Abstract
The range of smallmouth bass (Micropterus dolomieu) is expanding northward, creating new interactions with native predators, including walleye (Sander vitreus). We used a series of experiments to investigate competition between walleye (WAE) and smallmouth bass (SMB) at different life stages and light conditions, identified behaviors that allowed one fish to outcompete another, and evaluated whether prey switching mitigated competitive interactions. Juvenile and adult SMB appeared to outcompete WAE when fed during the daytime; neither species dominated when fed near dusk. Attack rates and capture efficiencies of both species were similar with an intra- or interspecific competitor, but SMB often exploited prey before the competitor had a chance to feed (exploitative competition) or displayed agonistic behaviors toward a potential competitor (interference competition). Prey selectivity of WAE or SMB did not differ when by themselves or with a potential competitor. These results indicate that SMB could outcompete WAE under limiting prey conditions due to the aggressive nature of SMB, but resources may be partitioned at least along a temporal scale.
Introduction
Competition is one mechanism by which introduced fishes may reduce or extirpate native fish populations (Moyle et al. Citation1986). The effects of interspecific competition for prey on the fitness, growth, fecundity, abundance, or feeding habits of a native fish in the presence of an introduced species have been extensively studied (Keddy Citation2001). For example, several studies have documented reduced growth, lipid reserves, and survival of native juvenile cutthroat trout (Oncorhynchus spp.) due to competition for food by non-native brook trout (Salvelinus fontinalis; Cummings Citation1987; Thomas Citation1996; Novinger Citation2000). Size structure of Eurasian perch (Perca fluviatilis) decreased after the introduction of pikeperch (Sander lucioperca) in Lake Grober Vätersee, Germany, due to the competition for shared prey (Schulze et al. Citation2006).
Competition may occur either directly (interference competition) or indirectly (exploitative competition). Interference competition for food occurs when one species or individual displays agonistic behaviors that decrease the use of prey by another predator. Exploitative competition occurs when the better competitor ultimately influences the ability of the lesser competitor to feed by consuming all available prey resources first. Understanding the mechanisms of competition provides useful information on the relative energetic cost of the interaction between two species, as more energy is typically expended during interference competition than during exploitative competition (Tilman Citation1987).
Competition may vary by life stage whereby competitive interactions are negligible or differ at the larval, juvenile, or adult stage as a result of ontogenetic niche shifts in morphology, physiology, or behavior (Werner and Gilliam Citation1984; Mougi and Nishimura Citation2005). Lifetime fitness may be established early in life as dominant individuals are able to garner territories and resources before subordinates; thus, younger fish may be more aggressive against competitors than during the adult stage after dominance hierarchies have been established among populations and communities (Berejikian et al. Citation2001). Environmental conditions such as light intensity could also alter the competitive ability of a species. For example, Schleuter and Eckmann (Citation2006) found that the competitive ability of Eurasian perch against ruffe (Gymnocephalus cernuus) was diminished during darkness compared to daylight due to special sensory adaptations of ruffe that allow the species to feed at low light levels. Prey switching may also mitigate competitive interactions between two species. In the presence of roach (Rutilus rutilus), age-0 Eurasian perch shifted from a diet of zooplankton to a diet of macroinvertebrates in August in a shallow eutrophic lake to avoid competition (Persson Citation1983); no such shift by perch was observed in the absence of roach (Persson Citation1987). While an abundance of research has examined the effects of competition on the life history and dynamics of fishes, far fewer tested the influence of the conditions that may influence the direction and magnitude of those effects.
As fishes are intentionally or unintentionally introduced into ecosystems, understanding mechanisms of competition and the influence of life stage, environmental conditions, and prey availability are important in predicting and assessing the consequences of those introductions on native fishes. In recent decades, the range of smallmouth bass (Micropterus dolomieu) has been expanding throughout North America due to intentional introductions and dispersal through adjacent networks (Jackson Citation2002). Consequently, the introduction of smallmouth bass (SMB) has been linked to reduced growth and survival of native fishes, including lake trout (Salvelinus namaycush) in Canadian shield lakes (Vander Zanden et al. Citation2004) and anadromous alewives (Alosa pseudoharengus) in the lower St. John River, New Brunswick (Hanson and Curry Citation2005), due to competition for shared prey resources. Walleye (Sander vitreus) is another native species that could potentially compete with SMB. Food habits studies of walleye (WAE) and SMB in sympatry describe at least some level of prey resources shared between the two species (Fedoruk Citation1966; Johnson and Hale Citation1977; Frey et al. Citation2003; Fayram et al. Citation2005; Bacula Citation2009; Wuellner et al. Citation2010). To date, no study has examined competitive mechanisms of WAE and SMB under controlled conditions (Wuellner Citation2009).
