ABSTRACT
Background: Following traumatic brain injury (TBI), optimization of cerebral physiology is recommended to promote more favourable patient outcomes. Accompanying pain and agitation are commonly treated with sedative and analgesic agents, such as opioids. However, the impact of opioids on certain aspects of cerebral physiology is not well established.
Objective: To conduct a systematic review of the evidence on the effect of opioids on cerebral physiology in TBI during acute care.
Methods: A comprehensive literature search was conducted in five electronic databases for articles published in English up to November 2017. Studies were included if: (1) the study sample was human subjects with TBI; (2) the sample size was ≥3; (3) subjects were given an opioid during acute care; and (4) any measure of cerebral physiology was evaluated. Cerebral physiology measures were intracranial pressure (ICP), cerebral perfusion pressure (CPP), and mean arterial pressure (MAP). Subject and study characteristics, treatment protocol, and results were extracted from included studies. Randomized controlled trials were evaluated for methodological quality using the Physiotherapy Evidence Database tool. Levels of evidence were assigned using a modified Sackett scale.
Results: In total, 22 studies met inclusion criteria, from which six different opioids were identified: morphine, fentanyl, sufentanil, remifentanil, alfentanil, and phenoperidine. The evidence for individual opioids demonstrated equally either: (1) no effect on ICP, CPP, or MAP; or (2) an increase in ICP with associated decreases in CPP and MAP. In general, opioids administered by infusion resulted in the former outcome, whereas those given in bolus form resulted in the latter. There were no significant differences when comparing different opioids, with the exception of one study that found fentanyl was associated with lower ICP and CPP than morphine and sufentanil. There were no consistent results when comparing opioids to other non-opioid medications.
Conclusion: Several studies have assessed the effect of opioids on cerebral physiology during the acute management of TBI, but there is considerable heterogeneity in terms of study methodology and findings. Opioids are beneficial in terms of analgesia and sedation, but bolus administration should be avoided to prevent additional or prolonged unfavourable alterations in cerebral physiology. Future studies should better elucidate the effects of different opioids as well as varying dosages in order to develop improved understanding as well as allow for tighter control of cerebral physiology.
Abbreviations: CPP: Cerebral Perfusion Pressure, GCS: Glasgow Coma Scale, ICP: Intracranial Pressure, MAP: Mean Arterial Pressure, PEDro: Physiotherapy Evidence Database, RCT: Randomized Controlled Trial, TBI: Traumatic Brain Injury
Introduction
In 2013, one in every 50 emergency department visits in the United States was related to traumatic brain injury (TBI); this totalled approximately 2.5 million visits, which was a dramatic increase from roughly 1.6 million in 2007 (Citation1). Further, there were approximately 282,000 TBI-related hospitalizations and 56,000 deaths within the same year (Citation1). Clinical management of TBI in the acute phase is focused on attending to primary injuries and preventing secondary injuries. Primary injury occurs as a direct result of forces at the time of impact, whereas secondary injury can be a consequence of the natural evolution of the primary injury or due to secondary insults, which produce additional damage (Citation2).
Given that TBI can lead to cerebral swelling and brain herniation, the Guidelines for the Management of Severe Traumatic Brain Injury recommend that patients with severe TBI be managed using intracranial pressure (ICP) monitoring to reduce both in-hospital and two-week post-injury mortality (Level II B) (Citation3). Through ICP monitoring, there may be an increase in favourable functional outcomes, and decreases in the rate of electrolyte disturbances and renal failure (Citation4). The guidelines suggest treatment of ICP above 22 mmHg, as values higher than this are associated with increased mortality (Level II B) (Citation3).
Cerebral perfusion pressure (CPP) drives cerebral blood flow (CBF), which is necessary for the delivery of oxygen to brain tissues. CPP is the difference between mean arterial pressure (MAP) and ICP (CPP = MAP – ICP) (Citation5). Following TBI, the brain’s ability to regulate CBF may be impaired, which can cause CBF to decrease and lead to brain ischemia (Citation5). Management of severe TBI using guideline-based recommendations for CPP is recommended to decrease two-week mortality (Citation3). In addition, the guidelines recommend a target CPP between 60 and 70 mmHg in order to promote survival and favourable outcomes (Level II B) (Citation3).
