ABSTRACT
Objective
We aimed to determine the incidence of pre-injury alcohol abuse in TBI at our neurointensive care unit (NICU), the relation to intracranial hemorrhage evolution, and clinical outcome.
Methods
Patients with TBI treated at our NICU at Uppsala university hospital, Sweden, 2008–2018, were included. Clinical, radiological, and outcome variables were evaluated.
Results
Of 844 patients with TBI, 147 (17%) had a history of pre-injury alcohol abuse and these patients were slightly older, but had a similar Charlson co-morbidity index as the other patients. They were more often injured by falls and more frequently developed acute subdural hematomas and cerebral contusions. Their platelets were lower and their IVY bleeding time slightly longer. Patients with pre-injury alcohol abuse more often exhibited an intracranial hemorrhage progression on the second computed tomography. Pre-injury alcohol abuse was an independent predictor of increased mortality (odds ratio = 2.96, p-value = 0.001) and decreased favorable outcome (odds ratio = 0.46, p-value = 0.001) in multiple regression analyses.
Conclusions
Pre-injury alcohol abuse was common in severe TBI, associated with coagulopathy, worse intracranial hemorrhage/injury evolution, and independently predicted poor clinical outcome. These patients deserve more attention in care and research to address specific challenges including disturbed hemostasis.
Introduction
Although alcohol overconsumption is a risk factor for acquiring a traumatic brain injury (TBI) (Citation1,Citation2), several reports indicate that alcohol consumption has contradictory effects after TBI – low to moderate doses of alcohol might even be neuroprotective, by reduced excitotoxicity, catecolaminergic response, decreased coagulopathy, and a lower mortality and more favorable outcome (Citation3–7). However, chronic alcohol overconsumption leads to several changes that worsen the consequences of TBI, e.g. increased N-methyl-D-aspartate receptor-sensitivity and excitotoxicity (Citation8), coagulopathy (reduced platelet count and function, coagulation factor deficiency, and increased fibrinolysis) (Citation9–11), and energy metabolic disturbances due to Vitamin B1-deficiency (Citation12). Alcohol abuse may cause systemic organ damage including the cardiovascular, liver, and immune system that make these patients more susceptible to complications after trauma (Citation13,Citation14). Chronic alcohol abuse is also associated with low pre-injury education level and alcohol-induced brain atrophy that indicate a reduced cognitive reserve with smaller margins for TBI recovery (Citation15–17). Furthermore, patients with pre-injury alcohol abuse tend to exhibit greater gray matter and hippocampal atrophy after TBI (Citation15,Citation18) and some (Citation19,Citation20), but not all (Citation21,Citation22), studies indicate that these patients have an increased mortality and a reduced rate of favorable outcome after TBI.
Considering the frequency of patients with TBI with pre-injury alcohol abuse, we need to increase our understanding of specific challenges in the neurosurgical intensive care management and clinical outcome for these patients. In the current study, our aim was to investigate the incidence of pre-injury alcohol abuse, risk for intracranial hemorrhage progression, and clinical outcome in severe TBI cases with alcohol abuse in an updated cohort from our neurointensive care unit (NICU).
Materials and methods
Patients
This retrospective study was conducted at the Department of Neurosurgery, Uppsala University Hospital, Sweden. There were 926 patients with TBI aged 15 or older who were treated at our NICU between 2008 and 2018 and who were eligible for inclusion in this study. Seventy-five patients were excluded because they had either been treated at another NICU before admission or had been discharged to a different catchment area/country. Seven patients were treated twice at our NICU and were also registered both times, but we only included data from their first visit to the NICU. In the final study population, 844 patients with TBI were included. Patients with pre-injury alcohol abuse were identified based on ICD codes (F10) in combination with medical records from our university hospital or the referring site.
Management protocol
Patients were treated in accordance with our standardized intracranial pressure (ICP)- and cerebral perfusion pressure (CPP)-oriented treatment protocol to avoid secondary insults, as previously described in detail (Citation23–25). Treatment goals were ICP ≤ 20 mm Hg, CPP ≥ 60 mm Hg, systolic blood pressure >100 mm Hg, central venous pressure 0–5 mm Hg, pO2 >12 kPa, arterial glucose 5–10 mmol/L (mM), hemoglobin (Hb) >100 g/L, electrolytes within normal ranges, normovolemia, and body temperature <38°C. Patients were initially mildly hyperventilated (4.0–4.5 kPa) and normoventilated as soon as ICP allowed. Patients with a history of alcohol abuse received thiamine-injections the first days as prophylaxis for Wernicke-Korsakoff encephalopathy. Alcohol-induced coagulopathy was treated conservatively or with platelets, desmopressin, vitamin K, prothrombin complex concentrate, and/or tranexamic acid, depending on the blood and coagulation status and the individual decision by the neurosurgeon.
