 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
The measurement of traumatic brain injury (TBI) ‘severity’ has traditionally been based on the earliest Glasgow Coma Score (GCS) recorded, however, the underlying parenchymal pathology is highly heterogonous. This heterogeneity renders prediction of outcome on an individual patient level inaccurate and makes comparison between patients both in clinical practice and research difficult. The complexity of this heterogeneity has resulted in generic all encompassing ‘traumatic brain injury protocols’. Early management and studies of neuro-protectants are often done irrespective of TBI type, yet it may well be that a specific treatment may be beneficial in a subset of TBI pathologies.
Methods
A simple CT-based classification system rating the recognised types of blunt TBI (extradural, subdural, subarachnoid haemorrhage, contusions/intracerebral haematoma and diffuse axonal injury) as mild (1), moderate (2) or severe (3) is proposed. Hypoxic brain injury, a common secondary injury following TBI, is also included. Scores can be combined to reflect concomitant types of TBI and predominant location of injury is also recorded. To assess interrater reliability, 50 patient CT images were assessed by 5 independent clinicians of varying experience. Interrater reliability was calculated using overall agreement through Cronbach’s alpha including confidence intervals for intra-class coefficients.
Results
Interrater reliability scores showed strong agreement for same score and same injury for TBIs with blood on CT and Cronbach’s alpha co-efficient (range 0.87–0.93) demonstrated excellent correlation between raters. Cronbach’s alpha was not affected when individual raters were removed.
Conclusions
The proposed simple CT classification system has good inter-rater reliability and hence potentially could enable better individual prognostication and targeted treatments to be compared while also accounting for multiple intracranial injury types. Further studies are proposed and underway.
Introduction
Classically, traumatic brain injury (TBI) has been described as mild, moderate and severe based on the first recorded Glasgow Coma Score (GCS − 13–15; 9–12 and <8 respectively.Citation1–3 This has served well over the last 40 yearsCitation4 especially in remote regions of the world where computer tomography (CT) scanning is not possible. However, on an individual patient level, it does not take into account the actual pathology when predicting outcome.Citation5 A postictal patient can be GCS 3 with no brain injury and a patient who dies of an extradural might initially have had a GCS of 15. The dramatic increase in CT scan availability since the 1970s offers more pathology and patient specific grading of injury severity. CT scans provide much greater indication as to the actual parenchymal nature of TBI rather than the functional (neurological) result.Citation6 It could be considered that extra-axial haematoma on their own, whilst they reflect head injury, are not in themselves brain injuries. As they expand, however, they may cause brain injury through underlying ischaemia or shift (e.g. Duret’s haemorrhages or conning) if not acted upon.
Lack of imaging may be the cause of a generic ‘traumatic brain injury’ diagnosis being pervasive throughout the patient pathway – from pre-hospital and critical care (where most services have a generic TBI protocol) through to death where a common cause of death is ‘traumatic brain injury’.Citation7 As pre-hospital imaging becomes potentially possible,Citation8 earlier diagnosis will enable more pathology specific protocols to be developed.
Applying treatments, be they pharmacological (such as neuroprotectants), physiological (such as targeted cerebral perfusion pressure) or surgical (such as decompression) generically across the TBI spectrum is illogical. When we study potential treatments, it may be that a potentially revolutionary treatment for one subtype of injury is discounted because its effects are diluted when multiple types of brain injury are included.
Current radiological classification systems
A distinction needs to be made between ‘classification’ and ‘prognostication’ tools. Although commonly referred to as classification tools, the MarshallCitation9 and RotterdamCitation10 systems are principally used for scoring (which can then be used to classify ‘severity’) but they do not classify the type of brain injury. Brain injury is often heterogenous, and an individual patient may have several different brain injuries (e.g. right subdural and left frontal contusion). A comprehensive system needs to account for these different injuries, not just report ‘the worst one’ as occurs in the abbreviated injury scoring (AIS) system.Citation11 The AIS system (as used by the Trauma Audit Research Network in the UK) is a form of radiological classification in that it reports the highest scoring brain injury, but it takes the fidelity of a location and size of injury and converts it to a number (/score). Not all of those numbers are comparable. For example, a cerebellar extradural (score = 3) is not the same as a cerebral laceration (score also = 3). A system that classifies and scores by pathologies may improve specificity.
