Abstract
We outline, in chronological sequence, the events and findings over 25 years that have shaped our understanding of Didymosphenia geminata (Lyngbye) M. Schmidt blooms. Starting with the first appearance of D. geminata mats in streams on Vancouver Island in the late 1980s and followed years later by blooms in Iceland, South Dakota and Poland, D. geminata blooms were enigmatic for nearly 20 years. Early papers exploring whether blooms were caused by environmental change consistently failed to identify any specific factor(s) associated with their onset. Following the D. geminata outbreak in New Zealand in 2004 that seemed to result from an introduction of the species, the possibility that blooms that had previously occurred elsewhere in the world might also be explained by the introduction and movement among watersheds of a new variant with a bloom-forming tendency was touted and widely accepted. Now, however, the identification of very low soluble reactive phosphorus (SRP; below ∼2 ppb) as the proximate cause of bloom formation, has led to the more likely explanation that D. geminata blooms are the result of large-scale human intervention in climatic, atmospheric and edaphic processes that favour this ultra-oligotrophic species. In this new view, blooms of D. geminata are not simply due to the introduction of cells into new areas. Rather, bloom formation occurs when the SRP concentration is low, or is reduced to low levels by the process of oligotrophication. Mechanisms that potentially cause oligotrophication on global and regional scales are identified.
The early years (1988–1998)
Modern episodes of Didymosphenia geminata (Lyngbye) M. Schmidt blooms in rivers were first systematically documented on Vancouver Island, British Columbia in the late 1980s (Sherbot & Bothwell Citation1993, Bothwell et al. Citation2009). British Columbia Ministry of Environment (BCMoE) officials conducting routine water-quality monitoring in rivers downstream of municipalities in 1988 noticed patchy accumulations of slimy looking material covering rocks in the Heber River, upstream of the town of Gold River. One year later, several kilometres of the Heber River were covered in heavy mats. Microscopic examination of the mats revealed the stalk-producing diatom D. geminata. The appearance of thick algal mats was unusual because the Heber River, like most rivers on Vancouver Island, is nutrient-poor and extensive algal accumulations upstream of known human nutrient inputs were unprecedented.
In 1993, an ad hoc group of biologists and chemists (M. Clark, R. Nordin, J. Deniseger, L. Erikson and C. Wightman) from the BCMoE was established to examine the blooms and determine their causes. Photographs of D. geminata-affected rivers on Vancouver Island were taken during site visits () and included on an early website describing the blooms (http://www.env.gov.bc.ca/wat/wq/didy_bcstrms.html).
Fig. 1. The British Columbia Didymosphenia ad hoc group viewing the D. geminata bloom in the Englishman River in 1993.

Didymosphenia geminata is native to British Columbia, as established by some of the earliest published records of this species (Lord Citation1866, Cleve Citation1894–1896). Because D. geminata was a native species, a pressing question in the 1980s was whether the blooms on Vancouver Island were new events, or simply newly reported. Because the dense benthic mats were occurring in more remote, sparsely populated areas of the island, this was not an easy question to address. Personal testimonies and scientific grey literature were evaluated. For example, mute evidence was available in the writings of Roderick Haig-Brown (1908–1976) (). Widely recognized as the 20th century's finest and most prolific angler writer, Haig-Brown lived on Vancouver Island most of his adult life and was a pioneering river conservationist. He fished Vancouver Island's rivers and wrote about them for four decades. In Fisherman’s Fall Haig-Brown (Citation1964) describes two of his favourite rivers, the Heber and the Stamp, in both of which D. geminata blooms were documented in the 1990s. If these blooms had occurred in his lifetime, Haig-Brown would most likely have seen them and written about them, but no mention appears in his works.
Fig. 2. Roderick Haig-Brown (1908–1976). A pioneer river conservationist, prolific author and resident fly fisherman of Vancouver Island for 42 years. No mention of nuisance benthic algal mats occurs in his writings. (Reproduced with permission of the Haig-Brown Institute.)

