ABSTRACT
A theory is proposed that views emotional feelings as pivotal for action control. Feelings of emotions are valued interoceptive signals from the body that become multimodally integrated with perceptual contents from registered and mentally simulated events. During the simulation of a perceptual change from one event to the next, a conative feeling signal is created that codes for the wanting of a specific perceptual change. A wanted perceptual change is weighted more strongly than alternatives, increasing its activation level on the cognitive level and that of associated motor structures that produced this perceptual change in the past. As a consequence, a tendency for action is generated that is directed at the production of the wanted perception.
Why do we have emotional feelings? Scholars have debated this question for centuries, if not millennia of years. From a biological perspective, the psychological analysis of mental phenomena, such as emotional feelings, is always complicated by the fact that the mental operates distantly from the ultimate evolutionary criterion of reproductive fitness. To serve this evolutionary purpose, the immaterial mental (feeling) must be connected to the materialisation of a functional behaviour in the physical world (action) – a connection that immediately raises the issue of why evolution has bothered with emotional feelings. If the evolutionary function of fear is, for example, defence against a source of threat, why has evolution selected feeling-generating systems if activation of the defensive action is all that matters for survival? Generally asked: Why are we not just Cartesian beast machines, simply performing the action without any accompanying feeling?
The straightforward answer to this question in my opinion is that emotional feelings evolved because they were functional for action control. This opinion is, however, far from uncontroversial, because many “control functions’ could be hypothesised for emotions in general, and for feelings of emotion in particular. For example, theories have proposed that emotions (conceptualised as a set of processes distinct from feelings) trigger action either directly (e.g. LeDoux, Citation2014) or indirectly via mediation by motivational systems (e.g. Frijda, Citation2004). According to these accounts, the animal can act “emotionally” without intervention by feelings. Other accounts highlighted functional roles of emotional feelings as a source of information which, among other sources of information, is utilised for action choices and judgments, but does not trigger actions directly (e.g. Baumeister et al., Citation2007).
For a theory about functions of feelings for action control, it is essential to make explicit how the concepts emotion, feeling, and action are used. Like many others before me (Izard, Citation2010), I will use the term emotion as the “umbrella” concept that includes affective, cognitive, behavioural, expressive, and a host of physiological changes. Feeling of an emotion (or the synonymous term affect) refers here to the experiential component that is grounded in perceptions of patterned changes in the body or memories thereof (Damasio & Carvalho, Citation2013). I hence view feelings, more than anything else, as a special class of bodily perceptions processed by the central nervous system. Feeling an emotion is, however, never constituted by bodily perceptions alone, but is always integrated with perceptual representations of external objects or events. It is this multimodal integration process that accounts for the intentionality of feelings (i.e. feeling about something). Furthermore, feelings of emotions can be distinguished from other bodily perceptions by an intrinsic coding of the pleasantness of the perception. Theories of emotion causation have proposed several mechanisms for how this affective coding is implemented on the algorithmic level (for an overview see Moors, Citation2022). For example, embodiment theories of emotion claimed that stimulus evaluation is an integral part of bodily reactions that follow on the (non-evaluative) perception of the stimulus (e.g. Craig, Citation2015). Other theories proposed a separate set of evaluative stimulus appraisals that continually trigger somatic reactions (among other reactions), which is collectively experienced as a particular emotion (e.g. Scherer, Citation2009). The present framework is not committed to a specific process model of emotion causation – as long as it is assumed that emotional feelings are represented in an interoceptively accessible (perceptual) format. Furthermore, the generation of an action tendency (the explanandum) must be separable from stimulus evaluation processes (as part of the explanans) on the mechanistic level. Just like one can analyse the function of the perception of a red traffic signal for a driver without making specific assumptions about the qualia, or light source, of the colour “red”, one can then analyse the functional role of feelings of emotions for action control without making specific assumptions about the qualia, and origins, of the involved feeling state.
For the definition of action, I also make reference to representations on the mental level, using an approach borrowed from Jeannerod (Citation2006) and Searle (Citation1983). For a long time, philosophers of action have discussed the importance of mental representations for action, as epitomised in Wittgenstein’s famous question: “What remains if I subtract the fact that my arm went up from the fact that I raised my arm?” Jeannerod’s answer is: the action representation. The concept is best explained by contrasting it with perceptual representations. In the realm of perception, representation typically refers to the end-product of the perceptual process and it has a mind to world direction of fit: the representation describes an external event in the world. In the realm of action control, by contrast, the goal of the action which is represented in the mind corresponds to a possible state of the world that is created with the action. Contrary to perceptual representation, the action representation has a world to mind direction of fit: the representation prescribes a possible state of the world that follows from a particular action.
The concept of action representation has two important implications: First, action representations are anticipatory, not only with respect to the execution of the action itself, but also with respect to the world that is intended with the action. Secondly, the notion that an action representation precedes execution of the action suggests that it can be detached from execution. It is the hidden part of the action that exists when the arm in Wittgenstein’s puzzle does not go up.
Psychology of emotional action: fundamental issues and problems
Before I go into details, fundamental issues and challenges should be highlighted that in my opinion must be addressed by a theory of emotional action. The first two problems constitute general problems for the study of action control. The third issue is specific to the study of emotional action.
The degrees of freedom problem
When emotion scientists theorised in the past about the mental organisation of emotional action, they often referred to “motor programs’ that are triggered by specific emotional events. Keele (Citation1968) defined motor program as “a set of muscle commands that are structured before a movement sequence begins, and that allows the entire sequence to be carried out uninfluenced by peripheral feedback” (p. 387). In emotion science, the concept was popularised in theories about “innate neuromotor programs” (Tomkins, Citation1962), “species-specific defence behaviours” (Bolles, Citation1970), “instinctual motor outputs” (Panksepp, Citation2005), and “emotive action programmes” (Damasio, Citation1999). The common theme is that emotional stimuli (or more precisely: particular neurocognitive appraisals thereof) trigger motoric actions that are biologically fixed because they were functional in coping with specific, recurrent challenges in the evolutionary history of the species. This idea of an emotional “motor program” is intuitively attractive because it provides a straightforward explanation of emotional action control; however, it becomes dauntingly complex when thought through in detail. A particular problem is the so-called degree of freedom problem: Typically, there are many ways to perform a movement to achieve a goal, leaving the question of how the brain chooses a course of action among infinite ones. For survival in a complex and dynamically changing environment, actions must be adapted to the affordances of the situation, which requires a substantial degree of behavioural plasticity.
A theoretical solution to the degree of freedom problem is that action representations do not code contractions on the muscular level but, rather, distal features of the movement or classes of movements. Feature-based action coding can drastically reduce the processing and storage loads for the action system, for example, by parametrisation of the kinematics (positions in time) and the kinetics (forces in time) of the movement. Following off-line parametrisation, parameters are filled online by perceptual processes during movement execution (Milner & Goodale, Citation2006). Notably, this action coding process is not limited to kinematic and kinetic features and also includes the coding of action goals, including their semantics (Aziz-Zadeh & Damasio, Citation2008).
The perceptual-motor integration problem
Another theoretical challenge is how perception and motor control are combined: How does perception affect movement, and how does movement affect perception? In emotion science, analogous questions came up with the so-called “sequence problem”: What comes first – the emotional feeling or the emotional action tendency? According to William James (Citation1884), the action tendency, conceived of as a bodily reaction to an emotional stimulus, comes first, and the feeling (the emotion), which is the perception of the bodily reaction, is second. From a motor control perspective, however, the sequence problem is a pseudo problem because motor control and perceptual processes are inextricably intertwined: Perception informs motor planning, and bodily movements change the perceived environment (including internal body states) via physical action, about which the action control system is again informed by virtue of sensory feedback to the actions. Action control and perception hence form a closed loop, in which the sequence problem covers only half of the circle. For an adequate account, the complete perception-action cycle must be considered, which can be formally done with cybernetic control theory.
For the present theory, I will use a cybernetic framework called perceptual control theory (PCT) for an account of feeling-action interactions (Powers, Citation1973; see also Mansell, Citation2020). PCT views organisms as living control systems that act to maintain perceived aspects of their environment in pre-selected (goal) states. The purpose of behaviour is to produce a perceptual state that matches a set goal state – or as Powers simply put it: behaviour is the control of perception.
The control precedence issue
Since the seminal work of Charles Darwin (Citation1872), emotional actions are theorised to differ from mundane activities in fundamental ways. Frijda et al. (Citation2014) highlighted several characteristics of impulses to emotional action: (1) They have a specific purpose or aim that are grounded in basic motivational concerns; (2) they have a certain strength or urgency, and persist over time until the end state has been reached; (3) they can be elicited by appraisals of events without prior deliberation or conscious representation of some action goal. In short, emotions can seize control over the action system, gaining precedence of control over other actions for the satisfaction of a basic concern.