Data acquisition and analysis
Demographic (including alcohol abuse), admission, and treatment variables were collected from the Uppsala TBI register (Citation26). The extent of comorbidities was evaluated according to the Charlson co-morbidity index (CCI) (Citation27). Routine blood and coagulation status including hemoglobin, platelets, international normalized ratio (INR), and activated partial thromboplastin time (APTT) at admission were evaluated. The testing was done at the accredited laboratory of the Department of Clinical Chemistry at Uppsala University Hospital. Bleeding time was calculated using the IVY bleeding time test on admission for patients with TBI with suspected or confirmed bleeding disorder and in individuals on antithrombotic agents admitted to the NICU according to a standardized protocol (Citation11). The Surgicutt© device which represents a refinement and a modification of the IVY bleeding test was used. The test is performed with a blood pressure cuff applied on the upper arm and inflated to 50 mmHg to control capillary tone, maintain constant capillary pressure, and better standardize the procedure. A sterile blood lancet performs a shallow intradermal incision over the bend of the supinated forearm. Every 30 seconds, filter paper is used to absorb the blood and check for hemostasis. The time period from skin incision until the bleeding has stopped is measured. Values below 480 s are considered normal, whereas values between 480 and 900 s are considered prolonged, and values above 900 s markedly prolonged (Citation11). Testing was done by experienced nurse assistants familiar with this procedure. For quality control of the bleeding time test, our full-time lab technician in the NICU teaches the participating nurse assistants how to perform the test and issues a permit to perform bleeding time on patients after demonstration of the technique. All test results are continuously monitored by the responsible technician to check for aberrant data clusters for each nurse assistant.
Computed tomography (CT) images of the brain were evaluated according to the Marshall classification (Citation28). The size of intracranial hemorrhages was evaluated and compared on the first two CT scans. Hemorrhage progression of intracranial lesions was defined similarly to Shin et al. (Citation29), as an increase on the second CT in 1) epidural hematoma (EDH) or acute subdural hematoma (ASDH) width, with more than 2 mm, 2) traumatic subarachnoid hemorrhage (tSAH) by visual inspection, 3) intraventricular hemorrhage (IVH) with more than 2 mm in lateral width, and/or 4) cerebral contusions with more than 6 ml or 33%. The contusion volume was calculated according to the ABC/2 formula (Citation30). However, some patients received emergency neurosurgery immediately after the first CT and some patients had large intracranial hemorrhages and were in such a poor clinical condition that they were not considered to benefit from surgery and developed total brain death before a second CT was done. To take into account these different clinical courses of progression of intracranial lesions, we divided the patients into four groups: (1) stable intracranial hemorrhages at follow-up CT, (2) progression of intracranial hemorrhages on the second CT, as defined above, (3) immediate intracranial surgery after the first CT, and (4) brain death soon after the first CT. Groups 2, 3, and 4 were considered to have different clinical trajectories of a severe hemorrhage evolution, and for statistical purposes we dichotomized the groups into significant intracranial hemorrhage/injury evolution (groups 2, 3, and 4) and stable intracranial lesions at follow-up CT (group 1).
Outcome
Clinical outcome was assessed by specially trained personnel with structured telephone interviews at 6 months post-injury using the Extended Glasgow Outcome Scale (GOS-E), containing eight categories of global outcome, from death to upper good recovery (Citation26,Citation31,Citation32). The interviews were held with the patients if they had recovered sufficiently, otherwise with their next of kin. Favorable/unfavorable outcome was defined as GOS-E 5–8/1-4.
Statistical analysis
Patients with and without pre-injury alcohol abuse were compared in clinical and outcome variables in accordance with the aims of the study.
Demography, admission status, coagulation status, treatments, and clinical outcome were described as median (interquartile range) or number (proportion). The Mann–Whitney U-test and Pearson’s Chi squared test were used for statistical comparisons between groups of patients.
Differences in hemorrhage/injury progression of intracranial lesions (stable, progression, emergency neurosurgery, or immediate death) in relation to pre-injury alcohol abuse were evaluated with Pearson’s Chi-square test and simple logistic regression.