The weakness of Haig-Brown's testimony is that absence of evidence is not evidence of absence. For this reason, confirmation from long-time observers of Vancouver Island rivers was sought. In 1976, the Fish and Wildlife Branch of BCMoE engaged a team of professional fisheries biologists as a Steelhead Snorkel Survey Team (SSST) to conduct annual censuses of steelhead escapement in response to diminishing returns to Vancouver Island rivers. For 13 years, the SSST annually snorkel-surveyed the calibrated reaches of many rivers and filed reports on steelhead numbers. Starting in the early 1990s, the team noted that benthic surfaces were being covered with thick brown mucilaginous mats that had not occurred in the preceding decade (L. Carswell, G. Horncastle, R. Hooton, R. Axford and S. Hay, pers. commun.). Notes from the SSST helped document the development of D. geminata proliferations among rivers on Vancouver Island.
In the late 1970s, Fisheries and Oceans Canada conducted a study of the impact of nutrients discharged by fish hatcheries into rivers in British Columbia by quantifying algal biomass and diatom species abundance (Munroet al. Citation1985). The Puntledge River on Vancouver Island was included in this study. Between 1978 and 1980, D. geminata was never sufficiently abundant to make the list of quantifiable diatom taxa, although it was occasionally present (Munro et al. 1985). Starting in 1991 and continuing to the present, D. geminata accumulations persist in varying degrees in the Puntledge River. Munro et al. (1985) corroborate that D. geminata was present in Vancouver Island rivers prior to 1989, but in low abundance.
The most likely factors for increased benthic algae in streams are typically either eutrophication (i.e., increased concentration of nutrients, most often phosphorus) or declines in the intensity of physical disturbance or scour (Biggs & Close Citation1989, Biggs Citation2000). In 1993, we examined water chemistry (BCMoE Environmental Monitoring System Web Reporting) and hydrological (Water Survey ofCanada, HYDAT Citation1992) databases for rivers on Vancouver Island. Although the analysis was hampered by a 3 ppb minimum detection limit for SRP, there was no indication that the onset of blooms resulted from either an increase in SRP or benign hydrological conditions (Sherbot & Bothwell1993).
In the 1990s, ground level increases in solar ultraviolet radiation (UVR) resulting from global ozone depletion were identified as a potential threat to aquatic ecosystems at mid and higher latitudes (Häder & Worrest Citation1991). Unshaded, shallow, clear water streams were especially vulnerable (Bothwell et al. Citation1994). Although the inhibition of attached riverine diatom communities by natural UVR was significant, of most relevance was the finding that some species of stalked diatoms in the genera Gomphoneis and Cymbella appeared to be preferentially favoured under exposure to UV light (Bothwell et al. Citation1993). Didymosphenia geminata, however, was not found in the experiment.
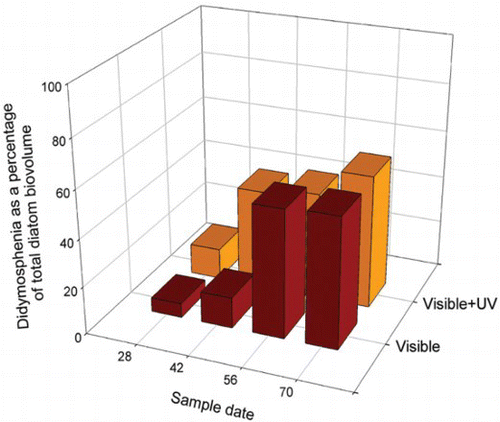
A later, in situ, experiment was run in 1995 to assess the deleterious effects of UVR on aquatic insects (Kelly Citation2001). During a 70-day experiment, diatom samples were collected and enumerated, but at the time, the data were not analysed. We recently analysed the Kelly data. Two-way analysis of variance (ANOVA) revealed no consistent effect of solar UVR on the abundance of D. geminata over a 70-day period relative to other diatom taxa (p=0.71) (). Although a positive relationship would have provided a plausible explanation for D. geminata blooms, the data do not support this conclusion.