The theoretical construct of an emotion-driven, “impulsive” action is closely linked to the notion of automaticity, and as such, it inherits the problems associated with discrete views of automaticity. The difficulty becomes particularly obvious in discussions of the purposiveness or adaptiveness of emotional actions (Moors & Fischer, Citation2019). On the one hand, it is highlighted that emotional actions are purposive and functional for reaching a particular end state (e.g. flight, fight, or escape). This purposiveness would qualify the emotional behaviour as “goal-directed”. Yet, it is also emphasised that emotional action is an “involuntary” stimulus- or appraisal-driven response that does not require conscious planning or deliberations (e.g. a cost–benefit calculus). The challenge hence lies in the conceptualisation of an action-generating process that is purposive yet impulsive.
For the present theory, I will use modern ideomotor theory to achieve this aim (Hommel et al., Citation2001; for a related view see Ridderinkhof, Citation2017). Specifically, I will argue that actions are selected, instigated, and controlled by cognitive anticipations of sensory effects that were contiguously produced by the action in the past. A sensory action effect becomes selected as a goal for action if the anticipated state represents a significant improvement in comparison to the existing situation (Eder & Hommel, Citation2013). The state comparison, referred to here as “emotional weighting” of action effects, is driven by basic motivational mechanisms, presumably subserved by dopaminergic and serotonergic brain systems (Berridge, Citation2018). I view this situation-based, emotion-driven weighting process as the counterpart to an endogenous, intention-driven weighting process, which both constitute routes to action (Hommel & Wiers, Citation2017).
In the following, I will present cybernetic control theory as a general framework for the analysis of how emotional action becomes selected, instigated, and controlled. Specifically, I will refer to Perceptual Control Theory (PCT), which claims that the purpose of behaviour is the control of perception. Following a discussion of the perceptual basis of emotion and action, and their overlap on the representational level, I will discuss motivational mechanisms operating on these representations that arouse actions effecting approach to wanted perceptions and/or avoidance of unwanted perceptions. In a supplement to this article, I will also discuss how emotionally processed action effects are learned and a genetic transmission of perceptual biases that forms the basis of a biological preparedness for specific actions. In a nutshell, I will argue that the purpose of emotional action is the control of emotional feelings – the perceptual aspect of emotion – that function as a sensorium for the individual’s needs and concerns and how these are related to the current situation.
Perceptual control theory (PCT)
PCT was developed by the engineer William T. (Bill) Powers in the sixties as an alternative to the then-dominant behaviouristic stimulus-response model of human action (Powers, Citation1973). The theory has been revised several times and it is now applied in diverse research fields ranging from neuroscience to robotics (for a recent overview see Mansell, Citation2020). Like other cybernetic approaches, PCT distinguishes between input functions, output functions, and comparators that are located inside the organism (see ). The input function is carried out by the relevant sense organ(s) and responsible for coding the aspect of the environment to be controlled. It transforms energy from the environment into a continuous perceptual signal that is proportionate to the current amount of the controlled variable. The perceptual signal is subtracted from the reference signal, to create the error signal by the comparator. The signal is transformed by the output function. For example, it may be amplified (by a gain value) and is ultimately sent to muscles in the body. This signal is then transformed by a feedback function in the physical environment, shifting the controlled variable towards the desired direction. This change is sensed by the input function, and checked, inside the organism, by the comparator, which closes the loop.
Figure 1. Outline of a perceptual control system that uses actions on the physical environment to reduce the discrepancy between registered (p) and a wanted (p’) perceptual signals. Emotional feelings about perceptual changes code for the wanting of a simulated change, increasing the activation level of wanted perceptual codes and that of associated motor structures via ideomotor processes. s1 … sn = low-level sensory codes; m1 … mn = low-level motor codes; P = current perceptual state; P′1 … P′n = simulated perceptual states or events.
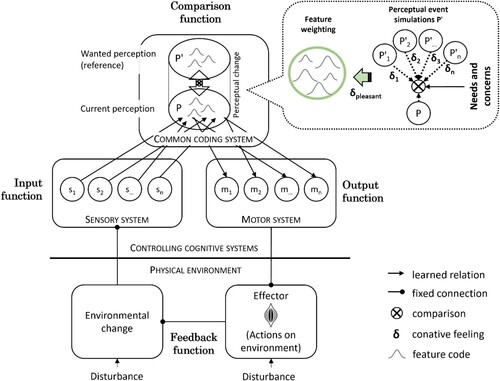
According to PCT, the purpose of a perceptual control system is to shift, and maintain, a to-be-controlled perceptual variable to a selected perceptual state by means of behaviour. For example, if a predator intends to catch a prey, the perceived distance to the prey would be the controlled variable that is reduced by movement towards the prey until the prey is in striking reach. In contrast, the prey will attempt to maintain a safe distance to the predator. The controlled perceptual variable thus is the same for both predator and prey, but the motivated actions are opposites because they serve different reference standards (establishing a small versus large distance, respectively). Understanding action control hence means knowing what perceptions are being controlled, how they are being controlled, and why.
The example above sketches the basic principle how behaviour is analysed with PCT. However, it is also an oversimplification because many perceptual signals must be controlled for adaptive action at a given time. For example, the lurking predator may close the distance to its prey by following a trail of smell or its distant sound. At the same time, the animal must move silently, and with appropriate speed, in the prey’s blind spot to catch it in surprise. For adaptive action control, several perceptual variables (maximising the smell; masked approach without making noise) must be controlled, and maintained, at the same time during the action episode. Meeting each selection criterion significantly narrows down the range of appropriate behaviours. Furthermore, the perceptual variables must be brought into an arrangement that reflects their relative importance, or “weight”, for action selection. For example, speed of movement could be prioritised in comparison to slow, sneaky movement, and probably even more so at the end of the predation episode when the prey is already close. The relevance of perceptual signals for action control hence can change dynamically during the episode change, reordering the control behaviour. According to the present account, feelings of emotion have a key function in this process because they determine which perceptual signal should be weighted more, or less, during action control.
Specifically, I propose that emotions influence the operation of control systems because emotional feelings, conceptualised as valued bodily reactions to a personally significant event, are also processed as perceptual signals in the brain. These interoceptive signals become integrated with ongoing perceptual activities from other senses (vision, audition, touch, etc.), coding for whether a perceptual event feels good or bad at a given instant in time. Importantly, an analogous evaluative coding process is also triggered during mental simulation of expected events, which generate future-oriented feelings that can be distinct from feelings about the current situation. By sensing differences in emotional feelings about perceptual states, the embodied mind creates a new perceptual signal (a “conative” feeling) that codes for the attractiveness or desirability of the event. If a perceptual change from one event to another feels pleasant, the perceptual change is coded as “wanted” or “desirable”. This coding increases the activation level of the selected perceptual representation, spreading activation to motor structures that generated this particular perceptual change in the past. Energised by this ideomotor process, a readiness for action is generated that is directed at the production of the selected perceptual change.
In the following sections, I will describe with more details the input, output and comparator functions proposed by the present theory. In closing, I will discuss the relationship between emotions and intentions, and a few testable implications of the theory that could be helpful for future avenues of research.
The input function: perceiving with feeling
According to the present theory, emotional feelings constitute a special class of perceptions that are grounded in physiological changes within the body accessible by interoceptive processes (Craig, Citation2015; Critchley & Garfinkel, Citation2017). Just as changes in the external world are displayed in dedicated sensory regions as neural maps of the external world (exteroceptive maps), afferent body signals feed into interoceptive body maps that encode, moment by moment, the physical state of the organism (Damasio & Carvalho, Citation2013). These interoceptive signals include information for homeostatic reflexes and allostatic control and they are inherently valued. In fact, people typically refer to internal bodily sensations in their reports of emotional experiences, with substantial consistencies across different cultures (Nummenmaa et al., Citation2014). The emotional quality of rudimentary interoceptive signals, or “raw feelings’, has been extensively discussed and a broad consensus has been reached that they represent graded intensities of pleasantness which form building blocks for more complex feeling states (Barrett & Russell, Citation2014). These feelings unfold dynamically during a given emotional episode.
Interoceptive signals (raw feelings) and sensory signals from other channels (e.g. taste, touch, vision) become multisensorially integrated to a representation about an external event. In fact, there is much cross-talk between neural channels of interoceptive information with exteroceptive information from other senses (including proprioception), at multiple nodes of the neuroaxis (Craig, Citation2015). After multimodal integration, the feeling is referenced to “something”, which could be an object or event, a state of affairs, or even a salient bodily reaction such as shortness of breath or a heart palpitation (Goldie, Citation2002). Research suggests several mechanisms for a binding of sensory information across space, time, and modalities that results in a unified perceptual representation (see e.g. Damasio, Citation1989; Singer, Citation2001). More important for the present discussion is the implication that interoceptive and somatic signals can enter these bindings as value signals, coding for the pleasantness of the perceived event (Critchley & Garfinkel, Citation2017). This means the individual can experience several feelings depending on the event to which attention is directed. In addition, intensities of pleasurable feelings about different events can be compared on a common metric (Levy & Glimcher, Citation2012).