Pain and agitation are common post TBI and are often treated with various sedatives and analgesics. Similar medications may be used to prevent and treat elevations in ICP. Roberts et al. (2011) conducted a systematic review examining the effects of sedative agents on ICP and CPP, and found that sedation generally improved ICP and CPP. The review found three studies showing that opioids given in bolus or short infusions resulted in clinically and statistically significant increases in ICP as well as decreases in CPP and MAP (Citation6). A more recent review by Alnemari et al. (2017) reported that there was a lack of understanding concerning the effects of fentanyl and other opioids on ICP reduction. The review also found that sufentanil did not have an effect on ICP unless there were also changes in MAP, in which case sufentanil may actually increase ICP (Citation7).
The impact of opioids on ICP, CPP, and MAP requires further clarification. To the authors’ knowledge, no review comprehensively examining this topic has been conducted. Therefore, the objective of this systematic review was to examine the evidence regarding the effect of opioids on cerebral physiology in TBI during acute care.
Methods
The current review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Citation8).
Search strategy
A comprehensive literature search was conducted in five electronic databases (i.e., PubMed, Scopus, Embase, CINAHL, and PsycINFO) for articles published from database inception up to November 2017. Filters were applied in each database to restrict searches to articles published in English. The following keywords were used: brain injury, head injury, narcotic, analgesic, opioid, and opiate. Variations of keywords were tailored to each database. References of included studies were scanned to ensure no relevant articles were missed in the original search.
Study selection
Studies were included in the current review if they met the following four a priori criteria:
the entire study population was human subjects with TBI;
the sample had three or more subjects;
subjects were given an opioid during acute care (e.g., emergency department, trauma center, intensive care, critical care setting); and
any one or combination of measures of cerebral physiology (i.e., ICP, CPP, and/or MAP) was evaluated.
After removal of duplicates, studies were screened for eligibility based on title and abstract. Full-text articles were retrieved for the remaining studies and further screened for eligibility. Studies were not included if there was insufficient information to extract regarding subject characteristics, methods, and/or results.
Study appraisal
Randomized controlled trials (RCTs) were evaluated for methodological quality using the Physiotherapy Evidence Database (PEDro) tool () (Citation9). The PEDro tool consists of 11 items, each answered with a “yes” (score = 1) or “no” (score = 0). The first item is not used in calculating the final score, thus the tool yields a maximum score of 10. PEDro scores were then used to categorize RCTs as poor (<4), fair (4–5), good (6–8), or excellent (9–10) quality (Citation10). Studies were assigned levels of evidence using a modified Sackett scale, which simplifies the original ten-level scale into five levels () (Citation11).
Table 1. Physiotherapy evidence database (PEDro) tool (Citation9).
Table 2. Modified sackett scale (Citation11).
Data extraction and synthesis
Study characteristics (i.e., authors, year of publication, country of origin, study design, and sample size), subject characteristics (i.e., age, sex, and TBI severity), study setting (i.e., hospital unit and baseline medications), treatment protocol (i.e., drug, dosage, and delivery), and results were extracted from the included studies. Data were organized into tables and grouped by intervention. Levels of evidence assigned to each study were used to determine the strength of the evidence for each intervention.