The association between pre-injury alcohol abuse and mortality and favorable clinical outcome at 6 months was evaluated with Pearson’s Chi-square test. Multiple logistic regression analyses were also performed for mortality and favorable outcome, respectively, as dependent variables, and pre-injury variables (age, sex, CCI, antithrombotic agents, chronic substance abuse, and alcohol abuse) in combination with mechanism of injury as explanatory variables. These regressions were repeated with the remaining IMPACT core variables (Citation33) Glasgow Coma Scale Motor (GCS M) score and pupillary status (normal vs. anisocoria/unreactive) at admission. The degree of intracranial hemorrhage/injury evolution was also included into the regression to evaluate if increased hemorrhage risk could explain the association between alcohol abuse and clinical outcome. A p-value <0.05 was considered statistically significant.
Ethics
All procedures performed in the study involving humans were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments. The study was approved by Uppsala University Regional Ethical Board. Informed consent was obtained during NIC from the next of kin or by the patient after recovery at follow-up.
Results
Demography, admission status, and treatments in relation to pre-injury alcohol abuse
There were 844 patients with TBI included in the study, of whom 147 (17%) had a pre-injury history of alcohol abuse (). These 147 patients were slightly older (median 56 years IQR 48–65 vs. 53 IQR 30–68, p-value 0.05) and were more often men (86% vs. 75%, p-value 0.004). They had a similar CCI (median 0 IQR 0–1 vs. 0 IQR 0–1, p-value 0.24) as the other patients and were to a lower extent treated with antithrombotic agents prior to injury (10% vs. 21%, p-value = 0.003). The mechanism of injury differed between the groups, as those with pre-injury alcohol abuse were more often injured by falls (82% vs. 54%, p-value = 0.001) and less often by other mechanisms. They had more often acute subdural hematomas (ASDH; 64% vs. 42%, p-value = 0.001), cerebral contusions (30% vs. 20%, p-value 0.01), and had a higher Marshall grade (median 4 IQR 2–5 vs. 2 IQR 2–5, p-value = 0.001). There was no difference between the groups regarding GCS M (median 5 IQR 5–6 vs. 5 IQR 5–6, p-value = 0.40) or pupillary abnormalities (14% vs. 16%, p-value = 0.56) at admission. Furthermore, those with pre-injury alcohol abuse had slightly lower platelet count (median 198 ×109/L IQR 134–261 vs. 218 IQR 167–275, p-value = 0.005) and higher bleeding time according to the IVY test (median 570 s IQR 360–900 vs. 480 IQR 360–750, p-value = 0.04), but similar hemoglobin (median 125 g/L IQR 114–140 vs. 127 IQR 113–138, p-value = 0.68), INR (median 1.1 IQR 1.0–1.2 vs. 1.1 IQR 1.0–1.2, p-value = 0.07), and APTT (median 33 s IQR 31–37 vs. 33 IQR 31–37, p-value = 0.71) at admission. The IVY bleeding time was more frequently assessed in the group with pre-injury alcohol abuse than the other patients (86 (59%) vs. 242 (35%), p-value = 0.001).
Table 1. Demographic, admission, treatment, and outcome in relation to pre-injury alcohol abuse
Slightly, but not significantly, more patients received treatment to improve hemostasis in the alcohol abuse group (31 (21%) vs. 113 (16%), p-value = 0.15). The specific treatments are described in detail in .
Table 2. Pre-injury conditions and treatments to improve hemostasis
Patients with a pre-injury alcohol abuse history were more often treated with craniotomies (52% vs. 43%, p-value = 0.05), less often with thiopental (3% vs. 9%, p-value = 0.04), and with a similar rate of decompressive craniectomy (4% vs. 7%, p-value = 0.16).
Pre-injury alcohol abuse in relation to intracranial hemorrhage/injury evolution
Patients with pre-injury alcohol abuse had an increased risk of significant intracranial hemorrhage/injury evolution (odds ratio (95% confidence interval) = 1.6 (1.1–2.3), p-value = 0.01). We found that pre-injury alcohol abuse was specifically associated with a lower degree of patients with a stable follow-up CT (41% vs. 54%, p-value = 0.007) and a higher degree of significant hemorrhage progression on follow-up CT (33% vs. 22%, p-value = 0.005), but a similar degree of emergency neurosurgery after the first CT (25% vs. 23%, p-value = 0.63) and death in the NICU after the first CT (1% vs. 2%, p-value = 0.67), as shown in .