Raw feelings are also evoked during imaginations and mental simulations of events. According to the bio-informational model of emotional imagery (Lang, Citation1979), mental imagery of emotional stimuli triggers physiological reactions that are comparable to the direct experience with the imagined stimuli. Systems previously involved in the emotional process are reactivated during the vivid recall of an emotional event, triggering associated parasympathetic and sympathetic reactions (for a review see Cuthbert et al., Citation1991). Damasio (Citation1999) proposed that bodily reactions to events can be simulated neurally, because mental imagery will make use of much the same neural substrates as perception in the same sensory modality. By simulation in the so-called “as-if body loop”, somatovisceral representations of bodily responses can guide decision-making without waiting for slow feedback from the peripheral physiology (“somatic marker hypothesis’; for a review see Poppa & Bechara, Citation2018).
Just like other perceptual contents, raw feelings become further processed on cognitive levels, being categorised and enriched by conceptual knowledge about the event. Barsalou (Citation1999) argued that these cognitive abstractions and high-level perceptions are grounded in re-enactments of previous sensorimotor experiences with these events. Specifically, it is proposed that knowledge about emotions and conceptual processing of emotional events is based on neural simulations of sensorimotor states in modality-specific brain areas, including simulations of interoceptive and proprioceptive states (Moseley et al., Citation2012). Based on these re-enactive simulations, it should make a striking difference whether a movement is situated as a distance-regulating motion “towards or away from something”, re-enacting an approach and avoidance movement, or as a vertical motion “in the upward or downward direction”, which presumably involves the re-enactment of action episodes related to ascension and decline (for evidence see e.g. Eder & Rothermund, Citation2008; Meier & Robinson, Citation2004). It should be highlighted that this grounded view of cognition is largely compatible with PCT. PCT proposes a hierarchy of perceptual systems levels in which controlled perceptual variables at lower levels (e.g. the intensity of force of a finger grip) provide the input for a regulatory loop at a higher level (e.g. the grasping of a tea cup) that can again serve as input for an even higher control loop (e.g. having tea time) and so on. By a hierarchical layering of perceptions, control of perception at higher system levels is grounded in the regulation of sensory signals at lower levels that operate on reference signals set by the output of the higher controller.
The described somatic version of the input function has notable gaps and challenges that could be only superficially addressed in this article. Firstly, it is not clarified which processes elicit somatic responses in the first place. They could be triggered by automatic appraisals of external stimuli, such as failing an exam, but also by effects of internal stimuli, such as hormone actions. This specification is however not necessary for the present theory as long as the resulting raw feeling is accessible to interoception. Secondly, the undifferentiated nature of somatic responses only allows for a coarse coding of pleasantness (Kreibig, Citation2010). Additional processes must come into play for a fine-grained differentiation into specific emotions. The present framework proposes a differentiation of emotional experiences by conative feelings: trajectories between pleasant and unpleasant states that feel different depending on the involved affective contrast (for details, see the comparator function below). Thirdly, individuals differ in the sensibility to signals from the body and how easily they become aware of a bodily reaction (Garfinkel et al., Citation2015). If emotional signals from the body can be perceived non-consciously and in the absence of focal awareness, this raises the issue whether feelings can be unconscious and which functional role consciousness has in the present framework. I will address these issues in a separate section (Emotion, Intention, and Consciousness).
To sum up the input function: After multisensorial integration with interoceptive feelings, a central representation of a perceived event is created that includes a crude affective coding of the event in terms of whether it is good or bad for the individual based on (simulated) interoceptive feelings. The format of the event representation is perceptual and the coding of the event is distributed across different sensory maps in the brain (including affective-interoceptive ones) that become loosely connected to what Hommel (Citation2004) called an “event file”. Event files are created by the processes implementing the input function, and as elaborated in the next section, they also form the representational basis for processes operating in the output function.
The output function: action with feeling
According to the action definition by Jeannerod (Citation2006), the goal of the action represented in the mind corresponds to a possible state of the world that is created with the action. This implies that perceptual states referring to possible world states are part of the mental action representation, and that these perceptual states are used for the preparation and control of the action. This is a key assumption of the Theory of Event Coding (TEC; Hommel et al., Citation2001), which I present here as a model for the output function.
TEC integrates the common coding hypothesis of Prinz (Citation1990) with the ideomotor principle developed by Lotze (Citation1852), Harleß (Citation1861), and James (Citation1890). Ideomotor theory suggests that actions are stored in memory by their sensory effects, and that action planning uses the anticipation of these effects to automatically retrieve the associated action. The common coding hypothesis proposes that cognitive representations of actions and sensory action effects share a common representational domain (i.e. a common code).Footnote1 Hence, TEC assumes that due to the representational integration of actions and their effects, anticipating the outcome of a certain action, or processing a stimulus that shares features with learned action effects, will automatically activate the corresponding action due to the bidirectional nature of the action-effect associations. This hypothesis was supported by many neurophysiological and behavioural findings (for reviews see Badets et al., Citation2016; Shin et al., Citation2010; Waszak et al., Citation2012).
The common coding principle has three general implications: (1) Motor processes should be involved in perceptual coding. This hypothesis is in line with numerous demonstrations of embodied cognitive processes in perception and action preparation (Barsalou, Citation1999; Pulvermüller & Fadiga, Citation2010). (2) The coding of perceptual contents in a shared representational domain should prime overlapping actions in the representational domain, inducing a readiness for action. This hypothesis is in line with research on so-called “mirror neurons” in the premotor cortex that discharge both when individuals perform a given motor act and when they observe other perform that same motor act (Bonini et al., Citation2022). (3) An action is prepared by the selection of a perceptual event that the person intends to create with the action (Prinz et al., Citation2004).
The extension of the ideomotor and common-coding principles to feelings suggests that feelings can become an integrated part of the action representation when the feeling is contingent upon the production of the behaviour. This assumption is central to Damasio’s somatic marker hypothesis (Poppa & Bechara, Citation2018). According to Damasio, action decisions become associated with the somatic reactions produced by the outcome of the action, forming a “somatic marker signal” that will guide decision-making if retrieved during the decisional phase. In pioneering studies (Bechara et al., Citation1994, Citation1997), patients with prefrontal brain lesions performed disadvantageously in a simple card game with punitive losses presumably because they failed to develop anticipatory somatic reactions to the threat of a loss. The somatic marker hypothesis hence suggests that memorised emotional consequences of actions are actively recruited before making a decision (for a discussion of similarities between decision-making and action selection processes see Wolpert & Landy, Citation2012).
Automatic retrieval of integrated action effect knowledge during action selection was also demonstrated with studies that used a two-stage research paradigm in which participants first learned to associate particular actions (e.g. a keypress) with a specific emotional consequence (e.g. the administration of a painful electric shock). In a subsequent second stage, stimuli resembling the emotional consequence were then presented as response cues and the time needed to initiate the action was recorded. Several studies found that actions linked to a pleasant response effect were initiated faster, or more frequently, in response to pleasant compared to unpleasant a response cue, and vice versa with actions producing an unpleasant action effect (Beckers et al., Citation2002; Eder et al., Citation2015; Strohmaier & Veling, Citation2019). Latter priming of negative action effects clearly suggests a priming of affective-sensory aspects of emotional feelings and not of motivational components.
Once it is accepted that codes representing feelings can become linked to action structures in a common coding area, cross-talk between these codes should be observed within and across perceptual and action-related modalities. In line with this hypothesis, studies found perception-action (Eder & Rothermund, Citation2008), action-perception (Eder & Klauer, Citation2007, Citation2009), and action-action (Eder et al., Citation2012) interactions on affective dimensions. Affective codes hence are shared between perceptual and action-related representations, and processes selecting actions consider codes that predict affective consequences of the action (for evidence see Eder et al., Citation2017).
To sum up this section, TEC can provide a model of the output function in line with the assumptions of PCT. Specifically, TEC’s ideomotor hypothesis suggests that an action is selected by the activation of its associated sensory effect. This means the action control system generates motor commands indirectly by specification of appropriate perceptual inputs, which is a central tenet of PCT. The present theory extends this account to emotional perceptions, proposing that emotional feelings that come after, or are produced by the behavioural activity, become linked to the cognitive action representation controlling the activity. Like other features of action outcomes, these integrated emotional effects are retrieved during action selection, affecting the choice of action.