Current radiological and prognostic tools assessing TBI severity have improved outcome prediction on populations of patients, but beyond predicting survival at 24 h, they lack precision at an individual patient level.Citation12,Citation13 Accurately predicting outcome could support the setting of limits of further interventions, however, there is no tool that can provide this accurately and hence this is often done on experience and multi-disciplinary consensus.
Saatman et al. demonstrated the heterogeneity of brain injury resulting in GCS < 8 across a range of conditions – extradural (epidural), subdural, contusions, subarachnoid haemorrhage, diffuse axonal injury and general swelling (reflecting hypoxic injury).Citation14 They propose the development of a multidimensional classification model. However, the beauty and universality of GCS has largely been achieved because of its simplicity and a radiological classification system, to achieve universal adoption, should similarly be usable by clinicians and non-clinicians alike.
The history of development of the Marshall and Rotterdam systems may explain why they are not pathology based. The Marshall CT classification () was introduced in 1991 principally to categorise severity of diffuse injury but it was accepted for its descriptive and predictive value.Citation9 The IMPACT (International Mission for Prognosis and Analysis of Clinical Trials in TBI) utilises the Marshall CT score for 6-month outcome prediction in moderate to severe TBI. However, the Marshall CT classification system was not designed for outcome prediction hence in 2005 Maas et al. redesigned it for 6-month mortality prediction resulting in the Rotterdam scoreCitation10 (). Traumatic subarachnoid blood was added as it had been shown to be a strong independent predictor of outcome.Citation15
Table 1. the Marshall computer tomography (CT) classification.
Table 2. The Rotterdam computer tomography classification.
The Helsinki CT score () takes the Rotterdam scoring system one stage further and proportions a ‘score’ specifically for the type of injury (mass lesion) and its size.Citation16 The authors report that this provides a more accurate 6-month outcome prediction.
Table 3. The Helsinki computerized tomography score chart.
A comparative study of the Rotterdam and Marshall scoring systems found that both were good at predicting early death in TBI in Nepal, though the authors suggested that other factors (subarachnoid blood, midline shift and the status of the peri-mesencephalic cisterns) were also predictive.Citation17 In conclusion, they suggested these individual characteristics gave better prognostication than the scoring systems themselves. Chun et al. demonstrated that both Rotterdam and Marshall classification systems had good interobserver reliability when assessed by neuroradiologists and neurosurgeons.Citation18 Whilst these systems do not necessarily grade the underlying pathology, they do grade resulting potential indicators of severity such as midline shift.
All the above are scoring not classification systems. They do not allow comparison between patients as the same score can be achieved through different types of brain injury. None of the current systems consider the location of the injury. It is clearly appreciated that areas of the brain have different function and eloquence and hence comparable injuries in one area can potentially have considerable different effects to injuries in another. It should also be noted that systems requiring calculation of intracerebral blood volume, are advised to use the ABC/2 system as a good formula of volume measurement.Citation19
It could be argued that an MRI classification system would be more predictive, however, CT scanning is still the workhorse of acute brain injury and is more ubiquitous around the world. For this reason, an injury specific classification based on CT is highly desirable.
The two core principles of the proposed classification system are that it should be simple to use and grade specific pathologies. This would make it suitable for research purposes (to enable comparisons between patients) globally and may in the future with evidence, guide clinical management personalised to the severity of those specific pathologies.
The proposed SBNS TBI CT classification system
The ubiquity and speed of image acquirement with CT scans make them the ideal tool on which to base a uniform classification system. The need for a simple classification was discussed within the British Neurotrauma Group (BNTG) of the Society of British Neurological Surgeons (SBNS) and then developed by MW and PH. Key factors in the proposed system were agreed in that the new system should not require a radiologist to report it and it should not require additional measures such as biomarkers. The variables that can be used to describe an injury are its type, severity and location. The cut off boundaries of the ‘mild’, ‘moderate’ and ‘severe’ categories for the different pathologies were agreed by members of the BNTG. The proposal was then reviewed with the key stakeholders and presented to the SBNS council who endorsed its publication.