Results
Study characteristics
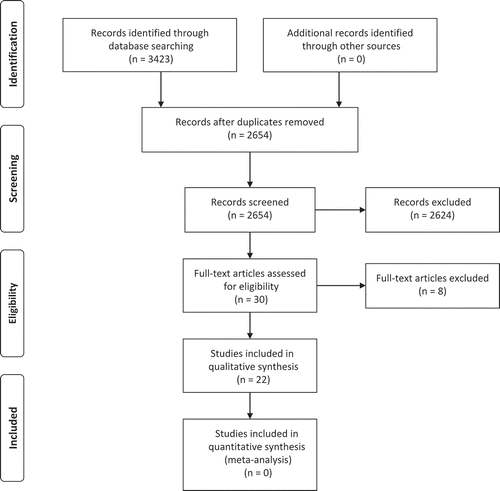
For the current review, 22 studies met inclusion criteria (); no studies were excluded due to an inability to extract data. The characteristics of the included studies are summarized in . Nine studies (Citation13,Citation15,Citation16,Citation20,Citation23,Citation24,Citation27,Citation30,Citation33) were RCTs, of which four (Citation13,Citation20,Citation30,Citation33) utilized a crossover design. Three RCTs (Citation15,Citation20,Citation30) were good quality (PEDro = 6–8) and six RCTs (Citation13,Citation16,Citation23,Citation24,Citation27,Citation33) were fair quality (PEDro = 4–5). Three studies (Citation22,Citation26,Citation31) were prospective controlled trials. One study (Citation25) was a prospective cohort study. Nine studies (Citation12,Citation14,Citation17–Citation19,Citation21,Citation28,Citation29,Citation32) were pre-post test studies. The total pooled sample size of all included studies was 792, with study sample sizes ranging from 6 to 161.
Table 3. Study and subject characteristics.
Subject characteristics
Subject characteristics are summarized in . The majority of studies (N = 15) reported a mean/median age between 20 and 40 years old; two studies (Citation32,Citation33) did not report age. There were considerably more male (N = 536) than female (N = 187) subjects overall; four studies (Citation16,Citation22,Citation27,Citation33) did not report sex. Injury severity was measured using the Glasgow Coma Scale (GCS), and ranged from moderate (GCS = 9–12) to severe (GCS = 3–8). Four studies (Citation23,Citation24,Citation26,Citation31) included subjects with moderate to severe TBI. One of these studies (Citation26) did not report GCS scores, but specified that injuries were moderate to severe. The remaining studies included subjects with severe TBI only.
Study setting
All included studies were conducted in intensive care units, such that all patients were receiving mechanical ventilation. Sedation was administered prior to intervention in the majority of studies: midazolam in 12 studies (Citation15,Citation16,Citation19,Citation20,Citation23,Citation26–Citation32), propofol in 6 studies (Citation12,Citation13,Citation17,Citation18,Citation21,Citation23), etomidate in 1 study (Citation14), and diazepam in 1 study (Citation33). Four studies (Citation22–Citation25) reported that no sedatives were administered before at least one of the interventions. Analgesia administered prior to intervention was reported as morphine in four studies (Citation19,Citation20,Citation27,Citation33), fentanyl in four studies (Citation17,Citation18,Citation29,Citation32), and sufentanil in two studies (Citation21,Citation28). As well, four studies (Citation22,Citation25,Citation28,Citation33) investigated treatments during endotracheal suctioning.
Outcomes
For the purpose of this review, three analyses on cerebral physiology were conducted: (1) the pre-post effects of individual opioids; (2) the comparative effects of different opioids; and (3) the comparative effects of opioids versus other medications. Cerebral physiology was evaluated in terms of change in ICP, CPP, and/or MAP, as available in each study.
1) Opioids. Six different types of opioids were identified in the included studies: morphine, fentanyl, sufentanil, remifentanil, alfentanil, and phenoperidine ().
Table 4. Effect of opioids on cerebral physiology.
Morphine was evaluated in six studies. One study (Citation20) reported that a bolus injection of morphine was associated with a significant increase in ICP and significant decreases in CPP and MAP. Three studies (Citation24,Citation27,Citation31) reported no significant changes to ICP, CPP, or MAP following morphine infusion. The pre-post effects of morphine infusion were not reported in two comparative studies (Citation23,Citation25).