Life stage, light level, and prey availability could alter competitive interactions between WAE and SMB. Both juvenile and adult SMB are known to display agonistic behaviors in the presence of a competitor, but juveniles may be more aggressive than adults (Sabo et al. Citation1996). Agonistic behaviors by WAE at any life stage have not been documented in any study to our knowledge. SMB feed opportunistically over a 24 h period (Warren Citation2009), but peak activity in feeding has been documented near sunrise and sunset (Stewart Citation1978; Kwak et al. Citation1992). Conversely, WAE tends to feed nocturnally or crepuscularly in clear water (Schlick Citation1978); a specialized tapetum lucidum and enhanced odor sensors may provide advantages when feeding at low light levels (Rottiers and Lemm Citation1985). Feeding at different times of the day may help partition shared prey resources along a temporal scale (Pianka Citation1973). Finally, SMB is considered to be a generalist feeder, readily consuming both fish and invertebrates (Clady Citation1974; Hubert Citation1977; Johnson and Hale Citation1977; Winemiller and Taylor Citation1987; Frey et al. Citation2003; Lott Citation1996; Gangl et al. Citation1997; Bacula Citation2009), while WAE is considered to be more piscivorous in its feeding habits (Knight et al. Citation1984; Lyons Citation1987). Plasticity in food habits of at least one species may allow interspecific competitors to co-exist by reducing demand for a prey resource (Gerking Citation1994).
The goals of this study were to determine whether WAE and SMB compete in a controlled environment and whether certain factors influence competitive interactions. A series of laboratory experiments was used to address the following questions.
| 1. | What are the direction and magnitude of competition between adult and juvenile WAE and SMB? Do the direction and magnitude of competition differ by life stage? | ||||
| 2. | Does light level (i.e., day versus night) affect competitive interactions between WAE and SMB? | ||||
| 3. | If two prey types are offered, will WAE or SMB shift feeding in the presence of a competitor to avoid competition? | ||||
| 4. | Is competition between WAE and SMB direct (interference competition) or indirect (exploitative competition)? | ||||
Materials and methods
Fish collection
All WAE and SMB used for these experiments were wild-caught fish obtained from several local lakes and ponds during the summers of 2007–2009; the source was the same for each experiment in the series. Both adult and juvenile WAE were captured using a combination of angling, electrofishing, and fyke netting. Adult and juvenile SMB were captured exclusively by angling. Life stage of fish was determined by total length (TL; adult WAE: 310–380 mm TL; juvenile WAE: 220–280 mm TL; adult SMB 230–350 mm TL; juvenile SMB: 150–220 mm TL).
Direction and magnitude of competition by life stage and light level
Specific growth rate in weight (g of weight lost or gained/g of initial body weight/number of days of experiment) was used as a surrogate to test the relative influence of intra- and interspecific competitions between WAE and SMB at the adult and juvenile stages and at different light levels. All growth experiments were conducted in fiberglass tanks (274 × 76 × 61 cm3). Each of the tanks contained a biofiltering system, cobble substrate (64–110 mm diameter), two vegetation mats, and an aeration system. One-third of the water in each tank was replaced with fresh water every 3 days to maintain water quality. Water temperature was held constant at 20°C, and windows in the laboratory allowed for a natural photoperiod. We used a LiCor® LI-193 spherical quantum sensor and LI-250A light meter to verify differences in light levels in the tanks during daytime and dusk.
Separate trials were conducted for adult WAE and SMB fed at midday, juvenile WAE and SMB fed at midday, and juvenile WAE and SMB fed near dusk; experimental protocol was the same for all three trials. During the habituation period (at least 7 days), WAE and SMB were kept in separate tanks at densities similar to experimental conditions and fed fathead minnows (Pimephales promelas) ad libitum. Three days prior to the start of the experiments, uneaten prey items were removed from the tanks. Each WAE and SMB was weighed, measured (TL; mm), given a unique fin clip, and randomly assigned to one of the three treatments [i.e., WAE alone (allopatric WAE), SMB alone (allopatric SMB), and WAE and SMB together (sympatric WAE and SMB)]. Each treatment was randomly assigned to one of nine tanks. Fish were stocked at a density of six per tank. In the allopatric treatments, WAE and SMB were stocked at equal densities (three individuals of each species).
Once the experiments began, WAE and SMB were fed a limiting ration of fathead minnows every 3 days. Initially, rations were determined from bioenergetics models (Hanson et al. Citation1997) for each species run under maintenance conditions. However, the first experiments with adult WAE and SMB indicated that this ration was not limiting enough for competition to occur (i.e., nearly all fish, regardless of treatment, gained weight). Thus, maintenance rations were reduced by 30%, and this ration was also used for all subsequent juvenile growth experiments.
All growth experiments (e.g., adult, juvenile, day versus nighttime feeding) were run for at least 15 days or until a response (weight gain or loss, mortality) was detected. At the end of each trial, all fish were weighed and measured. Mean specific growth rate was calculated by species and treatment. In the case of the experiments with adult WAE and SMB, specific growth rate was calculated between days 30 and 43; in all other experiments, specific growth rate was calculated between first and last days of the trials. Analysis of variance (ANOVA) was used to determine whether specific growth was different from zero for each species within each treatment and whether differences in specific growth between all combinations of predators and treatments were significant. Differences between individual species treatments were determined using Tukey's multiple pairwise comparisons; significance was determined at the 0.05 level.