There are only a limited number of traumatic brain injury types, although they can occur in combination and in areas of brain of different eloquence. Traumatic brain injury types visible on CT are: extradural, acute subdural, intracerebral haematoma/contusion, traumatic subarachnoid haemorrhage, diffuse axonal injury and hypoxic injury.
The proposed SBNS Brain Injury Classification System () grades individual brain pathology severities. All brain injuries are scored between zero and three. Additionally, a score can be assigned to ‘mild TBI/concussion’ with a history of TBI but no evidence on CT. There is also a score for rating the signs of raised intracranial pressure or ‘tightness’ (sulcal, ventricular and basal cistern effacement). Hence possible scores are: mild TBI (m + ve/−ve), extradural (E 0–3), subdural (SD 0–3), subarachnoid (SA 0–3), contusion (C 0–3), diffuse axonal injury (DA 0–3), hypoxia (H 0–3), + ‘Tightness’ (0–3). The location of each injury can also be recorded (Frontal, Parietal, Occipital, Temporal, Cerebellum, Brainstem). Although not part of brain injury classification specifically, skull fractures can also be scored (1 – simple, 2 – depressed, 3 – compound).
Figure 1. The SBNS TBI CT Classification. All brain injuries are scored between zero and three. Additionally, a score can be assigned to ‘mild TBI/concussion’ with a history of TBI but no evidence on CT. There is also a score for ‘tightness’ representing the resulting raised ICP (sulcal, ventricular and basal cistern effacement). Hence, possible scores are: mild TBI (m + ve/−ve), extradural (E 0–3), subdural (SD 0–3), subarachnoid (SA 0–3), contusion (C 0–3), diffuse axonal injury (DA 0–3), hypoxia (H 0–3), + ‘Tightness’ (0–3).
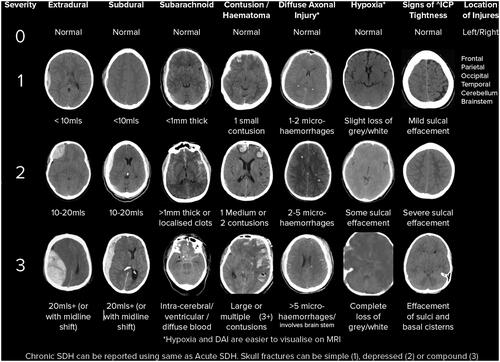
Where blood volumes are noted (e.g. extradural haematoma), this can be estimated or calculated using ABC/2.Citation20 For subarachnoid haemorrhage, the key thickness of >1mm of blood can be anywhere within the subarachnoid space. Small contusions are <2 cm in diameter with medium being 2–5 cm diameter. Large are >5 cm diameter.
The intention is not to sum the scores as there is no suggested linear relationship, however it can enable comparisons between patients (e.g. a group of patients with an acute subdural haematoma with no more than a 1 in any other category).
Inter-rater reliability
Methods
Data from the ‘Traumatic Injury to Brain Across London’ (TriBAL) dataset was used (http://www.imperialneurotrauma.co.uk/tribal.pdf).Citation21 This dataset comprised an audit across London’s Major Trauma Centres capturing data of all TBI patients in London (with a positive CT for acute blood or skull fracture) over a 4-month period. CT scan details and injury scoring using the SBNS brain injury classification (BIC) system were captured for the first 50 consecutive datasets.
The CT head scans were reviewed by five raters of different levels and types of experience, as has been done for other CT classification systems previously.Citation18,Citation22 The raters comprised a nurse, senior house officer (resident physician), neurosurgical registrar (chief resident), neurosurgical consultant (attending physician) and a consultant neuroradiologist. Raters were blinded to each other and to the patient demographics. Access was given to the initial CT head scan (soft tissue and bone windows) for each patient on admission and raters were asked to classify this according to the SBNS BIC.