While TEC offers solutions to the degrees-of-freedom and perceptual-motor integration problems, it does not address the control precedence issue. In fact, the classic framework treats action effects from different sensory modalities in the same way and does not distinguish between motivational qualities of action effects (Pfister, Citation2019). Ideomotor theory hence can explain how a motor activity is selected to achieve a particular effect (the action goal); however, it does not explain why that action effect was selected as a goal for action in the first place. Accordingly, additional processes must come in play that prioritize a specific action effect over other action effects available for action control. This set of processes is described as comparator function in the next section.
The comparator function: striving for a better feeling
According to PCT, the purpose of action is to reduce discrepancies between wanted and registered signals until the desired perception is reached. This cybernetic principle implies that the representational basis for action control is not so much a singular perception but, rather, a comparison between perceptual states. A similar conclusion was also reached in recent theorising on ideomotor mechanisms (Kunde et al., Citation2017). A problem associated with the classic ideomotor account is the so-called “circular reflex”: If motor actions are represented in terms of perceptual outcomes, perception of the generated action effect should reactivate the associated motor pattern, producing an infinite cycle of action priming that must be laboriously interrupted by an external stopping mechanism (Greenwald, Citation1970). An alternative proposal is that the ideomotor system operates on discrepancies between current and intended perceptions, namely the intended perceptual change. This means, representations of action effects code not only for the intended sensory effect but, even more so, for the transition from a current to an anticipated perceptual state. For example, intending to turn on a room light will refer to perceptual codes that specify a perceptual transition from dark to bright, generating an action tendency to turn on the light only if the room is dark but not when the room is already well-lit.
The ideomotor theorising about effect codes specifying a perceptual change fits with PCT’s claim that the purpose of action control is to reduce discrepancies between registered and intended perceptual states. Furthermore, it is also consistent with motivational models that claim that goal-directed action is driven by the expectancy of an action outcome that is desired by the individual (de Wit & Dickinson, Citation2009). In the present framework, expectancy of action effects is grounded in ideomotor cognitions that code for sensory changes produced by specific actions. The desire of an effect is grounded in evaluative operations of the comparator function that computes the attractiveness of a simulated sensory change in respect to the individual’s needs and concerns.
In the following, I will describe neuropsychological mechanisms how a sensory change is transformed to an incentive for action. Based on the affective operations in the input function, valuations of perceived and anticipated or simulated perceptual states produce dynamic difference signals that represent whether feelings will improve, be constant, or deteriorate, from the present to the simulated state. The comparator function uses this feeling-based difference signal as an internal readout whether a sensory change is desirable. I will refer to these readouts as “conative feelings’ because they represent feelings about the attractiveness, or “wanting”, of a specific perceptual change.
Organised by principles of hedonic contrast (Flaherty, Citation1996), conative feelings can vary in quality as well as in intensity. Specifically, a pleasant conative feeling is produced by changes from neutral to relatively pleasant states, changes from pleasant to even more pleasant states, or changes from an unpleasant to a relatively neutral or pleasant state. Unpleasant conative feelings code for changes in the opposite direction: from a pleasant or neutral state to an unpleasant state, or from an unpleasant to a state that is even more unpleasant. In short, conative feelings are meta-feelings about feeling differently in specific states, coding for a change in feeling. By the wanting of a perceptual change that feels better or best among a set of alternatives, living systems can select actions via the output function that have the potential to bring about an improvement.
shows a classification scheme of pleasant and unpleasant conative feelings based on expectations of an improvement or decline relative to the current situation (for a similar scheme based on reinforcement contingencies, see Rolls, Citation2005). Anticipated positive and negative events could be rewards and punishments, respectively, or the omission and expected termination of these events. It should be noted that the scheme is neither an exhaustive map of all emotional feelings nor is it meant to be a dimensional scheme. Some emotions do not fit neatly into the proposed classification scheme (e.g. surprise), and others have relations to several types of events (e.g. anxiety). Accordingly, the scheme only portrays logical possibilities of ways in which predictions of emotionally relevant events may be related to conative feelings.
Figure 2. Some emotions associated with predictive processes about positive and negative events or their termination and omission. A pleasant conative feeling is generated by the anticipation of an improved state and an unpleasant conative feeling by the expectation of a declined state with respect to the individual’s current needs and concerns. Intensity of the conative feeling depends on the magnitude of the feeling change and on the estimated probability of the event (i.e. large changes and likely events generate more intense feelings). Figure modified after Rolls (Citation2005, p. 14).
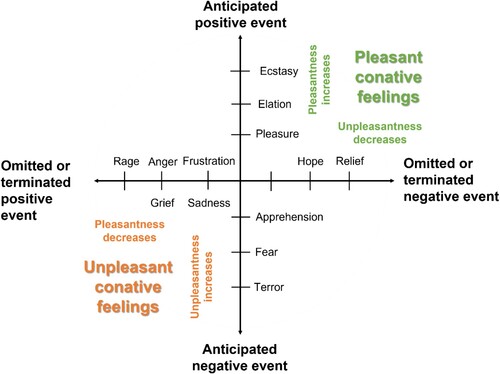
Dimensionality is however assumed for feeling changes based on hedonic contrasts. This means feeling better will inevitably lead to feeling less worse and vice versa. Intensities of conative feelings will also change with perceptual events during an emotional episode. For example, the terror of getting attacked by a sighted dog will increase with perceptions that the dog is approaching (a negative event). Conversely, the relief about keeping the scary dog at bay (the avoidance or omission of a negative event) will increase with perceptions of increased distance. Operating in this way, feelings about perceptual changes that have happened or that are expected to happen during an episode function as internal readouts whether the situation has improved or will improve from one moment to the next.
Several cognitive mechanisms can generate conative feelings, with mental imagery and expectancies based on previous experience being the most important ones. According to grounded views of cognition (Barsalou, Citation1999), knowledge about an object is based on simulations of previous sensorimotor interactions with that object. Papies et al. (Citation2020) extended this framework to temptations and desires, proposing that external cues will activate nonconscious simulations, or re-enactments, of previous sensorimotor interactions with that object. For example, reading about an attractive food will activate mental images of eating and enjoying consumption of that food. An analogous cognitive simulation of anticipated emotional experiences is also involved in fear conditioning. According to Lang’s (Citation1979) bio-informational model, mental imagery of emotional events elicits physiological reactions that are comparable to direct experience with the imagined stimuli. In line with this model, fear conditioning studies demonstrated that CS automatically evoke perceptual mental images as conditioned responses that trigger autonomic responses which can potentiate, or diminish, the conditioned emotional reaction (for a review see Mertens et al., Citation2020).
Conative feelings can be generated quickly using a neural simulation mode, described by Damasio (Citation1999) as the “as-if-body loop”, or they can change the body milieu directly and more slowly, by triggering a complex set of peripheral reactions in the body. The body reaction is further processed by the brain, in line with the input function, and in preparation of the organism for the upcoming challenge or task (a process described as “allostasis’; Schulkin & Sterling, Citation2019). This primitive set of “Pavlovian” emotional reactions, however, has only a global effect on the body milieu and is not generative of an “instrumental” action impulse tailored to a specific situational challenge.
For this tailoring, the “wanting signal” generated by conative feelings must first be relayed to the output function for a specification which perceptual change is selected as a target for action control. It is proposed that conative feelings, generated during the simulation of a perceptual change, become integrated with the mental representation of the simulated effect, coding for whether the perceptual effect represents an improvement (pleasant conative feeling) or “not wanting” (unpleasant conative feeling) of the associated perceptual effect. Specifically, a sensory change that is coded by a pleasant conative feeling as “wanted”, or “attractive”, is weighted more strongly, so that perceptual codes on dimensions that are constitutive to the wanted sensory change are activated more strongly. Weighted sensory codes will then automatically activate the associated motor code stored in ideomotor memory, priming actions that lead to a desired perceptual effect. In contrast, a sensory change marked by an unpleasant conative feeling as “not wanted”, or “repulsive”, can affect the activation level of the associated perceptual representation only indirectly by rendering alternative perceptions more attractive. Via hedonic contrasting with an unpleasant conative feeling, even neutral perceptual changes become attractive in a relative comparison, which increases their respective activation weights. As a consequence, the alternative perceptual effect is selected as a target for action and alternative actions become suppressed via lateral inhibition (Mostofsky & Simmonds, Citation2008). This account of avoidance behaviour is in line with classic research on fear and avoidance learning (e.g. McAllister & McAllister, Citation1992), although the explanations differ in the underlying assumptions (for a detailed discussion see the supplement).