Fentanyl was evaluated in 11 studies. Four studies (Citation13,Citation19,Citation20,Citation30) reported that fentanyl was associated with a significant increase in ICP and significant decreases in CPP and MAP, three by bolus and one by infusion. Two studies (Citation17,Citation18) found that fentanyl infusion resulted in significantly decreased ICP, but did not report on CPP or MAP. Three studies (Citation26,Citation27,Citation33) reported no significant changes to cerebral physiology following fentanyl administration, two by infusion and one by bolus. The pre-post effects of fentanyl were not reported in two comparative studies (Citation23,Citation25).
Sufentanil was evaluated in eight studies. Three studies (Citation12,Citation13,Citation30) reported that sufentanil was associated with a significant increase in ICP and significant decreases in CPP and MAP, one by bolus and two by infusion. One study (Citation29) found that sufentanil infusion resulted in significantly decreased ICP and MAP, and found no significant change in CPP. Four studies (Citation15,Citation16,Citation27,Citation32) reported no significant changes to cerebral physiology following sufentanil administration, three by infusion and one by bolus.
Remifentanil was evaluated in three studies (Citation21,Citation23,Citation28). One study (Citation28) reported that remifentanil infusion was associated with a significant increase in ICP and significant decreases in CPP and MAP. One study (Citation21) found no significant changes in cerebral physiology following remifentanil infusion. The pre-post effects of remifentanil were not reported in one comparative study (Citation23).
Alfentanil was evaluated in two studies (Citation13,Citation22), which reported that alfentanil, delivered intravenously by bolus or infusion, was associated with increased ICP and decreased CPP and MAP.
Phenoperidine was evaluated in a single study (Citation14), which demonstrated significant decreases in CPP and MAP, but no significant changes in ICP.
Levels of evidence
Bolus:
There is Level 1 evidence that bolus morphine, fentanyl, and sufentanil each increase ICP and decrease CPP and MAP.
There is Level 2 evidence that bolus alfentanil increases ICP and decreases CPP and MAP.
There is Level 4 evidence that bolus phenoperidine does not alter ICP but decreases CPP and MAP.
Infusions:
There is Level 1 evidence that sufentanil infusion does not alter ICP, CPP, or MAP, which conflicts with Level 2 evidence that it increases ICP and decreases CPP and MAP.
There is Level 2 evidence that fentanyl infusion does not alter ICP, CPP, or MAP, which conflicts with Level 2 evidence that it increases ICP and decreases CPP and MAP.
There is Level 2 evidence that morphine infusion does not alter ICP, MAP, or CPP.
There is Level 2 evidence that alfentanil infusion increases ICP and decreases CPP and MAP.
There is Level 4 evidence that remifentanil infusion increases ICP and decreases CPP and MAP, which conflicts with Level 4 evidence that it does not alter ICP, CPP, or MAP.
2) Opioid vs Opioid. Seven comparisons between different opioids were identified in the included studies (). All studies reported on ICP, CPP, and MAP.
Table 5. Cerebral physiology: Comparison of different opioids.
Only one study (Citation27) reported a significant difference between opioids, which found that fentanyl infusion was associated with significantly lower ICP and CPP compared to infusion of either morphine or sufentanil. Another study (Citation20) found no significant differences between boluses of fentanyl and morphine. Two other studies found no significant differences between fentanyl and sufentanil, either by bolus (Citation30) or infusion (Citation13).
The remaining comparisons demonstrated that morphine infusion was not significantly different than infusion of sufentanil (Citation27) or remifentanil (Citation23) in terms of its effects on cerebral physiology, and that fentanyl infusion was not significantly different than infusion of remifentanil (Citation23) or alfentanil (Citation13). As well, one study (Citation13) found no significant differences between infusions of sufentanil and alfentanil.
Levels of evidence
Bolus:
There is Level 1 evidence that bolus morphine and fentanyl have similar effects on ICP, CPP, and MAP.
There is Level 1 evidence that bolus fentanyl and sufentanil have similar effects on ICP, CPP, and MAP.