Data were analysed using SPSS 24.0 (IBM, Armonk, NY: IBM Corp). Inter-rater reliability and overall agreement of injury type and severity were calculated through the Cronbach’s alpha including confidence intervals for intra-class coefficients. Cronbach’s alpha was also calculated with each individual rater’s score removed to check the weighting of each rater on agreement. Cronbach’s alpha measures internal consistency and reliability, a score above 0.7 shows acceptable consistency, above 0.8 shows good consistency and above 0.9 shows excellent consistency. The approach has been used to be consistent with other studies. Cohen’s Kappa was calculated to assess agreement between individual raters. Landis and Koch guidelines were used to interpret agreement where 0 is poor, 0–0.2 is slight, 0.2–0.4 is fair, 0.4–0.6 is moderate, 0.6–0.8 is substantial and 0.8–1.0 is almost perfect.Citation23 The agreement of the reviewer's interpretation was determined by comparison to the consensus opinion once all reviewers had scored the CT scans as defined in the methods of a previous study.Citation18 Consensus was defined as when 80% or more of the blinded scores agreed for the presence or absence of injury. Average inter-item correlation was calculated to analyse internal consistency and reliability. It is a measure of consistency of individual classification types (e.g. each injury type). To show that the values of Cronbach’s alpha were not heavily weighted on one particular rater, values were calculated with each rater removed. This was calculated for the removal of each rater, in turn. Skull fractures were not considered part of the brain injury classification and for the purposes of inter-rater reliability were considered present or absent.
Results
The 50 patients had a median age of 54.5 years with an interquartile range (IQR) of 35.3–72.1 years and were predominantly (84%) male. The median pre-hospital GCS was 12 (IQR 8–15)), and in-hospital GCS was 11 (IQR 6–15). The most common mechanism of injury was falls of less than 2 m (32%) followed by falls greater than 2 m (30%) (). As a mean number of diagnoses from raters, 24 of the 50 patients had acute subdurals (20.7%), 7 had extradural haematomas (6.1%), 21 had subarachnoid haemorrhage (18.1%), 5 had chronic subdural haematomas (3.9%), 16 had evidence of tightness (13.9%), 23 had skull fractures (19.3%), 2 (1.4%) patients had evidence of diffuse axonal injury, and nil with overt hypoxia (hypoxia and DAI are normally seen on magnetic resonance imaging; ).
Table 4. Patient demographics.
Table 5. Injury pathology as identified by all raters inter-rater reliability.
Agreement by percentage was measured for the same injury and the same score for each individual pathology. Results above 70% were shown for all injuries indicating good agreement. However, some injuries were low in prevalence (such as DAI and chronic subdurals) and therefore these results should be analysed with caution (). Scores for agreement of absence or presence of injury (by consensus) were above 80% agreement. All injuries showed good or excellent consistency other than DAI (which is usually diagnosed on MRI imaging) and tightness (). Inter-item correlation was lower for DAI and for tightness ().
Table 6. A table to show the percentage agreement of raters for same injury and same score, and for absence or presence of injury my majority vote.
Removing individual raters when calculating the Cronbach’s alpha did not impact the score significantly using a non-parametric test for significance (p > 0.05) and therefore it can be assumed the Cronbach’s alpha is accurate (Supplementary Table 1).
Kappa coefficients were calculated between individual raters and as a whole group of raters. The grouped values were compared to a previous study which also used 50 CT scans of traumatic brain injury and interobserver variability when assessing for injury.(182010) Previous inter-observer and inter-rater reliability studies for CT scoring tools, not just those for head CTs, used patient numbers between 41 and 50 for inter-rater reliability and 5 separate raters.Citation18,Citation22 The Kappa co-efficient for the SBNS classification are lower than those of the other rating tools (Marshall, Rotterdam and individual assessment), however, as highlighted this is dependent of prevalence of injuries (). The Median Kappa coefficients for injuries between raters was also calculated (Supplement Table 2).
Table 7. Comparison of average Kappa coefficients for individual injuries with the literatureCitation18.