The “emotional weighting mechanism” described above explains how a perceptual event is singled out as a target for action control. A plausible neurophysiological correlate of this process could be the dopaminergic coding of action outcomes in the midbrain. Many neuropsychological studies demonstrated that dopamine neurons in the midbrain are activated by rewarding events that are better than predicted, remain uninfluenced by events that are as good as predicted, or are depressed by events that are worse than expected (for reviews see Bromberg-Martin et al., Citation2010; Schultz, Citation2007). The noted relativity of the dopamine response fits with the present theorising about a generation of conative feeling by comparative processes, although the exact relationship between reward processing and emotional feelings is not clear (Sander & Nummenmaa, Citation2021). The “incentive salience hypothesis’ additionally proposed that dopaminergic activities would also underlie the transformation of a sensory perception into a “wanted” perception. Berridge and Valenstein (Citation1991) described this process as “the active assignment of salience and attractiveness to visual, auditory, tactile, or olfactory stimuli that are themselves intrinsically neutral” (p. 9). By incentive salience attribution, a perceptual event could hence be transformed from a mere sensory representation into a “wanted” perception that grabs attention and motivates primitive appetitive reactions (Warlow & Berridge, Citation2021). It must be highlighted, however, that the incentive salience hypothesis also suggested that the “wanting” system, subserved by dopaminergic activities in the midbrain, is separable from a “liking” system, underlying the hedonic experience of a reward. This distinction appears to be at odds with the present theorising about a functional role of feelings for action strivings. A solution is that only specific feelings, here identified as conative feelings, are connected to wanting. Consistent with this argument, a large review of human studies concluded that expected pleasantness is a confounding factor in measures of wanting and liking, arguing that anticipatory affects – and not consummatory reactions – are systematically related to wanting states (Pool et al., Citation2016). With this reservation, hedonic hotspots underlying anticipatory feelings could have the central function for the control of behaviour in line with the present theorising.
To sum up, conative feelings, presumably subserved by dopaminergic brain processes, code perceptual representations for their attractiveness. This basic motivational coding is already capable of triggering rudimentary organismic changes along an appetitive-aversive axis that, after integration with other perceptual activities, are registered as a wanting of, or aversion to, a specific percept. In the PCT framework, the wanting signal serves as a reference for perceptual changes induced by ideomotor actions that were in the past effective in controlling the perceptual signal.
Emotion, intention, and consciousness
The present theory describes general-purpose mechanisms that are not specifically about “emotional actions”, however they are identified, but about action motivation in general. In fact, many of its assumptions were derived from research on reinforcement learning and reward processing and can hence be applied to motivational phenomena as well. The theory eschews commonly used, typically binary, classifications of actions into instrumental versus emotional, goal-directed versus habitual, or controlled versus automatic that appear to have a stronger grounding in the behaviour analyst’s head than in the head of the analysed person (cf. Hommel & Wiers, Citation2017). According to the present approach, the action-generating machinery is the same for generated actions during emotional and non-emotional episodes and only the mode of operation will differ between these episodes.
The present framework hence posits that all human action is ultimately motivated by sensations of pleasant and unpleasant affects. This hedonic account is, however, not without problems, because people often engage voluntarily in unpleasant yet necessary activities (Taquet et al., Citation2016). Sometimes they even strive for unpleasant emotions if instrumental for the task at hand, for example, when preparing for a confrontation (Tamir, Citation2016). Accordingly, maximisation of pleasure and minimisation of pain is not always the proximal goal of human behaviour and other perceptual features can be intentionally weighted higher than feeling better at the next moment.
Importantly, this intentional “reweighting” of wanted action effects can be described with a unitary mechanistic framework: “emotional” action is spurred bottom-up by conative feelings that prime perceptual codes associated with a motor action (the “action effect”). In “voluntary” action control, by contrast, a sensory action effect is selected in a top-down fashion via endogenously initiated weighting mechanisms (Memelink & Hommel, Citation2013). Because both mental routes have access to the same perceptual codes, they can interact during action control, producing conflicting or synergetic action tendencies. Furthermore, emotional feelings, conceptualised as registered signals from the body, can be selected intentionally as a target for action for the purpose of emotion regulation (Tamir, Citation2016). This means, the feeling itself can become a controlled variable in a perceptual control system that has the purpose to increase or decrease a particular feeling quality. Conative feelings operating in this control system would then function as meta-feelings that code for the attractiveness of feeling better or worse. Following Lewin’s (Citation1926) theorising about intentions as “quasi-needs’, one can even speculate that intentional processes recruit feeling-generating systems for selective motivation of action. In fact, many studies showed that signals perceived during action control are affectively processed with respect to whether they are beneficial or obstructive for reaching set goals (see e.g. Brendl et al., Citation2003; Ferguson, Citation2007). Accordingly, affective processes are an integral part of the cognitive machinery underlying voluntary action control, in line with a unitary system for action control.
An issue not discussed so far concerns the role of consciousness and whether feelings of emotion must be conscious to influence behaviour. With the grounding of phenomenal feelings of emotions in (neuro-)physiological states, it can be argued that feelings can be sensed and processed without being aware of them. In fact, research demonstrated that individuals differ in the extent of which they are sensitive to and become aware of internal body states (Garfinkel et al., Citation2015). Damasio (Citation1999) distinguished between “having a feeling” – essentially the neural representation of the bodily changes that constitute the emotion – and “feeling a feeling,” a matter of knowing that one has that feeling, which involves relating the feeling to the sense of self. Lambie and Marcel (Citation2002) made a similar distinction between first-order phenomenological experience (what it’s like) and second-order awareness of it (a kind of knowledge). These distinctions entail that people can have phenomenal experiences of emotions without being aware of them. However, the question is whether neural patterns subserving interoception will qualify as “emotional” in the sense that they can create a hedonic experience characteristic of emotions. After all, a strong heartbeat in isolation is not sufficient to create an emotional experience. For this experience, a binding process appears to be necessary that ties interoceptive and exteroceptive elements together to a unified experience. Singer (Citation2001) proposed that this phenomenal experience requires a binding of perceptual features distributed in the brain by neural synchronisation processes. This binding functions independently of conscious awareness, but it is facilitated by attentional processes. Computational results provided by binding at lower brain levels are relayed to higher levels for monitoring functions. Higher cortical areas treat output of lower-order cortical areas in the same way as these treat input from the sensory periphery. This idea of a cascaded processing of perceptual results largely corresponds with the hierarchical layering of perceptual systems proposed by PCT.
Feelings of emotions, generated by the integration of information from the body, hence can be generated at low levels without being consciously aware of them. These unconscious “raw feelings’ are then relayed to higher levels for further refinement, where they eventually become conscious (Cunningham et al., Citation2013). The interesting question is whether raw feelings can be relayed to the common coding system for the generation of an action tendency without reaching consciousness. Empirical research suggests that this is indeed possible. For example, masked presentations of affective stimuli can activate behavioural responses even when they were presented below the threshold of consciousness (see e.g. Klauer et al., Citation2007). These unconscious response activations are however conditional because they depend on specific preparatory states, such as the pre-existing intention or action desire to react to the (masked) signal with a particular behaviour (Kunde et al., Citation2003). This means, once a perceptual system was set, even a complex action could be activated by perceptual signals without further conscious intent. Affective signals are no exception, and they could have energising properties that go beyond the mere activation of pre-configured stimulus-response mappings (for a discussion see Custers & Aarts, Citation2010; Winkielman & Berridge, Citation2004).
Implications of the theory
In this section, I will describe a few notable implications of the present theory that could be useful for future avenues of research.
The theory claims that emotional action is preceded and controlled by predictions of associated sensory effects. This predictive process could be studied with measurements of action-specific perceptual representations before action initiation. For example, Dignath et al. (Citation2020) recorded brain activity before an action choice using electroencephalography. Each action produced a distinctive flickering visual action effect. Results showed neural patterns characteristic of the associated flickering action effect before initiation of the action. Using related approaches, it is possible to study predictive processes of emotional action effects without resort to self-report measures, and how these predictions are causally involved in the production of the behaviour.
Another claim of the theory concerns the role of learning and how affective and sensory properties of action outcomes affect action selection. A powerful research paradigm with this respect is the so-called Pavlovian-to-instrumental transfer (PIT) paradigm. In the outcome-selective variant of this paradigm, organisms first learn to associate stimuli and actions with particular rewards (e.g. sucrose and food pellets) in separate Pavlovian and operant conditioning phases. In a subsequent transfer phase, action choices are then made in the presence of a stimulus cue for a particular outcome and in extinction. A typical finding is that humans and animals work harder to obtain the reward that was signalled by the stimulus cue (for reviews see Cartoni et al., Citation2016; Holmes et al., Citation2010). The PIT task can be used for a study of outcome-specific routes to action, mediated by sensory properties of the outcome (e.g. the colour of a food reinforcer), as well as for a study of outcome-general appetitive and aversive motivations, mediated by the affective-hedonic properties of the outcome (e.g. the hedonic value of the food). By the joint analysis of affective and sensory features of outcomes, the PIT paradigm provides an ideal testbed for the study of multimodal outcome representations and how affective and sensory features become integrated during action choice.