Fig. 3. Didymosphenia geminata as a percentage of total diatom biovolume during an in situ solar UVR exclusion experiment in the Little Qualicum channel on Vancouver Island during 1995 (Kelly Citation2001). The presence or absence of UVR had no affect on the abundance of D. geminata relative to other diatom taxa (two-way ANOVA, p=0.71).
More recent research in New Zealand demonstrated an initial, inhibitory effect of UVR on D. geminata cell division that diminished after six days (Kilroy & BothwellCitation2011b). Stalk lengths are also often reduced under exposure to UVR (Kilroy and Bothwell Citation2014). No positive relationship between UVR and D. geminata growth has been documented, despite ongoing speculation that a relationship exists (James et al. Citation2013). Although greater solar exposure can increase D. geminata stalk length (Kilroy & BothwellCitation2011a) and has been associated with greater bloom development (James et al. Citation2013), it appears that the UVR portion of the solar spectrum does not play a major role in D. geminata blooms (Kilroy & Bothwell 2011b, Kilroy & Bothwell2014).
The net result of investigating D. geminata blooms on Vancouver Island in the early years was that, although it was a native species, the blooms appeared to be a new phenomenon, and there was no obvious change in environmental conditions that might explain them.
On the boots of fishermen
The onset of D. geminata blooms in New Zealand in 2004 was widely attributed to the incursion of a new species (Kilroy Citation2004). This suggestion reignited questions about the cause of blooms on Vancouver Island 15 years previously. Was the explanation for blooms that resulted from an introduction in New Zealand also applicable to Vancouver Island? Didymosphenia geminata was a native species in British Columbia, but could a new bloom-forming variant have been introduced in the 1990s? Might this be the reason we had been unable to make sense of the blooms in the northern hemisphere?
On Vancouver Island, the British Columbia Steelhead Harvest Questionnaire database was used to quantify angler activity on selected rivers between 1968 and 2003 and revealed a three- to fourfold increase in angler days on some rivers in the mid-1980s leading up to the D. geminata blooms (Bothwell et al. Citation2009). Increased river use coincided with the commercial introduction of felt-soled waders, the expansion of the guided fishing industry, and an increase in the number of visiting anglers fishing the rivers of Vancouver Island. The analysis of these data entitled, ‘On the Boots of Fishermen: The a History of Didymo Blooms on Vancouver Island, BC’ was published in Fisheries (Bothwellet al. 2009), with the statement:
… .all of the evidence suggesting that recreational fishermen have played a role in the movement of Didymo regionally and globally is circumstantial.
Nevertheless, the publication was widely accepted as an important step in initiating management actions aimed at controlling the spread of aquatic invasive species. Yet, the explanation for the spatial and temporal occurrence of blooms of D. geminata as the result of human vectors was based on coincidental timing.
The Didymo trilogy
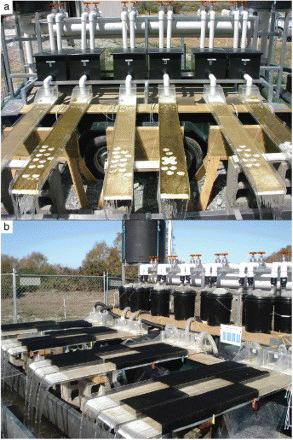
Early in the incursion of D. geminata into New Zealand, it became clear that bloom formation must involve more than the simple introduction of cells into new areas. The observation that blooms did not form in spring-fed creeks discharging into D. geminata-affected rivers on the South Island, in spite of repeated introduction of cells, was demonstrated multiple times (Sutherland et al. Citation2007). To address experimentally the role of environmental factors (light intensity and nutrient concentration of N and P) leading to bloom formation, a flume apparatus was built on the bank of the D. geminata-affected Waitaki River, adjacent to the confluence with the D. geminata-free, Otiake Spring Creek (). This facility was utilized in a collaborative research project between Environment Canada (EC) and the New Zealand Institute for Water and Atmospheric Research (NIWA) during 2008–2010. Results from the NIWA–EC studies were published (Bothwell & Kilroy Citation2011, Kilroy &Bothwell 2011a, Kilroy & Bothwell 2011b Kilroy &Bothwell 2012). We refer to these three papers as the ‘Didymo Trilogy’ because each contains solutions to pieces of the puzzle and, collectively, they provide the evidence that the proximate cause of D. geminata blooms in rivers is low concentration of P. We outline the ‘Didymo Trilogy’ as three chapters in our understanding.