Figure 1. Intracranial hemorrhage/injury evolution in relation to pre-injury alcohol abuse
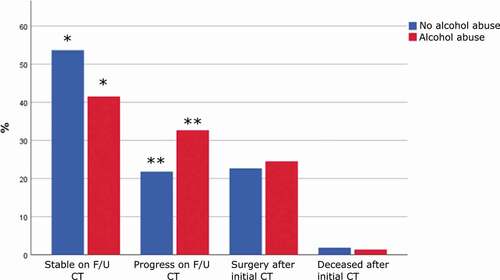
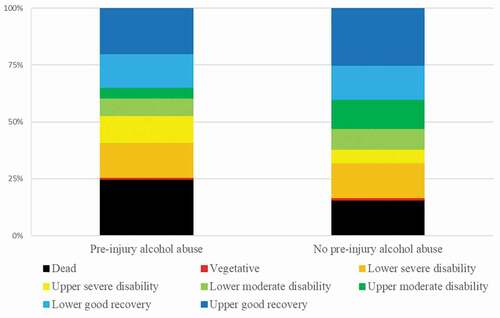
Figure 2. Clinical outcome (GOS-E) after 6 months in relation to pre-injury alcohol abuse
Pre-injury alcohol abuse in relation to mortality and favorable clinical outcome
Patients with pre-injury alcohol abuse had a higher mortality (25% vs. 16%, p-value = 0.01) and a lower degree of favorable outcome (47% vs. 62%, p-value = 0.002) 6 months after injury (). In a multiple logistic outcome regression analysis with mortality as the dependent variable and age, sex, CCI, chronic substance abuse, alcohol abuse, and mechanism of injury, alcohol abuse (odds ratio = 2.29, p-value = 0.003) was a significant predictor of mortality (). In as similar regression for favorable outcome, alcohol abuse (odds ratio = 0.55, p-value = 0.008) was independently associated with a lower rate of favorable outcome. This also held true after inclusion of the IMPACT core admission variables GCS M and pupillary abnormalities, in addition to a worse intracranial hemorrhage/injury evolution. Consistently, higher age, higher CCI, lower GCS M score, pupillary abnormalities, and intracranial hemorrhage/injury evolution were independently associated with increased mortality and decreased rate of favorable outcome. However, sex, chronic substance abuse, and mechanism of injury were not associated with mortality and favorable outcome.
Table 3. Mortality and favorable clinical outcome in relation to pre-injury alcohol abuse – multiple logistic regression analyses
Discussion
Alcohol abuse leads to increased susceptibility for unfavorable outcome after TBI. In the current study of 844 patients with severe TBI treated at our NICU, we found that pre-injury alcohol abuse was common (17%) and an independent predictor of increased mortality and reduced rate of favorable clinical outcome. Pre-injury alcohol-induced brain damage likely contributes to worse clinical outcome, but other mechanisms also play a part. In our study, these patients had worse coagulation status and increased intracranial hemorrhage progression. This highlights that optimizing hemostasis may be particularly important in these patients. However, pre-injury alcohol abuse remained a significant predictor for worse clinical outcome even after adjustment for hemorrhagic procession, indicating that other aspects are also important. Future studies are needed to better address specific challenges to improve outcome in these patients.
Pre-injury alcohol abuse and the risk of intracranial hemorrhage progression after traumatic brain injury
Development of intracranial hemorrhages after head trauma may lead to severe neurological sequelae and possibly death. Pre-injury co-morbidities, drug addiction, and TBI-induced coagulopathy could increase the risk of severe intracranial hemorrhage evolution(Citation34). Pre-injury alcohol abuse is associated with disturbances in both primary (thrombocytopenia and thrombocyte dysfunction) and secondary (coagulation factor deficiency) hemostasis (Citation10), although some studies indicate that alcohol at the time of trauma is associated with improved coagulation status (Citation4). In accordance with previous studies, we found that patients with pre-injury alcohol abuse both had lower thrombocyte levels and higher IVY, indicating disturbed primary hemostasis. However, this study also demonstrates that this pre-injury condition predisposed for an increased risk of significant intracranial hemorrhage/injury evolution with (odds ratio = 1.6) and more often required surgical evacuation with craniotomy (52% vs. 43%). These findings suggest a pathophysiological pathway of coagulopathy, intracranial hemorrhage evolution, and worse clinical outcome for patients with chronic alcohol abuse and TBI. However, our study lacks data on the level of acute alcohol intake and there remains uncertainty how this would contribute to the level of hemostasis.