Discussion
We have described a novel and simple classification system that enables comparisons based on discreet CT pathology and assessed its inter-rater reliability amongst healthcare professionals with varying experience of analysing CT scans. The results demonstrate strong agreement between raters for all injuries except for DAI, which shows fair agreement justified by its lower prevalence compared to other injuries. MRI may be more useful in quantifying DAI and hypoxia. Other scoring systems in the literature have found comparative results, in particular in response to low prevalence of injuries.Citation18 When compared with the results from Chun et al. (), the new proposed classification showed comparable levels of agreement using Kappa coefficients for ASDH and DAI. We have attempted to mirror their inter-rater work to provide comparison. Agreement was stronger using the proposed classification for contusions and skull fractures. There was weaker agreement using the new system than other classifications for EDH and SAH. This may relate to the differences in the sampling of the study populations which were random CT positive head injuries in this study and < GCS 12 for Chun et al. There was no comparison for tightness, midline shift, effacement of basal cisterns and CSDH. These results therefore show that the proposed classification, when compared to other scores is comparable, if not better at diagnostically categorising some injuries. The benefit of the new classification, however, is the simplicity and the reporting of actual brain injury pathology enabling patient and treatment comparisons. The inclusion of a score for signs of raised ICP (‘Tightness’) should additionally support additional comparisons between patients especially where ICP monitoring is not routine.Citation24 Chun et al. also found that contusions and DAI had the lowest sensitivity for detection by CT imagining, whereas EDH, intraventricular haemorrhage and parenchymal hematomas had the highest. It was also found that there was poor agreement between raters for SDH and contusion in particular in relation to measuring volumes of blood using the classification and scoring tools. One reason for this may be due to the accuracy of volumes needed for the tools which has been mitigated by the proposed new classification using broader scoring categories for volumes of blood.Citation18 Chun et al. only used experienced neurosurgeons and neuroradiologists whilst in this current study we wanted to demonstrate agreement across a breadth of experiences.
This study has some limitations. The sample size was comparable to those of similar studies, however, due to the number of injuries within the scoring system, this resulted in a small prevalence of some injuries, meaning that the statistics may have been skewed. Despite this limitation, it is accepted that Kappa scores as low of 0.41 may be acceptable in healthcare applications.Citation25
Future work
This is a proposed classification system that is now being used in several studies. As increasing data is accumulated, it is fully expected that the system will be modified. Additionally, this study has focused on blunt TBI. It could be possible to extend the classification system into penetrating injuries by adding in penetrating (low velocity = 1, high velocity = 2) and the location affected. Penetrating TBI from gun/knife crime, terrorism, conflict and work-related injury can contribute a large burden of TBI care.Citation26,Citation27 This can also be complex pathologically with low and high velocity components, the effects of blast and the ensuing risks of infection.Citation28 Therefore, the utility of this scoring system for such additional injuries has not been assessed and it is expected that it will be modified as future work in this field progresses.
Converting this classification system into a prediction tool
When applied in a larger population, it is expected it will be possible to provide a weighting to the classification of different injury types to provide a clinical prognosis or outcome. For example, the outcome of patients who suffer from DAI varies significantly to those who have an extradural haematoma. Scores for injuries can be weighted to reflect these differences in prognosis to predict outcome, in the way that current systems such as the Marshall CT score do. Similarly, assessment in changes of scores over time may be a prognostic indicator and identify the need for intervention.
Radiological severity of brain injury is just one factor in outcome prognostication. The constitutional factors of the patient (age/comorbidities), extent of other injuries and neurological and physiological factors can also be influential, hence these should be factored in:
It is envisaged that utilising large data and outcome registries we will be able to populate an equation and develop the factors a,b,c,d,e,f,g to weight individual pathological elements and more accurately predict not just of mortality but also functional outcome.
E.g.
where E is the Extradural score, SD is the Subdural score, DA is the Diffuse Axonal Score, H is the hypoxia score and T is the tightness score.
Additionally physiological parameters could subsequently be included:
E.g.
Overtime these components will be refined. For example, a biomarker level may give a further indication of amount of neuronal damage, which when put into the above will enable even greater prognostication.
Conclusion
Current CT classification of TBI systems is not injury pathology specific and is difficult to remember and apply. Here we present a simple (mild, moderate and severe) system for different types of brain injury that allows for concomitant brain injuries. The system has good inter-rater reliability across a broad range of experienced users. Utilising such a classification has the potential to assist in clinical management and enables more specific investigation of potential neuroprotectant, physiological and surgical treatments and more accurate prognostication for individual patients.