Fig. 4. The experimental flume apparatus used during the collaborative research project between EC and NIWA during 2008–2010 was located on the bank of the Waitaki River, South Island, New Zealand, adjacent to the confluence with the Otiake Spring Creek. (a) The initial design in 2008 had six flumes, all initially colonized with D. geminata using Waitaki River water. Subsequently, three of the flumes were shifted to Otiake Spring Creek water (treatment), while the other three remained as controls and continued to receive Waitaki River water (details in Bothwell & Kilroy Citation2011). (b) In 2009–2010, a total of 12 flumes were employed to test additions of P or N and light intensity using shading cloth (details in Bothwell & Kilroy Citation2011; Kilroy & Bothwell Citation2011a).
Chapter 1: phosphorus limitation (Bothwell & KilroyCitation2011)
The summary points from this paper are:
(1) The frequency of dividing cells (FDC) is used as a metric for P-limited growth rates in D. geminata. FDC has been used to measure aquatic microbial growth for decades, but its application to D. geminata was key to understanding the blooms.
(2) Exposed to high concentrations of P, D. geminata cells divide rapidly for short periods, but colonies and blooms eventually dissipate.
(3) Experiments conducted year-round identified a continuing high level of P-limitation in D. geminata in situ while cells remained dominant in the Waitaki River.
(4) FDC was the same whether or not cells were associated with colonies and we concluded that D. geminata cells do not necessarily access P from within benthic mats.
Chapter 2: environmental control of stalk length (Kilroy& Bothwell 2011a).
The summary points from this paper are:
(1) When cell division rates are low, the much longer stalks characteristic of blooms are produced. Such diversion of photosynthetically fixed carbon into extracellular compounds under conditions of nutrient limitation is characteristic of some diatoms (Myklestad Citation1995).
(2) Higher light conditions also result in longer stalks.
Chapter 3: relation of cell growth rates and bloom formation to dissolved phosphorus concentrations (Kilroy & Bothwell 2012)
The summary points from this paper are:
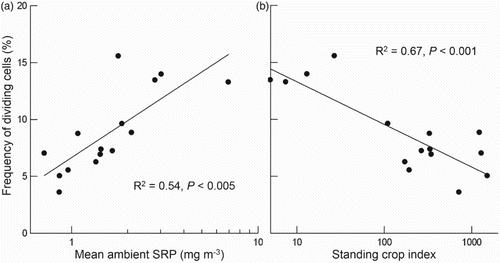
(1) Synoptic surveys of rivers on South Island, New Zealand show that the distribution of D. geminata blooms can be predicted from the SRP concentration. Blooms do not form if the mean SRP concentration over a 24-month period (monthly sampling) exceeds ∼2 ppb ().
(2) A direct positive relationship exists between ambient SRP concentration in the water and D. geminata FDC (a), whereas an inverse relationship exists between FDC and D. geminata biovolume, measured as a standing crop index (b). This is also a likely explanation for the absence of D. geminata on the North Island of New Zealand (Kilroy& Unwin 2011, Rost et al. Citation2011) where nearly all rivers have naturally high SRP, >2 ppb, shown to prevent bloom establishment in rivers on the South Island (Kilroy & Bothwell Citation2012).