Altogether, our results call for increased vigilance for detecting clinical deterioration due to hemorrhage progression that might require emergency neurosurgery in these patients. It is also necessary to further develop the evidence regarding management to optimize hemostasis after TBI. The CRASH-3 trial supported that tranexamic acid may be beneficial in some subgroups of patients with TBI (Citation35). However, more studies are needed to determine the optimal indications for different hemostatic agents (tranexamic acid, prothrombin complex concentration, platelets, desmopressin, etc.) in case of TBI and this may be particularly relevant for those with pre-injury alcohol abuse.
Pre-injury alcohol abuse and clinical outcome after traumatic brain injury
In the current study, we found that pre-injury alcohol abuse was independently associated with increased mortality and reduced rate of favorable outcome. These findings are in line with previous studies that have also found an increased mortality and a reduced rate of favorable outcome after TBI in these patients (Citation19,Citation20), although other studies have found little or no such associations (Citation21,Citation22). The role of alcohol in TBI pathophysiology and outcome prediction is complex. Alcohol intake in the acute setting may increase the risk of trauma, but low to modest doses may also be neuroprotective by a reduction in exctitoxicity, catecholaminergic response, better coagulation, and that may ultimately lead to better clinical outcome in the case of TBI (Citation3–7). However, for alcohol abuse, alcohol tolerance rather predisposes for increased excitotoxicity and catecholaminergic response (Citation3,Citation8). The long-term effects of alcohol may also worsen hemostasis and predispose for more severe post-traumatic intracranial hemorrhage evolution and also generate systemic co-morbidities with a reduced capacity to cope with severe trauma (Citation9,Citation13). Furthermore, these patients typically have a lower educational levels and alcohol-induced brain atrophy prior to the trauma that limit their capacity for recovery (Citation15–17). It has also been found that patients with alcohol abuse suffer from greater gray matter and hippocampal atrophy after TBI (Citation15,Citation18). Altogether, this supports that patients with TBI with pre-injury alcohol abuse are particularly vulnerable for TBI with a reduced chance of recovery. This also indicates the need for special attention for the risk of complications and the need for rehabilitation. Although intracranial hemorrhage evolution was one pathophysiological aspect, it cannot explain the entire association with worse outcome. There is hence a need to better target pathophysiological and social aspects in future research to improve the outcome of these patients.
Limitations
First, the inclusion criteria for the pre-injury alcohol abuse group were based on medical records and ICD codes, but no strict criteria or questionnaire were applied. Alcohol abuse was therefore considered as one entity, although it is rather a spectrum regarding daily alcohol intake and years of abuse. It is also possible that some patients with alcohol abuse were missed, if they had not sought medical attention for this condition before the injury. Second, our study focused on the NICU and included some important pre-injury variables, but alcohol abuse is also associated with other pre- and post-injury variables, such as socio-economic status, education level, and participation in rehabilitation, which could partly explain the association to worse clinical outcome (Citation36–38). Third, although tests for substances and alcohol were analyzed in many patients, this was not performed consistently. Due to the inconsistent testing and reporting of acute intoxications, we abstained from including such data into the study. Future studies are needed to determine if alcohol intake at the time of trauma could attenuate the relation to intracranial hemorrhage progression and worse clinical outcome in patients with pre-injury alcohol abuse. Fourth, groups 3 and 4 (emergency neurosurgery and immediate brain death after the first CT, respectively) in the categorization of intracranial hemorrhage/injury evolution, may represent progression of brain edema rather than hemorrhage progression in some cases, which limits the validity of the dichotomization of severe intracranial hemorrhage/injury evolution concept to some extent. However, pre-injury alcohol abuse was first and foremost associated with hemorrhagic progression of intracranial lesions on the second CT (group 2) compared to the other patients, which supports the detrimental effect on hemostasis in case of alcohol abuse.
Conclusions
Pre-injury alcohol abuse was a frequent co-morbidity in severe traumatic brain injury and independently associated with increased mortality and a reduced rate of favorable outcome. This was partly, but not exclusively, explained by worse intracranial hemorrhage evolution for these patients. However, it is likely that other pathomechanisms including pre-injury alcohol-related brain alterations, excitotoxicity, energy metabolic disturbances, systemic effects of alcohol abuse and participation in rehabilitation care also contribute to worse outcome. This calls for preventive measures of alcohol abuse and TBI, but also for future studies to address pathophysiological pathways and treatments that could improve the clinical outcome for these patients.
