuits as a cortical basis for language. Nature Reviews Neuroscience, 11, 351–360. doi:10.1038/nrn2811
- Raven, J. C. (1962). Coloured progressive matrices. Sets A, AB & B. London: Lewis.
- Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120, 2008–2039. doi:10.1016/j.clinph.2009.08.016
- Rosso, C., Perlbarg, V., Valabregue, R., Arbizu, C., Ferrieux, S., Alshawan, B., … Samson, Y. (2014). Broca’s area damage is necessary but not sufficient to induce after-effects of cathodal tDCS on the unaffected hemisphere in post-stroke aphasia. Brain Stimulation, 7, 627–635. doi:10.1016/j.brs.2014.06.004
- Sandars, M., Cloutman, L., & Woollams, A. M. (2016). Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural Plasticity, 2016, 21. doi:10.1155/2016/8428256
- Saur, D., Baumgaertner, A., Lange, R., Schraknepper, V., Rijntjes, M., & Weiller, C. (2005). Dynamics of reorganisation in the language system after stroke: An fMRI-follow-up study from the acute to the chronic phase. Brain and Language, 95, 8–9. doi:10.1016/j.bandl.2005.07.006
- Shah-Basak, P. P., Norise, C., Garcia, G., Torres, J., Faseyitan, O., & Hamilton, R. H. (2015). Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Frontiers in Human Neuroscience, 9, 201. doi:10.3389/fnhum.2015.00201
- Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17, 37–53. doi:10.1177/1073858410386614
- Swinburn, K., & Byng, S. (2006). The communication disability profile. London: Connect Press.
- Swinburn, K., Porter, G., & Howard, D. (2005). The comprehensive aphasia test. Hove, East Sussex: Psychology Press.
- Szaflarski, J. P., Allendorfer, J. B., Banks, C., Vannest, J., & Holland, S. K. (2013). Recovered vs. not-recovered from post-stroke aphasia: The contributions from the dominant and non-dominant hemispheres. Restorative Neurology and Neuroscience, 31, 347–360. doi:10.3233/RNN-120267
- Szekely, A., Jacobsen, T., D’Amico, S., Devescovi, A., Andonova, E., Herron, D., … Bates, E. (2004). A new on-line resource for psycholinguistic studies. Journal of Memory and Language, 51, 247–250.
- Torres, J., Drebing, D., & Hamilton, R. H. (2013). TMS and tDCS in post-stroke aphasia: Integrating novel treatment approaches with mechanisms of plasticity. Restorative Neurology and Neuroscience, 31, 501–515.
- Turkeltaub, P. E., Coslett, H. B., Thomas, A. L., Faseyitan, O., Benson, J., Norise, C., & Hamilton, R. H. (2012). The right hemisphere is not unitary in its role in aphasia recovery. Cortex, 48, 1179–1186. doi:10.1016/j.cortex.2011.06.010
- Turkeltaub, P. E., Messing, S., Norise, C., & Hamilton, R. H. (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology, 76, 1726–1734. doi:10.1212/wnl.0b013e31821a44c1
- Vestito, L., Rosellini, S., Mantero, M., & Bandini, F. (2014). Long-term effects of transcranial direct-current stimulation in chronic post-stroke aphasia: A pilot study. Frontiers in Human Neuroscience, 8. doi:10.3389/fnhum.2014.00785
- Volpato, C., Cavinato, M., Piccione, F., Garzon, M., Meneghello, F., & Birbaumer, N. (2013). Transcranial direct current stimulation (tDCS) of Broca’s area in chronic aphasia: A controlled outcome study. Behavioural Brain Research, 247, 211–216. doi:10.1016/j.bbr.2013.03.029
- Whiteside, S. P., Inglis, A. L., Dyson, L., Roper, A., Harbottle, A., Ryder, J., … Varley, R. A. (2012). Error reduction therapy in reducing struggle and grope behaviours in apraxia of speech. Neuropsychological Rehabilitation, 22, 267–294. doi:10.1080/09602011.2011.639614