A novel assumption of the present theory concerns the role of conative feelings that index wanted perceptual changes. Accordingly, analyses of emotional actions should take the dynamic unfolding of feelings during an action episode into account. This process can be examined with the study of the emotional antecedents and consequences of actions, and how the timing of these events is related to action motivation. For example, a perceptual event that signals the onset of pain (a negative event) will evoke unpleasant feelings, whereas the same event will trigger a relatively pleasant feeling (relief) if it signals the termination of pain. If action is motivated by a predicted change towards a relatively pleasant feeling state after action execution (the conative feeling), then perceptual events associated with relief should acquire motivational properties (for evidence see Kim et al., Citation2006; Yarali et al., Citation2008).
Defensive action can take on many forms, ranging from active behaviour to withholding a particular action (passive avoidance). A particular challenge is the analysis of passive avoidance. According to the present framework, a deliberate nonaction should be coded in a similar way in terms of sensory effects that are contingent upon not acting (Kühn et al., Citation2009). This means, events contingent upon intentionally withholding an action (e.g. the non-detection by a predator) should be mentally represented during the choice for a nonaction.
A related challenge concerns learning of punishments and the suppression of action after punishment. There is evidence that a rewarding action effect becomes stronger linked to the producing action (Eder, Erle, et al., Citation2020); hence, it is possible that ideomotor cognitions with adverse action outcomes are less stable. Nevertheless, knowledge about adverse action outcomes is often expressed in the correction of maladaptive behaviour, which indicates learning of punishment. Guthrie (Citation1935) argued that “punishment achieves its effects … by forcing the animal or the child to do something different” (p. 158; as cited in Church, Citation1963). The present framework makes a similar proposal. In comparison with the adverse action outcome, effects of alternative actions become more attractive, in line with the suggested principle of hedonic contrast. Spurred by the generated conative feeling, the action producing an adverse action outcome is overridden by the alternative action tendency, resulting in a reduced frequency of the punished action. According to this framework, a punished action is not actively suppressed but only indirectly by the prioritisation of a competitive action.
From a PCT perspective, understanding behaviour is largely a matter of identifying the perceptions that organisms want to control. A test for discovering these input variables is the Test for the Controlled Variable (Marken & Mansell, Citation2013). This test requires a hypothesis about a proposed variable an individual is controlling and then systematically applying disturbances or perturbations to the variable while observing the responses of the individual. For example, if an aggressive person intends to harm another person, the purpose of her actions is to inflict harm and the only way how she can know whether she has succeeded with harm-doing is to monitor the target of aggression for perceptual indications of inflicted harm. Depending on the circumstances, perceptual indicators of harm-doing can be expression of suffering (e.g. a moan), signs of physical injuries (e.g. a bruise), or symbols indicating social damage (e.g. a derogatory commentary from others), among many others. Which perception of harm is actually controlled with the aggressor’s behaviour can then be inferred by variations of the hypothesised perceptual states, which could be, depending on the specific hypotheses, perceptions of the other’s facial display, perceptions of injuries, and so forth (see e.g. Eder, Mitschke, et al., Citation2020). Intentions underlying emotional behaviour hence can be inferred by systematically applying perturbances to perceptions that are hypothesised to control the behaviour.
Conclusion
In the introduction to this article, I identified three basic challenges to a theory of emotional action: the degree-of-freedom problem, the perceptual-motor integration problem, and the issue of control precedence. The proposed PCT model addresses these problems in the following ways: (1) a bodily movement is not coded on a muscular level but on a distal level in terms of the perceptual changes that should be produced with this movement. This distal coding principle allows for flexibility in the choice of action, because depending on the situation, different movements can effect similar perceptual changes (equifinality) and similar movements can lead to different perceptual changes (multifinality). The specification of a muscular pattern hence will depend on the prediction which perception it will produce. (2) Codes of sensory effects are an integral part of action representations (common-coding principle) and an action is selected indirectly by activation of the sensory effect that is contingent on the action (ideomotor principle). Action and perception are thus inextricably fused, forming a closed loop in which actions change perceptions that control the impulse for new action (perceptual control system). (3) Emotional feelings are valued signals from the body that become multimodally integrated with perceptual contents from other senses. By coding the attractiveness, or “wanting”, of a (simulated) perceptual change, conative feelings influence the salience and activation weights of selected perceptual feature maps (incentive salience attribution), selecting which perceptual feature or aspect of the sensed environment should be controlled with behaviour. By highlighting which perceptual change is most desirable for the current situation, emotional feelings can prioritize specific actions over others.
Emotional feelings, and conative feelings in particular, hence play a dual role in to the present theory. Conceptualised as interoceptive signals from the body, they operate as sensations that become continuously integrated with perceptual activities from other senses. Conceptualised as signals of value, they function as an inner sensorium for the current wants and needs of the person and about the progress that was made in addressing these concerns. It is this dual role why living systems experience feelings of emotion: by feeling the future they spring into action.
Supplemental Material
Download MS Word (54.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes
1 The term “code” refers here to the internal representation of features of perceived objects or planned movements. For example, the spatial position of a perceived tea cup could be internally coded as “LEFT” when it is located on the left side relative to the mid-sagittal body line (egocentric frame) or as “RIGHT” to one’s left hand that is used for preparation of grasping the tea cup (allocentric frame). It is assumed that relevant or salient features of the perceived or to-be-produced event become temporarily linked together to networks subserving object identification and/or action preparation (Hommel, Citation2004).
References
- Aziz-Zadeh, L., & Damasio, A. (2008). Embodied semantics for actions: Findings from functional brain imaging. Journal of Physiology-Paris, 102(1), 35–39. https://doi.org/10.1016/j.jphysparis.2008.03.012
- Badets, A., Koch, I., & Philipp, A. M. (2016). A review of ideomotor approaches to perception, cognition, action, and language: Advancing a cultural recycling hypothesis. Psychological Research, 80(1), 1–15. https://doi.org/10.1007/s00426-014-0643-8
- Barrett, L. F., & Russell, J. A. (2014). The psychological construction of emotion. Guilford Publications.
- Barsalou, L. W. (1999). Perceptual symbol systems. Behavioral and Brain Sciences, 22(4), 577–660. https://doi.org/10.1017/S0140525X99002149
- Baumeister, R. F., Vohs, K. D., DeWall, C. N., & Zhang, L. (2007). How emotion shapes behavior: Feedback, anticipation, and reflection, rather than direct causation. Personality and Social Psychology Review, 11(2), 167–203. https://doi.org/10.1177/1088868307301033
- Bechara, A., Damasio, A. R., Damasio, H., & Anderson, S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1–3), 7–15. https://doi.org/10.1016/0010-0277(94)90018-3
- Bechara, A., Damasio, H., Tranel, D., & Damasio, A. R. (1997). Deciding advantageously before knowing the advantageous strategy. Science, 275(5304), 1293–1295. https://doi.org/10.1126/science.275.5304.1293
- Beckers, T., De Houwer, J., & Eelen, P. (2002). Automatic integration of non-perceptual action effect features: The case of the associative affective Simon effect. Psychological Research, 66(3), 166–173. https://doi.org/10.1007/s00426-002-0090-9
- Berridge, K. C. (2018). Evolving concepts of emotion and motivation. Frontiers in Psychology, 9, 1647. https://doi.org/10.3389/fpsyg.2018.01647
- Berridge, K. C., & Valenstein, E. S. (1991). What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behavioral Neuroscience, 105(1), 3–14. https://doi.org/10.1037/0735-7044.105.1.3
- Bolles, R. C. (1970). Species-specific defense reactions and avoidance learning. Psychological Review, 77(1), 32–48. https://doi.org/10.1037/h0028589
- Bonini, L., Rotunno, C., Arcuri, E., & Gallese, V. (2022). Mirror neurons 30 years later: Implications and applications. Trends in Cognitive Sciences, 26(9), 767–781. https://doi.org/10.1016/j.tics.2022.06.003
- Brendl, C. M., Markman, A. B., & Messner, C. (2003). The devaluation effect: Activating a need devalues unrelated objects. Journal of Consumer Research, 29(4), 463–473. https://doi.org/10.1086/346243
- Bromberg-Martin, E. S., Matsumoto, M., & Hikosaka, O. (2010). Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron, 68(5), 815–834. https://doi.org/10.1016/j.neuron.2010.11.022
- Cartoni, E., Balleine, B., & Baldassarre, G. (2016). Appetitive Pavlovian-instrumental Transfer: A review. Neuroscience & Biobehavioral Reviews, 71, 829–848. https://doi.org/10.1016/j.neubiorev.2016.09.020
- Church, R. M. (1963). The varied effects of punishment on behavior. Psychological Review, 70(5), 369–402. https://doi.org/10.1037/h0046499
- Craig, A. D. (2015). How do you feel? An interoceptive moment with your neurobiological self. Princeton University Press.