Supplemental Material
Download MS Word (13 KB)Supplemental Material
Download MS Word (15.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jennett B, Teasdale G, Braakman R, Minderhoud J, Heiden J, Kurze T. Prognosis of patients with severe head injury. Neurosurgery 1979;4:283–9.
- Jennett B, Teasdale G, Knill-Jones R. Prognosis after severe head injury. Ciba Foundation symposium, 1975;34:309–24.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness a practical scale. Lancet 1974;304:81–4.
- Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 2014;13:844–54.
- Carter EL, Hutchinson PJA, Kolias AG, Menon DK. Predicting the outcome for individual patients with traumatic brain injury: a case-based review. Br J Neurosurg 2016;30:227–32.
- Nayebaghayee H, Afsharian T. Correlation between Glasgow Coma Scale and brain computed tomography-scan findings in head trauma patients. Asian J Neurosurg 2016;11:46–9.
- Wilson MH, Hinds J, Grier G, Burns B, Carley S, Davies G. Impact brain apnoea – A forgotten cause of cardiovascular collapse in trauma. Resuscitation 2016;105:52–8.
- Schwindling L, Ragoschke‐Schumm A, Kettner M, et al. Prehospital imaging‐based triage of head trauma with a mobile stroke unit: first evidence and literature review. J Neuroimaging 2016;26:489–93.
- Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg 1991;75:S14–S20.
- Maas AIR, Hukkelhoven CWPM, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2005;57:1173–82.
- Gennarelli TA, Wodzin E. AIS 2005: A contemporary injury scale. Injury 2006;37:1083–91.
- Stevens RD, Sutter R. Prognosis in severe brain injury. Crit Care Med 2013;41:1104–23.
- Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 2008;5:e165.
- Saatman KE, Duhaime A-C, Bullock R, Maas AIR, Valadka A, Manley GT, et al. Classification of traumatic brain injury for targeted therapies. Workshop Scientific Team and Advisory Panel Members. J Neurotrauma 2008;25:719–38.
- Kakarieka A, Braakman R, Schakel EH. Clinical significance of the finding of subarachnoid blood on CT scan after head injury. Acta Neurochir 1994;129:1–5.
- Raj R, Siironen J, Skrifvars MB, Hernesniemi J, Kivisaari R. Predicting outcome in traumatic brain injury: development of a novel computerized tomography classification system (Helsinki Computerized Tomography Score. Neurosurgery 2014;75:632–47. ).
- Munakomi S. A comparative study between Marshall and Rotterdam CT scores in predicting early deaths in patients with traumatic brain injury in a major tertiary care hospital in Nepal. Chin J Traumatol 2016;19:25–7.
- Chun KA, Manley GT, Stiver SI, et al. Interobserver variability in the assessment of CT imaging features of traumatic brain injury. J Neurotrauma 2010;27:325–30.
- Newman GC. Clarification of abc/2 Rule for ICH Volume. Stroke 2007;38:862.
- Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–5.
- Pan-London_Neurotrauma_Group. 2018. Traumatic Injury to Brain Across London Audit Report [online]. undefined. [Accessed 29 Mar 2021]. http://www.imperialneurotrauma.co.uk/tribal.pdf
- Khan L, Mitera G, Probyn L, et al. Inter-rater reliability between musculoskeletal radiologists and orthopedic surgeons on computed tomography imaging features of spinal metastases. Curr Oncol n.d.;18:282–7.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74.
- Chesnut RM, Temkin N, Carney N, et al. A Trial of intracranial-pressure monitoring in traumatic brain injury. Global Neurotrauma Research Group. N Engl J Med 2012;367:2471–81.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82.
- Chang VC, Guerriero EN, Colantonio A. Epidemiology of work‐related traumatic brain injury: a systematic review. Am J Ind Med 2015;58:353–77.
- Sears JM, Graves JM, Blanar L, Bowman SM. Case identification of work-related traumatic brain injury using the occupational injury and illness classification system. J Occup Environ Med 2013;55:507–13.
- Magnuson J, Leonessa F, Ling GSF. Neuropathology of explosive blast traumatic brain injury. Curr Neurol Neurosci Rep 2012;12:570–9.