(3) A transect across a braid in the Waitaki River just below a spring tributary indicated that the SRP concentration that inhibits blooms might even be lower (<1 ppb) than indicated in the synoptic survey of South Island.
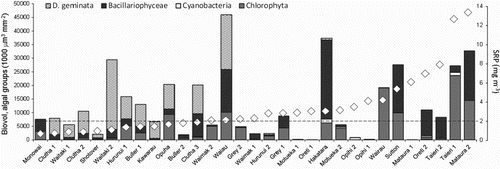
Fig. 5. Algal biovolume expressed as standing crop index (% cover×mat thickness in mm) at each of 31 South Island, New Zealand, river sites surveyed between January and March 2010, in terms of broad algal groups and D. geminata, with mean SRP (diamonds) shown for each river. The dashed horizontal line indicates 2 ppb SRP.
Our epiphany
The publications that we refer to as the ‘Didymo Trilogy’ made clear to us that the proximate cause of blooms or, more precisely, excessive extracellular stalk production, is low P. There is precedence for a mucopolysaccaride production response to low P. For example, photosynthetic release of structural or non-structural exudates is a common stress response by some diatoms to low nutrient conditions (Hoagland et al. Citation1993, Myklestad Citation1995). Moreover, the most rapid increase in diatom-specific growth rate occurs below 1 ppb dissolved P, so changes in dissolved P below 1 ppb are highly relevant to diatom cell division (BothwellCitation1988, Citation1989). The unexpected conclusion is that D. geminata blooms do not occur in spite of P limitation, they occur because of it! Stalk production in response to very low P may be a strategy to move cells out of the benthic boundary layer and into the water column where there is greater delivery of growth-limiting P. We propose that P concentrations in relatively unaffected rivers around the world are declining and D. geminata blooms are actually the result of that decline, not primarily the result of recent introduction.
At this point, it is important to highlight the difference between blooms of D. geminata and geographic distribution of this species. New Zealand is the best example of an introduction of D. geminata (Kilroy & Unwin Citation2011). However, while the presence of cells is obviously prerequisite, introduction alone is not the cause of bloom formation.
The ultimate causes of D. geminata blooms
With low SRP identified as the proximate cause of D. geminata blooms, we propose four mechanisms operating at global or regional scales that could potentially result in a decline of P, i.e., oligotrophication, entering fluvial systems. We outline the following as hypotheses of the potential ultimate causes of D. geminata blooms: (1) atmospheric deposition of reactive nitrogen resulting from the burning of fossil fuels and urbanization; (2) climate-induced shifts in the timing of snowmelt and growing season that decreases P inputs to rivers; (3) N-enrichment of landscapes during agricultural and silvicultural activities that result in greater retention of terrestrial P; and (4) a decline in marine-derived nutrients, particularly P, resulting from widespread depletion in spawning salmon. Although these processes do not apply to all regions, they are not mutually exclusive and could act synergistically. These processes are in need of further investigation for their role in driving blooms of D. geminata.
The above potential ultimate drivers for the formation of D. geminata blooms are consistent with the worldwide occurrence of blooms. In the case of Vancouver Island, it is now known that the onset of D. geminata blooms in the early 1990s followed the commencement of an island-wide annual urea-N fertilization silvicultural programme that was among the largest of its kind in North America (British Columbia Ministry of Forests, Lands, and Natural Resource Operations; http://www.for.gov.bc.ca/hfp/publications/00001/2-2-silv-stand-index.htm). Increased N inputs to landscapes will likely lead to increased P assimilation, reducing its availability for run-off into streams. A detailed discussion of these processes is presented elsewhere.