Acknowledgments
We express our gratitude to the personnel at the NICU, Uppsala University Hospital, for meticulous patient care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dikmen SS, Machamer JE, Donovan DM, Winn HR, Temkin NR. Alcohol use before and after traumatic head injury. Ann Emerg Med. 1995;26:167–76.doi:https://doi.org/10.1016/S0196-0644(95)70147-8.
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta neurochirurgica. 2006;148:255–68.doi:https://doi.org/10.1007/s00701-005-0651-y.
- Opreanu RC, Kuhn D, Basson MD. Influence of alcohol on mortality in traumatic brain injury. J Am Coll Surg. 2010;210:997–1007.doi:https://doi.org/10.1016/j.jamcollsurg.2010.01.036.
- Lustenberger T, Inaba K, Barmparas G, Talving P, Plurad D, Lam L, Konstantinidis A, Demetriades D. Ethanol intoxication is associated with a lower incidence of admission coagulopathy in severe traumatic brain injury patients. J Neurotrauma. 2011;28:1699–706.doi:https://doi.org/10.1089/neu.2011.1866.
- Türeci E, Dashti R, Tanriverdi T, Sanus GZ, Oz B, Uzan M. Acute ethanol intoxication in a model of traumatic brain injury: the protective role of moderate doses demonstrated by immunoreactivity of synaptophysin in hippocampal neurons. Neurol Res. 2004;26:108–12.doi:https://doi.org/10.1179/016164104773026633.
- O’Phelan K, McArthur DL, Chang CW, Green D, Hovda DA. The impact of substance abuse on mortality in patients with severe traumatic brain injury. J Trauma. 2008;65:674–77.
- Raj R, Skrifvars MB, Kivisaari R, Hernesniemi J, Lappalainen J, Siironen J. Acute alcohol intoxication and long-term outcome in patients with traumatic brain injury. J Neurotrauma. 2015;32:95–100.doi:https://doi.org/10.1089/neu.2014.3488.
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–40.
- Cowan DH. Effect of alcoholism on hemostasis. Semin Hematol. 1980;17:137–47.
- Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol. 2005;123(Suppl):S96–105.
- Tsitsopoulos PP, Marklund N, Rostami E, Enblad P, Hillered L. Association of the bleeding time test with aspects of traumatic brain injury in patients with alcohol use disorder. Acta neurochirurgica. 2020;162:1597–606.doi:https://doi.org/10.1007/s00701-020-04373-y.
- Ferguson RK, Soryal IN, Pentland B. Thiamine deficiency in head injury: a missed insult? Alcohol Alcohol (Oxford Oxfordshire). 1997;32:493–500.
- Jurkovich GJ, Rivara FP, Gurney JG, Fligner C, Ries R, Mueller BA, Copass M. The effect of acute alcohol intoxication and chronic alcohol abuse on outcome from trauma. Jama. 1993;270:51–56.doi:https://doi.org/10.1001/jama.1993.03510010057029.
- De Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest. 2010;138:994–1003.doi:https://doi.org/10.1378/chest.09-1425.
- Wilde EA, Bigler ED, Gandhi PV, Lowry CM, Blatter DD, Brooks J, Ryser DK. Alcohol abuse and traumatic brain injury: quantitative magnetic resonance imaging and neuropsychological outcome. J Neurotrauma. 2004;21:137–47.doi:https://doi.org/10.1089/089771504322778604.
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–12.doi:https://doi.org/10.1001/archpsyc.55.10.905.
- Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76:302–09.doi:https://doi.org/10.1016/S0003-9993(95)80654-7.
- Unsworth DJ, Mathias JL. Traumatic brain injury and alcohol/substance abuse: a Bayesian meta-analysis comparing the outcomes of people with and without a history of abuse. J Clin Exp Neuropsychol. 2017;39:547–62.doi:https://doi.org/10.1080/13803395.2016.1248812.
- Ruff RM, Marshall LF, Klauber MR, Blunt BA, Grant I, Foulkes MA, Eisenberg H, Jane J, Marmarou A. Alcohol abuse and neurological outcome of the severely head injured. J. Head Trauma Rehabil. 1990;5(3):21–31. doi:https://doi.org/10.1097/00001199-199009000-00006.