Infusions:
There is Level 2 evidence that morphine versus sufentanil or remifentanil infusions have similar effects on ICP, CPP, and MAP.
There is Level 2 evidence that fentanyl versus remifentanil or alfentanil infusions have similar effects on ICP, CPP, and MAP.
There is Level 2 evidence that sufentanil versus alfentanil infusions have similar effects on ICP, CPP, and MAP.
There is Level 2 evidence that fentanyl infusion results in lower ICP and CPP but similar MAP when compared to morphine infusion.
There is Level 2 evidence that fentanyl infusion results in lower ICP and CPP but similar MAP when compared to sufentanil infusion, which conflicts with Level 2 evidence that fentanyl and sufentanil have similar effects on ICP, CPP, and MAP.
3) Opioid vs Other Medication. Seven comparisons between opioids and other medications were identified in the included studies ().
Table 6. Cerebral physiology: Comparison of opioids and other medications.
Morphine was compared to propofol in two studies (Citation24,Citation31); both studies administered the medications by infusion. One study (Citation24) reported that morphine was associated with significantly higher ICP than propofol, but similar MAP, while the other study (Citation31) found no significant differences in ICP, CPP, or MAP between the two medications.
Sufentanil was compared to ketamine in two studies (Citation15,Citation16). Both studies reported that infusions of either medication were similar in terms of their effects on cerebral physiology. One study (Citation26) compared fentanyl and ketamine infusions, and found that fentanyl was associated with lower ICP and CPP, as well as higher MAP.
Opioids were compared to saline in two studies (Citation22,Citation33); both studies administered the medications by bolus injection. One study (Citation33) reported that fentanyl was associated with significantly lower ICP than saline, while the other study (Citation22) found that alfentanil resulted in significantly lower CPP.
A single study (Citation25) evaluated the effects of morphine or fentanyl infusion alone or in combination with vecuronium bromide, a neuromuscular blocker. The combined regimen was found to result in significantly smaller changes to ICP, CPP, and MAP than opioids alone. However, the study did not draw a direct comparison between the opioids and the neuromuscular blocker.
Levels of evidence
Bolus:
There is Level 2 evidence that, compared to saline, bolus fentanyl results in lower ICP, but similar CPP and MAP.
There is Level 2 evidence that, compared to saline, bolus alfentanil results in lower CPP, but similar ICP and MAP.
Infusions:
There is Level 1 evidence that sufentanil versus ketamine infusions have similar effects on ICP, CPP, and MAP.
There is Level 2 evidence that, compared to ketamine, fentanyl infusion results in lower ICP and CPP, and higher MAP.
There is Level 2 evidence that morphine versus propofol infusions have similar effects on ICP, CPP, and MAP, which conflicts with Level 2 evidence that morphine infusion results in higher ICP when compared to propofol.
Discussion
TBI often requires immediate emergency medical attention; the regulation of both ICP and CPP is frequently a primary objective. In the acute care setting, these patients typically receive medications to alleviate pain, provide comfort, and control agitation as nociceptive stimuli can lead to increases in ICP. Benzodiazepines and opioids are often given together to provide sedating, analgesic, and anxiolytic effects. They can also serve to reduce the metabolic demands being placed on critically-injured or at-risk neurons in the brain. Much controversy exists regarding the relationship between opioids and measures of cerebral physiology including ICP, CPP, and MAP. Given their widespread use, a synthesis of the research literature is necessary to develop a better understanding.
Among 22 studies, this systematic review evaluated six different opioids either alone or in comparison to another opioid or a non-opioid during the acute care management of patients with TBI. In studies examining the pre-post effects of individual opioids, ICP was shown to increase (N = 11) or remain stable (N = 11); conversely, CPP and MAP were shown to decrease (N = 12 and N = 13, respectively) or remain stable (N = 11 and N = 10, respectively). There were no significant differences when comparing the effectiveness between two opioids, with the exception of one study (Citation27) that found fentanyl to be associated with lower ICP and CPP when compared to morphine and sufentanil.