In addition to the large-scale processes we outline as the ultimate drivers of oligotrophication, understanding that low SRP is the proximate cause of bloom formation, affords new interpretations of how local-scale factors, which potentially alter P concentration, can explain smaller scale spatial variability of blooms. For example, the well-known tendency for D. geminata bloom formation to occur below lakes and dam outlets (Kirkwood et al. Citation2009) takes on a new significance in the light of dissolved P depletion in lake surface waters during the summer. Likewise, variations in D. geminata bloom formation along river channels have been shown to follow regions of upwelling and downwelling exchange with the hyporheic zone, with blooms being absent in zones of upwelling, nutrient-rich, hyporheic water, but forming in potentially P-depleted downwelling regions (Wyatt et al. Citation2008). Low dissolved P as the proximate cause of D. geminata bloom formation provides a parsimonious explanation for D. geminata blooms across spatial scales.
As we wrote elsewhere, ‘Didymosphenia geminata blooms end when cell division rates are not limited by P, and blooms do not begin unless these rates are P-limited’ (Bothwell et al. Citation2012). Much of the focus on D. geminata has been on identifying favourable potential-habitat conditions, not on what causes bloom formation. Once the suite of physical (higher light, low turbidity, stable substratum and flow) and chemical (specific pH range, minimum Ca and Si, maximum Na and Cl) prerequisites are met, we have shown that it is the concentration of soluble available P that explains the timing and spatial distribution of blooms where D. geminata cells are present.
Why was low P overlooked?
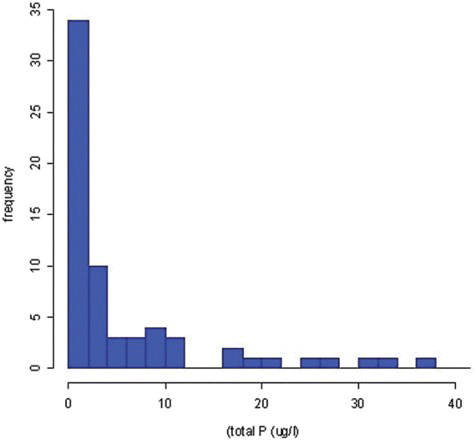
Parameters associated with the presence of D. geminata were outlined for the United States (Spaulding & ElwellCitation2007). Using data from the EPA Environmental Monitoring and Assessment Programme, Spaulding & Elwell illustrated that D. geminata is most frequently found in low-nutrient water (). Why was the simplest explanation for the increase in D. geminata abundance and the appearance of blooms as low P missed? A likely answer to this question is that aquatic biologists typically associate higher algal biomass with increases in nutrients, not decreases.
Fig. 7. The relationship between the frequency of occurrence of D. geminata cells and total phosphorus concentration. Data from the EPA Environmental Monitoring and Assessment Programme (EMAP), 2000–2003. Taken from Spaulding & Elwell (2007).
However, Eugene F. Stoermer, voiced the possibility of low phosphorus. In 1994, he was contacted by the Didymosphenia ad hoc group in British Columbia. His response to their query was prescient, indicating that he thought that the nuisance D. geminata blooms on Vancouver Island probably meant that the water quality in rivers was improving, that is, that P was declining (). Although Gene's view of exotic diatom species later changed (Smol & Stoermer Citation2010), his initial reaction to the nuisance blooms of D. geminata on Vancouver Island is consistent with our recent research, indicating that it is actually low P that causes the blooms. It is also consistent with Gene's recollection that D. geminata was once common throughout the Laurentian Great Lakes, but following decades of eutrophication it is now restricted to Lake Superior ().
Acknowledgements
This paper is based on an invited Plenary Lecture to the International Didymo Conference at the Biltmore Hotel in Providence, RI on 13 March 2013. John Smol and an anonymous reviewer provided comments on an earlier draft of this manuscript.