- Ponsford J, Tweedly L, Taffe J. The relationship between alcohol and cognitive functioning following traumatic brain injury. J Clin Exp Neuropsychol. 2013;35:103–12.doi:https://doi.org/10.1080/13803395.2012.752437.
- De Guise E, Leblanc J, Dagher J, Lamoureux J, Jishi AA, Maleki M, Marcoux J, Feyz M. Early outcome in patients with traumatic brain injury, pre-injury alcohol abuse and intoxication at time of injury. Brain Inj. 2009;23:853–65.doi:https://doi.org/10.1080/02699050903283221.
- Vickery CD, Sherer M, Nick TG, Nakase-Richardson R, Corrigan JD, Hammond F, Macciocchi S, Ripley DL, Sander A. Relationships among premorbid alcohol use, acute intoxication, and early functional status after traumatic brain injury. Arch Phys Med Rehabil. 2008;89:48–55.doi:https://doi.org/10.1016/j.apmr.2007.07.047.
- Elf K, Nilsson P, Enblad P. Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care. Crit Care Med. 2002;30:2129–34.doi:https://doi.org/10.1097/00003246-200209000-00029.
- Wettervik TS, Lenell S, Nyholm L, Howells T, Lewen A, Enblad P. Decompressive craniectomy in traumatic brain injury: usage and clinical outcome in a single centre. Acta neurochirurgica. 2018;160:229–37.doi:https://doi.org/10.1007/s00701-017-3418-3.
- Svedung Wettervik T, Lenell S, Enblad P, Lewén A. Pre-injury antithrombotic agents predict intracranial hemorrhagic progression, but not worse clinical outcome in severe traumatic brain injury. Acta neurochirurgica. 2021;163:1403–13.doi:https://doi.org/10.1007/s00701-021-04816-0.
- Nyholm L, Howells T, Enblad P, Lewén A. Introduction of the Uppsala Traumatic Brain Injury register for regular surveillance of patient characteristics and neurointensive care management including secondary insult quantification and clinical outcome. Ups J Med Sci. 2013;118:169–80.doi:https://doi.org/10.3109/03009734.2013.806616.
- Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–94.doi:https://doi.org/10.1016/j.jclinepi.2004.03.012.
- Marshall LF, Marshall SB, Klauber MR, van Berkum Clark M, Eisenberg HM, Jane JA, Luerssen TG, Marmarou A, Foulkes MAJ. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75:S14–S20.doi:https://doi.org/10.3171/sup.1991.75.1s.0s14.
- Shin SS, Marsh EB, Ali H, Nyquist PA, Hanley DF, Ziai WC. Comparison of traumatic intracranial hemorrhage expansion and outcomes among patients on direct oral anticoagulants versus vitamin k antagonists. Neurocrit Care. 2020;32:407–18.doi:https://doi.org/10.1007/s12028-019-00898-y.
- Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–05.doi:https://doi.org/10.1161/01.STR.27.8.1304.
- Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma. 1998;15:587–97.doi:https://doi.org/10.1089/neu.1998.15.587.
- Wilson JL, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85.doi:https://doi.org/10.1089/neu.1998.15.573.
- Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5. doi:https://doi.org/10.1371/journal.pmed.0050165.
- Maegele M, Schochl H, Menovsky T, Marechal H, Marklund N, Buki A, Stanworth S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16:630–47.doi:https://doi.org/10.1016/S1474-4422(17)30197-7.
- The CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet (London, England). 2019;394:1713–23. doi:https://doi.org/10.1016/S0140-6736(19)32233-0.
- Hoofien D, Vakil E, Gilboa A, Donovick PJ, Barak O. Comparison of the predictive power of socio-economic variables, severity of injury and age on long-term outcome of traumatic brain injury: sample-specific variables versus factors as predictors. Brain Inj. 2002;16:9–27.doi:https://doi.org/10.1080/02699050110088227.
- Calling S, Ohlsson H, Sundquist J, Sundquist K, Kendler KS. Socioeconomic status and alcohol use disorders across the lifespan: a co-relative control study. PloS One. 2019;14:e0224127.doi:https://doi.org/10.1371/journal.pone.0224127.
- Weil ZM, Corrigan JD, Karelina K. Alcohol use disorder and traumatic brain injury. Alcohol Rese. 2018;39:171–80.