The initial brain insult, especially in combination with additional distressing maneuvers such as intubation, can cause ICP to rise. Inadequate management of pain and agitation can also alter cerebral physiology and be associated with increases in ICP and secondary brain damage (Citation34,Citation35). Generally, the scientific evidence on the administration of opioids in acute care post TBI has demonstrated equally either: (1) no effect on ICP, CPP, or MAP; or (2) an increase in ICP with associated decreases in CPP and MAP. These differences may be related to the mode of administration (i.e., bolus versus infusion) (Citation12,Citation13). Among studies reporting increases in ICP and decreases in CPP, 63.6% reported on opioids given in bolus form; comparatively, 81.8% of studies reporting no change in ICP or CPP reported on opioids given as infusions. Over-sedation from high doses of bolus opioids can cause cerebral vasodilation, which accounts for increased ICP and decreased CPP (Citation6). However, these effects can be mitigated by obtaining optimal sedation level and maintaining constant MAP (Citation36).
Opioids certainly have a beneficial effect in terms of analgesia and sedation, and thus may be indicated during the acute management of TBI. Fentanyl was the most frequently studied drug of all the opioids, accounting for 50.0% of the studies included in the review. While morphine was once the most common opioid administered to patients with TBI, its use has declined in favour of the use of fentanyl, owing to its rapid onset and short duration of effect (Citation37). This trait is useful for clinicians requiring prompt and intermittent neurological assessment. At present, it is not clear whether one opioid is more effective than another, although according to the limited evidence available, this does not appear to be the case. Future research should prospectively and directly compare the effect of different opioid medications on cerebral physiology.
Another consideration when examining the evidence is in relation to drug dosing. Infusion dosing varied widely across studies; for example, fentanyl infusion doses ranged from 2 µg/kg/hr (Citation27) to 4.5 µg/kg/hr (Citation13). Other studies reported dose ranges per hour without regard for patient weight (25–550 µg/hr) (Citation17,Citation18). Even with individual patient variances aside, these ranges are still too broad; greater specificity in medication dosage is required to guide clinical decision-making surrounding opioid administration. Additional studies should better explore the effects of various dose regimens on cerebral physiology post TBI in acute care.
The current review is not without limitations. Despite a significant attempt to describe and organize the included studies in a logical manner, it was difficult to draw substantive comparisons. There were varying dosing regimens for each drug, and most studies included patients receiving various sedatives and/or analgesics at baseline. Furthermore, opioid administration was not always the primary intervention being studied, nor was cerebral physiology always the primary outcome assessed. As such, all reported findings and subsequent conclusions should be taken with caution.
Conclusion
Numerous studies have been conducted to assess the effect of opioids on cerebral physiology in the acute management of TBI. Owing to the heterogeneity of patients with TBI, the nature of the studies’ findings was diverse as well. In general, opioids given in bolus form were shown to increase ICP and decrease CPP and MAP, whereas those given as infusions did not appear to change these parameters. Future studies should elucidate the effects of different opioids and varying dosages in order to develop improved understanding as well as allow for tighter control of cerebral physiology. Moreover, studies should evaluate how opioids may improve outcomes over the short and long term with respect to unique patient subgroups; this type of analysis may facilitate the development of individualized treatment strategies.
Disclosure Statement
The authors report no conflicts of interest.
References
- Taylor C, Bell J, Breiding M, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. WWMR CDC. 2017;66(9):1–16.
- Yokobori S, Bullock M. Pathobiology of primary traumatic brain injury. In: Zasler N, Zafonte R, Arciniegas D, Bullock M, Kreutzer J, editors. Brain injury medicine: principles and practice. 2nd ed. New York: Demos Medical Publishing; 2013. p. 137–47.