References
- Biggs B.J.F. 2000. Eutrophication of streams and rivers: dissolved nutrient-chlorophyll relationships for benthic algae. Journal of the North American Benthological Society 19: 17–31. doi: 10.2307/1468279
- Biggs B.J.F. & Close M.E. 1989. Periphyton biomass dynamics in gravel bed rivers: the relative effects of flows and nutrients. Freshwater Biology 22: 209–231. doi: 10.1111/j.1365-2427.1989.tb01096.x
- Bothwell M.L. 1988. Growth rate responses of lotic periphytic diatoms to experimental phosphorus enrichment: the influence of temperature and light. Canadian Journal of Fisheries and Aquatic Sciences 45: 261–270. doi: 10.1139/f88-031
- Bothwell M.L. 1989. Phosphorus-limited growth dynamics of lotic periphytic diatom communities: areal biomass and cellular growth rate responses. Canadian Journal of Fisheries and Aquatic Sciences 46: 1293–1301. doi: 10.1139/f89-166
- Bothwell M.L. & Kilroy C. 2011. Phosphorus limitation of the freshwater benthic diatom Didymosphenia geminata determined by the frequency of dividing cells. Freshwater Biology 56: 565–578. doi: 10.1111/j.1365-2427.2010.02524.x
- Bothwell M.L., Sherbot D., Roberge A.C., & Daley R.J. 1993. Influence of natural ultraviolet radiation on lotic periphytic diatom community growth, biomass, accrual, and species composition: short-term versus long-term effects. Journal of Phycology 29: 24–35. doi: 10.1111/j.1529-8817.1993.tb00276.x
- Bothwell M.L., Sherbot D.M.J. & Pollock C.M. 1994. Ecosystem response of solar ultraviolet-B radiation: Influence of trophic-level interactions. Science 265: 97–100. doi: 10.1126/science.265.5168.97
- Bothwell M.L., Lynch D.R., Wright H. & deniseger J. 2009. On the boots of fishermen: The history of didymo blooms on Vancouver Island, British Columbia. Fisheries 34: 382–388. doi: 10.1577/1548-8446-34.8.382
- Bothwell M.L. Kilroy C., Taylor B.W., Ellison E.T., James D.A., Gillis C-A., Bladon K.D. & Silins U. 2012. Iron is not responsible for Didymosphenia geminata bloom formation in phosphorus-poor rivers. Canadian Journal of Fisheries and Aquatic Sciences 69: 1723–1727. doi: 10.1139/f2012-112
- Cleve P.T. 1894–1896. Synopsis of the Naviculoid Diatoms. Kongliga Sevenska Vetenskaps-Akademiens Handlingar. Stockholm. (Reprinted 1965, A. Asher and Co., Amsterdam).
- Häder D-P. & Worrest R.C. 1991. Effects of enhanced solar ultraviolet radiation on aquatic ecosystems. Photochemistry and Photobiology 53: 717–725. doi: 10.1111/j.1751-1097.1991.tb08502.x
- Haig-Brown R.L. 1964. Fisherman‘s Fall. Crown Publishers Inc, New York, NY. 279p. ISBN 0-517-52369-8.
- Hoagland K.D., Rosowski J.R., Gretz M.R. & Roemer S.C. 1993. Diatom extracellular polymeric substances: function, fine structure, chemistry and physiology. Journal of Phycology 29: 537–566. doi: 10.1111/j.0022-3646.1993.00537.x
- HYDAT. 1992. HYDAT De-ROM: Surface Water Data. Water Resource Branch, Inland Water Directorate, Ottawa, Canada.
- James D.A., Mosel K. & Chipps S.R. 2013. The influence of light, stream gradient, and iron on Didymosphenia geminata bloom development in the Black Hills, South Dakota. Hydrobiologia. 721: 117–127. doi: 10.1007/s10750-013-1654-y
- Kelly D.J. 2001. Effects of solar ultraviolet radiation on stream ecosystems and their application to forestry practices in British Columbia. Ph.D. Dissertation. University of Alberta, Edmonton, Canada.