- Critchley, H. D., & Garfinkel, S. N. (2017). Interoception and emotion. Current Opinion in Psychology, 17, 7–14. https://doi.org/10.1016/j.copsyc.2017.04.020
- Cunningham, W. A., Dunfield, K. A., & Stillman, P. E. (2013). Emotional states from affective dynamics. Emotion Review, 5(4), 344–355. https://doi.org/10.1177/1754073913489749
- Custers, R., & Aarts, H. (2010). The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science, 329(5987), 47–50. https://doi.org/10.1126/science.1188595
- Cuthbert, B. N., Vrana, S. R., & Bradley, M. M. (1991). Imagery: Function and physiology. In J. R. Jennings, M. Ackles, & G. H. Coles (Eds.), Advances in psychophysiology: A research annual (Vol. 4) (pp. 1–42). Jessica Kingsley Publishers.
- Damasio, A. (1989). The brain binds entities and events by multiregional activation from convergence zones. Neural Computation, 1(1), 123–132. https://doi.org/10.1162/neco.1989.1.1.123
- Damasio, A. (1999). The feeling of what happens: Body and emotion in the making of consciousness (Vol. xiv). Harcourt College Publishers.
- Damasio, A., & Carvalho, G. B. (2013). The nature of feelings: Evolutionary and neurobiological origins. Nature Reviews Neuroscience, 14(2), 143–152. https://doi.org/10.1038/nrn3403
- Darwin, C. (1872). The expression of emotion in man and animal. William Clows & Sons.
- de Wit, S., & Dickinson, A. (2009). Associative theories of goal-directed behaviour: A case for animal–human translational models. Psychological Research Psychologische Forschung, 73(4), 463–476. https://doi.org/10.1007/s00426-009-0230-6
- Dignath, D., Kiesel, A., Frings, C., & Pastötter, B. (2020). Electrophysiological evidence for action-effect prediction. Journal of Experimental Psychology: General, 149(6), 1148–1155. https://doi.org/10.1037/xge0000707
- Eder, A. B., Erle, T. M., & Kunde, W. (2020). Reward strengthens action–effect binding. Motivation Science, 6(3), 297–302. https://doi.org/10.1037/mot0000153
- Eder, A. B., & Hommel, B. (2013). Anticipatory control of approach and avoidance: An ideomotor approach. Emotion Review, 5(3), 275–279. https://doi.org/10.1177/1754073913477505
- Eder, A. B., & Klauer, K. C. (2007). Common valence coding in action and evaluation: Affective blindness towards response-compatible stimuli. Cognition & Emotion, 21(6), 1297–1322. https://doi.org/10.1080/02699930701438277
- Eder, A. B., & Klauer, K. C. (2009). A common-coding account of the bidirectional evaluation–behavior link. Journal of Experimental Psychology: General, 138(2), 218–235. https://doi.org/10.1037/a0015220
- Eder, A. B., Mitschke, V., & Gollwitzer, M. (2020). What stops revenge taking? Effects of observed emotional reactions on revenge seeking. Aggressive Behavior, 46(4), 305–316. https://doi.org/10.1002/ab.21890
- Eder, A. B., Müsseler, J., & Hommel, B. (2012). The structure of affective action representations: Temporal binding of affective response codes. Psychological Research, 76(1), 111–118. https://doi.org/10.1007/s00426-011-0327-6
- Eder, A. B., Pfister, R., Dignath, D., & Hommel, B. (2017). Anticipatory affect during action preparation: Evidence from backward compatibility in dual-task performance. Cognition and Emotion, 31(6), 1211–1224. https://doi.org/10.1080/02699931.2016.1208151
- Eder, A. B., & Rothermund, K. (2008). When do motor behaviors (mis)match affective stimuli? An evaluative coding view of approach and avoidance reactions. Journal of Experimental Psychology: General, 137(2), 262–281. https://doi.org/10.1037/0096-3445.137.2.262
- Eder, A. B., Rothermund, K., De Houwer, J., & Hommel, B. (2015). Directive and incentive functions of affective action consequences: An ideomotor approach. Psychological Research, 79(4), 630–649. https://doi.org/10.1007/s00426-014-0590-4
- Ferguson, M. J. (2007). On the automatic evaluation of end-states. Journal of Personality and Social Psychology, 92(4), 596–611. https://doi.org/10.1037/0022-3514.92.4.596
- Flaherty, C. F. (1996). Incentive relativity. Cambridge University Press.
- Frijda, N. H. (2004). Emotions and action. In A. Fischer, A. S. R. Manstead, & N. H. Frijda (Eds.), Feelings and emotions: The Amsterdam symposium (pp. 158–173). Cambridge University Press. https://doi.org/10.1017/CBO9780511806582.010.
- Frijda, N. H., Ridderinkhof, K. R., & Rietveld, E. (2014). Impulsive action: Emotional impulses and their control. Frontiers in Psychology, 5, 518. https://doi.org/10.3389/fpsyg.2014.00518
- Garfinkel, S. N., Seth, A. K., Barrett, A. B., Suzuki, K., & Critchley, H. D. (2015). Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biological Psychology, 104(Supplement C), 65–74. https://doi.org/10.1016/j.biopsycho.2014.11.004
- Goldie, P. (2002). Emotions, feelings and intentionality. Phenomenology and the Cognitive Sciences, 1(3), 235–254. https://doi.org/10.1023/A:1021306500055
- Greenwald, A. G. (1970). Sensory feedback mechanisms in performance control: With special reference to the ideo-motor mechanism. Psychological Review, 77(2), 73–99. https://doi.org/10.1037/h0028689
- Guthrie, E. R. (1935). The psychology of learning. Harper.
- Harleß, E. (1861). Der apparat des willens. Zeitschrift Für Philosophie Und Philosophische Kritik, 38(2), 50–73.
- Holmes, N. M., Marchand, A. R., & Coutureau, E. (2010). Pavlovian to instrumental transfer: A neurobehavioural perspective. Neuroscience & Biobehavioral Reviews, 34(8), 1277–1295. https://doi.org/10.1016/j.neubiorev.2010.03.007
- Hommel, B. (2004). Event files: Feature binding in and across perception and action. Trends in Cognitive Sciences, 8(11), 494–500. https://doi.org/10.1016/j.tics.2004.08.007
- Hommel, B., Müsseler, J., Aschersleben, G., & Prinz, W. (2001). The Theory of Event Coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences, 24(5), 849–937. https://doi.org/10.1017/S0140525X01000103
- Hommel, B., & Wiers, R. W. (2017). Towards a unitary approach to human action control. Trends in Cognitive Sciences, 21(12), 940–949. https://doi.org/10.1016/j.tics.2017.09.009
- Izard, C. E. (2010). The many meanings/aspects of emotion: Definitions, functions, activation, and regulation. Emotion Review, 2(4), 363–370. https://doi.org/10.1177/1754073910374661
- James, W. (1884). What is an emotion? Mind; A Quarterly Review of Psychology and Philosophy, 9, 188–205. https://doi.org/10.1093/mind/os-IX.34.188
- James, W. (1890). The principles of psychology, Vol I. Henry Holt and Co.
- Jeannerod, M. (2006). Motor cognition: What actions tell the self (Vol. 42). Oxford University Press.
- Keele, S. W. (1968). Movement control in skilled motor performance. Psychological Bulletin, 70(6, Pt.1), 387–403. https://doi.org/10.1037/h0026739
- Kim, H., Shimojo, S., & O’Doherty, J. P. (2006). Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biology, 4(8), e233. https://doi.org/10.1371/journal.pbio.0040233
- Klauer, K. C., Eder, A. B., Greenwald, A. G., & Abrams, R. L. (2007). Priming of semantic classifications by novel subliminal prime words. Consciousness and Cognition, 16(1), 63–83. https://doi.org/10.1016/j.concog.2005.12.002
- Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. https://doi.org/10.1016/j.biopsycho.2010.03.010
- Kühn, S., Elsner, B., Prinz, W., & Brass, M. (2009). Busy doing nothing: Evidence for nonaction—Effect binding. Psychonomic Bulletin & Review, 16(3), 542–549. https://doi.org/10.3758/PBR.16.3.542
- Kunde, W., Kiesel, A., & Hoffmann, J. (2003). Conscious control over the content of unconscious cognition. Cognition, 88(2), 223–242. https://doi.org/10.1016/S0010-0277(03)00023-4
- Kunde, W., Schmidts, C., Wirth, R., & Herbort, O. (2017). Action effects are coded as transitions from current to future stimulation: Evidence from compatibility effects in tracking. Journal of Experimental Psychology: Human Perception and Performance, 43(3), 477–486. https://doi.org/10.1037/xhp0000311
- Lambie, J. A., & Marcel, A. J. (2002). Consciousness and the varieties of emotion experience: A theoretical framework. Psychological Review, 109(2), 219–259. https://doi.org/10.1037/0033-295X.109.2.219
- Lang, P. J. (1979). A bio-informational theory of emotional imagery. Psychophysiology, 16(6), 495–512. https://doi.org/10.1111/j.1469-8986.1979.tb01511.x
- LeDoux, J. E. (2014). Coming to terms with fear. Proceedings of the National Academy of Sciences, 111(8), 2871–2878. https://doi.org/10.1073/pnas.1400335111
- Levy, D. J., & Glimcher, P. W. (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology, 22(6), 1027–1038. https://doi.org/10.1016/j.conb.2012.06.001
- Lewin, K. (1926). Vorsatz, Wille und Bedürfnis [Intention, will, and need]. Psychologische Forschung, 7(1), 330–385. https://doi.org/10.1007/BF02424365
- Lotze, R. H. (1852). Medizinische psychologie oder physiologie der seele. Weidmann’sche Buchhandlung.