- Carney N, Totten A, O’Reilly C, Ullman J, Hawryluk G, Bell M, Baratton S, Chesnut R, Harris O, Kissoon N, et al. Guidelines for the management of severe traumatic brain injury, Fourth edition. Neurosurgery. 2017;80(1):6–15. doi:10.1227/NEU.0000000000001432.
- Han J, Yang S, Zhang C, Zhao M, Li A. Impact of intracranial pressure monitoring on prognosis of patients with severe traumatic brain injury: a PRISMA systematic review and meta-analysis. Medicine. 2016;95(7):e2827. doi:10.1097/MD.0000000000004864.
- Gaddam S, Robertson C. Critical care. In: Zasler N, Zafonte R, Arciniegas D, Bullock M, Kreutzer J, editors. Brain injury medicine: principles and practice. 2nd ed. New York: Demos Medical Publishing; 2013. p. 343–56.
- Roberts D, Hall R, Kramer A, Robertson H, Gallagher C, Zygun D. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med. 2011;39(12):2743–51. doi:10.1097/CCM.0b013e318228236f.
- Alnemari A, Krafcik B, Mansour T, Gaudin D. A comparison of pharmacologic therapeutic agents used for the reduction of intracranial pressure after traumatic brain injury. World Neurosurg. 2017;106:509–28. doi:10.1016/j.wneu.2017.07.009.
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–69.
- Moseley A, Herbert R, Sherrington C, Maher C. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. 2002;48(1):43–49.
- Teasell R. 2016. Introduction and Methods. In: Teasell R, Hussein N, Foley N, Cotoi A editors. Evidence-based review of stroke rehabilitation. 17th ed. http://www.ebrsr.com/.
- Sackett D, Strauss S, Richardson W, Rosenberg W, Haynes R. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Edinburgh: Churchill Livingstone; 2000. p. 173–77.
- Albanese J, Durbec O, Viviand X, Potie F, Alliez B, Martin C. Sufentanil increases intracranial pressure in patients with head trauma. Anesthesiology. 1993;79(3):493–97.
- Albanese J, Viviand X, Potie F, Rey M, Alliez B, Martin C. Sufentanil, fentanyl, and alfentanil in head trauma patients: a study on cerebral hemodynamics. Crit Care Med. 1999;27(2):407–11.
- Bingham R, Hinds C. Influence of bolus doses of phenoperidine on intracranial pressure and systemic arterial pressure in traumatic coma. Br J Anaesth. 1987;59(5):592–95.
- Bourgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med. 2003;31(3):711–17. doi:10.1097/01.CCM.0000044505.24727.16.
- Bourgoin A, Albanese J, Leone M, Sampol-Manos E, Viviand X, Martin C. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005;33(5):1109–13.
- Colton K, Yang S, Hu P, Chen H, Bonds B, Scalea T, Stein D. Intracranial pressure response after pharmacologic treatment of intracranial hypertension. J Trauma Acute Care Surg. 2014;77(1):47–53. doi:10.1097/TA.0000000000000270.
- Colton K, Yang S, Hu P, Chen H, Bonds B, Stansbury L, Scalea T, Stein D. Pharmacologic treatment reduces pressure times time dose and relative duration of intracranial hypertension. J Intensive Care Med. 2016;31(4):263–69. doi:10.1177/0885066614555692.
- de Nadal M, Ausina A, Sahuquillo J, Pedraza S, Garnacho A, Gancedo V. Effects on intracranial pressure of fentanyl in severe head injured patients. Acta Neurochir (Wien). 1998;71:10–12.
- de Nadal M, Munar F, Poca M, Sahuquillo J, Garnacho A, Rossello J. Cerebral hemodynamic effects of morphine and fentanyl in patients with severe head injury: absence of correlation to cerebral autoregulation. Anesthesiology. 2000;92(1):11–19.
- Engelhard K, Reeker W, Kochs E, Werner C. Effect of remifentanil on intracranial pressure and cerebral blood flow velocity in patients with head trauma. Acta Anaesthesiol Scand. 2004;48(4):396–99. doi:10.1111/j.0001-5172.2004.00348.x.