- Kilroy C. 2004. A new alien diatom Didymosphenia geminata (Lyngbye) Schmidt: its biology, distribution, effects and potential risks for New Zealand fresh waters. NIWA Client Report CHC2004–128. 27 p.
- Kilroy C. & Bothwell M. 2011a. Environmental control of stalk length in the bloom-forming, freshwater benthic diatom Didymosphenia geminata (Bacillariophyceae). Journal of Phycology 47: 981–989. doi: 10.1111/j.1529-8817.2011.01029.x
- Kilroy C. & Bothwell M.L. 2011b. Experimental investigations into environmental factors affecting the growth and blooms of Didymosphenia geminata, Phase 4. June 2011. NIWA Client Report CHC2011–052. 39 p.
- Kilroy C. & Bothwell M.L. 2012. Didymosphenia geminata growth rates and bloom formation in relation to ambient dissolved phosphorus concentration. Freshwater Biology 57: 641–653.
- Kilroy C. & Bothwell M.L. 2014. Attachment and short-term stalk development of Didymosphenia geminata: effects of light, temperature and nutrients. Diatom Research. This volume.
- Kilroy C. & Unwin M. 2011. The arrival and spread of the bloom-forming freshwater diatom Didymosphenia geminata in New Zealand. Aquatic Invasions 6: 349–362. doi: 10.3391/ai.2011.6.3.02
- Kirkwood A.E., Jackson L.J. & Mccauley E. 2009. Are dams hotspots for Didymosphenia geminata blooms? Freshwater Biology 54: 1856–1863. doi: 10.1111/j.1365-2427.2009.02231.x
- Lord J.K. 1866. Naturalist in Vancouver Island and British Columbia. Vols. 1, 2. R. Bentley. Spottiswoode and New-Street Square, London. 375pp.
- Munro K.A., Samis S.C. & Nassichuk M.D. 1985. The effects of hatchery effluents on water chemistry, periphyton and benthic invertebrates of selected British Columbia streams. Canadian Manuscript Report of Fisheries and Aquatic Science 1830: xvii +203p.
- Myklestad S. 1995. Release of extracellular products by phytoplankton with special emphasis on polysaccharides. Science of the Total Environment 165: 155–164. doi: 10.1016/0048-9697(95)04549-G
- Rost A.L., Fritsen C.H. & Davis C.J. 2011. Distribution of freshwater diatom Didymosphenia geminata in streams in the Sierra Nevada, USA, in relation to water chemistry and bedrock geology. Hydrobiologia 665: 157–167. doi: 10.1007/s10750-011-0617-4
- Sherbot D.M.J. & Bothwell M.L. 1993. Didymosphenia geminata (Gomphonemaceae). A review of the ecology of D. geminata and the physiochemical characteristics of endemic catchments on Vancouver Island. NHRI Contribution No. 93005. National Hydrology Research Institute, Environment Canada, Saskatoon, Saskatchewan.
- Smol J.P. & Stoermer E.F. 2010. Epilogue: A view to the future. In: The diatoms: Application for environmental and earth sciences (Ed. by J.P. Smol, & E.F. Stoermer), pp. 611–613. 2nd Edition. Cambridge University Press, Cambridge, UK. ISBN: 9780521509961. 686p.
- Spaulding S.A. & Elwell L. 2007. Increase in nuisance blooms and geographic expansion of the freshwater diatom Didymosphenia geminata: U.S. Geological Survey Open-File Report 2007–1425, 38p.
- Sutherland S., Rodway M., Kilroy C., Jarvie B. & Hughes G. 2007. The survival of Didymosphenia geminata in three rivers and associated spring-fed tributaries in the South Island of New Zealand. June 2007. Prepared for MAF Biosecurity New Zealand.
- Wyatt K.H., Hauer F.R. & Pessoney G.F. 2008. Benthic algal response to hyporheic-surface water in an alluvial river. Hydrobiologia 607: 151–161. doi: 10.1007/s10750-008-9385-1