- Mansell, W.2020). The interdisciplinary handbook of perceptual control theory: Living control systems IV. Academic Press.
- Marken, R. S., & Mansell, W. (2013). Perceptual control as a unifying concept in psychology. Review of General Psychology, 17(2), 190–195. https://doi.org/10.1037/a0032933
- McAllister, W. R., & McAllister, D. E. (1992). Fear determines the effectiveness of a feedback stimulus in aversively motivated instrumental learning. Learning and Motivation, 23(1), 99–115. https://doi.org/10.1016/0023-9690(92)90025-H
- Meier, B. P., & Robinson, M. D. (2004). Why the sunny side is up: Associations between affect and vertical position. Psychological Science, 15(4), 243–247. https://doi.org/10.1111/j.0956-7976.2004.00659.x
- Memelink, J., & Hommel, B. (2013). Intentional weighting: A basic principle in cognitive control. Psychological Research, 77(3), 249–259. https://doi.org/10.1007/s00426-012-0435-y
- Mertens, G., Krypotos, A.-M., & Engelhard, I. M. (2020). A review on mental imagery in fear conditioning research 100 years since the ‘Little Albert’ study. Behaviour Research and Therapy, 126, 103556. https://doi.org/10.1016/j.brat.2020.103556
- Milner, D., & Goodale, M. (2006). The visual brain in action. Oxford University Press.
- Moors, A. (2022). Demystifying emotions: A typology of theories in psychology and philosophy. Cambridge University Press. https://doi.org/10.1017/9781107588882.
- Moors, A., & Fischer, M. (2019). Demystifying the role of emotion in behaviour: Toward a goal-directed account. Cognition and Emotion, 33(1), 94–100. https://doi.org/10.1080/02699931.2018.1510381
- Moseley, R., Carota, F., Hauk, O., Mohr, B., & Pulvermüller, F. (2012). A role for the motor system in binding abstract emotional meaning. Cerebral Cortex, 22(7), 1634–1647. https://doi.org/10.1093/cercor/bhr238
- Mostofsky, S. H., & Simmonds, D. J. (2008). Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience, 20(5), 751–761. https://doi.org/10.1162/jocn.2008.20500
- Nummenmaa, L., Glerean, E., Hari, R., & Hietanen, J. K. (2014). Bodily maps of emotions. Proceedings of the National Academy of Sciences, 111(2), 646–651. https://doi.org/10.1073/pnas.1321664111
- Panksepp, J. (2005). Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition, 14(1), 30–80. https://doi.org/10.1016/j.concog.2004.10.004
- Papies, E. K., Barsalou, L. W., & Rusz, D. (2020). Understanding desire for food and drink: A grounded-cognition approach. Current Directions in Psychological Science, 29(2), 193–198. https://doi.org/10.1177/0963721420904958
- Pfister, R. (2019). Effect-based action control with body-related effects: Implications for empirical approaches to ideomotor action control. Psychological Review, 126(1), 153–161. https://doi.org/10.1037/rev0000140
- Pool, E., Sennwald, V., Delplanque, S., Brosch, T., & Sander, D. (2016). Measuring wanting and liking from animals to humans: A systematic review. Neuroscience & Biobehavioral Reviews, 63, 124–142. https://doi.org/10.1016/j.neubiorev.2016.01.006
- Poppa, T., & Bechara, A. (2018). The somatic marker hypothesis: Revisiting the role of the ‘body-loop’ in decision-making. Current Opinion in Behavioral Sciences, 19, 61–66. https://doi.org/10.1016/j.cobeha.2017.10.007
- Powers, W. T. (1973). Behavior: The control of perception. Aldine Publishing Company.
- Prinz, W. (1990). A common coding approach to perception and action. In O. Neumann, & W. Prinz (Eds.), Relationships between perception and action: Current approaches (pp. 167–201). Springer-Verlag.
- Prinz, W., de Maeght, S., & Knuf, L. (2004). Intention in action. In G. Humphreys, & J. Riddoch (Eds.), Attention in action (pp. 93–108). Psychology Press.
- Pulvermüller, F., & Fadiga, L. (2010). Active perception: Sensorimotor circuits as a cortical basis for language. Nature Reviews Neuroscience, 11(5), 351–360. https://doi.org/10.1038/nrn2811
- Ridderinkhof, K. R. (2017). Emotion in action: A predictive processing perspective and theoretical synthesis. Emotion Review, 9(4), 319–325. https://doi.org/10.1177/1754073916661765
- Rolls, E. T. (2005). Emotion explained. Oxford University Press.
- Sander, D., & Nummenmaa, L. (2021). Reward and emotion: An affective neuroscience approach. Current Opinion in Behavioral Sciences, 39, 161–167. https://doi.org/10.1016/j.cobeha.2021.03.016
- Scherer, K. R. (2009). The dynamic architecture of emotion: Evidence for the component process model. Cognition & Emotion, 23(7), 1307–1351. https://doi.org/10.1080/02699930902928969
- Schulkin, J., & Sterling, P. (2019). Allostasis: A brain-centered, predictive mode of physiological regulation. Trends in Neurosciences, 42(10), 740–752. https://doi.org/10.1016/j.tins.2019.07.010
- Schultz, W. (2007). Behavioral dopamine signals. Trends in Neurosciences, 30(5), 203–210. https://doi.org/10.1016/j.tins.2007.03.007
- Searle, J. R. (1983). Intentionality: An essay in the philosophy of mind. Cambridge University Press. https://doi.org/10.1017/CBO9781139173452.
- Shin, Y. K., Proctor, R. W., & Capaldi, E. J. (2010). A review of contemporary ideomotor theory. Psychological Bulletin, 136(6), 943–974. https://doi.org/10.1037/a0020541
- Singer, W. (2001). Consciousness and the binding problem. Annals of the New York Academy of Sciences, 929(1), 123–146. https://doi.org/10.1111/j.1749-6632.2001.tb05712.x
- Strohmaier, N., & Veling, H. (2019). Bypassing the gatekeeper: Incidental negative cues stimulate choices with negative outcomes. Cognition and Emotion, 33(5), 1059–1066. https://doi.org/10.1080/02699931.2018.1523136
- Tamir, M. (2016). Why do people regulate their emotions? A taxonomy of motives in emotion regulation. Personality and Social Psychology Review, 20(3), 199–222. https://doi.org/10.1177/1088868315586325
- Taquet, M., Quoidbach, J., Montjoye, Y.-A. d., Desseilles, M., & Gross, J. J. (2016). Hedonism and the choice of everyday activities. Proceedings of the National Academy of Sciences, 113(35), 9769–9773. https://doi.org/10.1073/pnas.1519998113
- Tomkins, S. S. (1962). Affect, imagery, consciousness: Vol. I. The positive affects. Springer.
- Warlow, S. M., & Berridge, K. C. (2021). Incentive motivation: ‘Wanting’ roles of central amygdala circuitry. Behavioural Brain Research, 411, 113376. https://doi.org/10.1016/j.bbr.2021.113376
- Waszak, F., Cardoso-Leite, P., & Hughes, G. (2012). Action effect anticipation: Neurophysiological basis and functional consequences. Neuroscience & Biobehavioral Reviews, 36(2), 943–959. https://doi.org/10.1016/j.neubiorev.2011.11.004
- Winkielman, P., & Berridge, K. C. (2004). Unconscious emotion. Current Directions in Psychological Science, 13(3), 120–123. https://doi.org/10.1111/j.0963-7214.2004.00288.x
- Wolpert, D. M., & Landy, M. S. (2012). Motor control is decision-making. Current Opinion in Neurobiology, 22(6), 996–1003. https://doi.org/10.1016/j.conb.2012.05.003
- Yarali, A., Niewalda, T., Chen, Y., Tanimoto, H., Duerrnagel, S., & Gerber, B. (2008). Pain relief’ learning in fruit flies. Animal Behaviour, 76(4), 1173–1185. https://doi.org/10.1016/j.anbehav.2008.05.025