- Hanowell L, Thurston J, Behrman K, Disbrow E. Alfentanil administered prior to endotracheal suctioning reduces cerebral perfusion pressure. J Neurosurg Anesthesiol. 1993;5(1):31–35.
- Karabinis A, Mandragos K, Stergiopoulos S, Komnos A, Soukup J, Speelberg B, Kirkham A. Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial. Critical Care. 2004;8(4):R268–280. doi:10.1186/cc2896.
- Kelly D, Goodale D, Williams J, Herr D, Chappell E, Rosner M, Jacobson J, Levy M, Croce M, Maniker A, et al. Propofol in the treatment of moderate and severe head injury: a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999;90(6):1042–52. doi:10.3171/jns.1999.90.6.1042.
- Kerr M, Sereika S, Orndoff P, Weber B, Rudy E, Marion D, Stone K, Turner B. Effect of neuromuscular blockers and opiates on the cerebrovascular response to endotracheal suctioning in adults with severe head injuries. Am J Crit Care. 1998;7(3):205–17.
- Kolenda H, Gremmelt A, Rading S, Braun U, Markakis E. Ketamine for analgosedative therapy in intensive care treatment of head-injured patients. Acta Neurochir (Wien). 1996;138(10):1193–99.
- Lauer K, Connolly L, Schmeling W. Opioid sedation does not alter intracranial pressure in head injured patients. Can J Anaesth. 1997;44(9):929–33. doi:10.1007/BF03011963.
- Leone M, Albanese J, Viviand X, Garnier F, Bourgoin A, Barrau K, Martin C. The effects of remifentanil on endotracheal suctioning-induced increases in intracranial pressure in head-injured patients. Anesthesia Analg. 2004;99(4):1193–98. doi:10.1213/01.ANE.0000132546.79769.91.
- Scholz J, Bause H, Schulz M, Klotz U, Krishna D, Pohl S, Schulte Am Esch J. Pharmacokinetics and effects on intracranial pressure of sufentanil in head trauma patients. Br J Clin Pharmacol. 1994;38(4):369–72.
- Sperry R, Bailey P, Reichman M, Peterson J, Petersen P, Pace N. Fentanyl and sufentanil increase intracranial pressure in head trauma patients. Anesthesiology. 1992;77(3):416–20.
- Stewart L, Bullock R, Rafferty C, Fitch W, Teasdale G. Propofol sedation in severe head injury fails to control high ICP, but reduces brain metabolism. Acta Neurochir (Wien). 1994;60:544–46.
- Werner C, Kochs E, Bause H, Hoffman W, Schulte Am Esch J. Effects of sufentanil on cerebral hemodynamics and intracranial pressure in patients with brain injury. Anesthesiology. 1995;83(4):721–26.
- White P, Schlobohm R, Pitts L, Lindauer J. A randomized study of drugs for preventing increases in intracranial pressure during endotracheal suctioning. Anesthesiology. 1982;57(3):242–44.
- Mahmood S, Al-Thani H, El-Menyar A, Alani M, Al-Hassani A, Mathrdikkal S, Peralta R, Latifi R. Tramadol in traumatic brain injury: should we continue to use it? J Anaesthesiol Clin Pharmacol. 2015;31(3):344–48. doi:10.4103/0970-9185.161670.
- Gremmelt A, Braun U. Analgesia and sedation in patients with head-brain trauma. Anaesthesist. 1995;44(Suppl 3):S559–565.
- Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, Citerio G. Optimizing sedation in patients with acute brain injury. Crit Care. 2016;20(1):128. doi:10.1186/s13054-016-1362-x.
- Metz C, Gobel L, Gruber M, Hoerauf K, Taeger K. Pharmacokinetics of human cerebral opioid extraction: a comparative study on sufentanil, fentanyl, and alfentanil in a patient after severe head injury. Anesthesiology. 2000;92(6):1559–67.