Observations of exploitative feeding behaviors
Feeding observation experiments were conducted in vinyl tanks measuring 42 × 42 × 32 cm with a 40 × 30 cm observation window in the front of the tank. Two 60-W, 120-V incandescent light bulbs were anchored over each tank, and timers maintained a 15 : 9 light: dark cycle (0600–2100 h), similar to that of mid-summer. Water temperature was maintained at 20°C, and standard aquarium aerators were used to maintain dissolved oxygen. The substrate was white aquarium gravel (2–5 mm diameter). Water was completely changed between trials.
Tanks were assigned one of three treatments (i.e., allopatric WAE, allopatric SMB, and sympatric WAE and SMB), and there were five replications per treatment. Adult fish were randomly assigned to each tank; density of fish per tank was two. In the case where both fish in the tank were conspecifics, unique identifying physical characteristics were noted and used to distinguish individuals. Fish were allowed to habituate for at least 14 days and fed fathead minnows ad libitum. Two days prior to the start of the feeding observations, any remaining prey items were removed and predators were starved.
At the end of the starvation period, 10 fathead minnows were introduced into each tank. The same observer monitored each tank for 30 min and recorded the number of strikes (whether successful capture occurred or not) on prey by individual predators and the number of successful captures of prey by individual predators during that time. If no fish in the tank fed during that time period, prey items were removed and the experiment was repeated the following day; repetition continued until at least one predator fed during the experiment period or mortality was observed.
Attack rate (i.e., the number of strikes per minute) and capture efficiency (i.e., the number of successful captures of prey/total number of strikes) were calculated for each individual predator, and means were computed by species and treatment. Comparisons among treatments and species were done using ANOVA, and significance was determined at the 0.05 level. Differences between species and treatment combinations were made using Tukey's multiple pairwise comparisons; significance was determined at the 0.05 level.
Prey switching experiments and observations of interference behaviors
To test whether WAE and SMB would select different prey in the presence of a competitor, we conducted prey selection experiments on juvenile WAE and SMB using fathead minnow and crayfish (Orconectes spp. obtained from a local bait dealer) as prey. Tanks, water temperature, aeration, light regime, and substrate were the same as used in the feeding behavior experiments.
At the beginning of the experiment, tanks were divided in half using an opaque divider. Each tank was randomly assigned one of three treatments (allopatric WAE, allopatric SMB, or sympatric WAE and SMB), and there were four replications per treatment. Fish were randomly assigned to individual tanks and the side of the tank into which they were stocked; density of fish in each tank was two. If two fish of the same species were stocked into one tank, each fish was given a unique fin clip. Fish were habituated to the tanks for 3 days, during which time they were exposed to both prey types and allowed to feed ad libitum. After this period, all remaining prey items were removed and the predators were starved for 3 days.
When the starvation period ended, each fish was fed five fathead minnows and five crayfish during early morning (0600–0630 h) and allowed to feed for 3 h. All prey items consumed were enumerated, and any remaining prey items and the tank divider were removed. Fish were again starved for 3 days. On the third day, the divider was replaced in the tank so as to sequester both fish on the same side of the tank. The following morning (0600–0900 h), 10 fathead minnows and 10 crayfish were introduced on the opposite side of the tank. The divider was then removed, and fish were filmed for 3 h to determine which predator consumed which prey items and to record any agonistic behaviors. Agonistic behavior was defined as any action (e.g., nipping, ramming, wig-wag display) by one individual that resulted in the movement or flight of another individual (Savino and Kostich Citation2000). Specific behaviors were classified according to Noakes (Citation1980).
Prey selectivity of WAE and SMB was determined for each individual when alone and when in competition by calculating Chesson's (Citation1983) coefficient of selectivity (α):
Results
Direction and magnitude of competition by life stage and light level
Adult growth experiments were initially run for 30 days. Nearly all fish, regardless of treatment, gained weight (), indicating that prey rations were not limiting. Fish were placed back into their original tanks, prey rations were reduced by 30%, and the experiments were run for an additional 13 days. Fish weights at the end of this 13 day period indicated that the modified rations were limiting. Both WAE and SMB in allopatry significantly increased in weight (WAE: t = 2.20, p = 0.03; SMB t = 2.73, p = 0.01); the difference in specific growth rate between the two species was not significant (t = 0.33, p = 0.74).
Figure 1. Specific growth rates at 30 and 45 days of adult WAE (white bars) and SMB (gray bars) in allopatry and sympatry fed during daytime. Specific growth rates at 30 days were calculated from the initial weights of fish at the beginning of the experiments; specific growth rates at 43 days were calculated between 30 and 43 days. The treatments were: allopatric WAE, allopatric SMB, and sympatric WAE and SMB. Error bars represent one standard error. Means at 43 days with the same letter are not significantly different at α = 0.05.
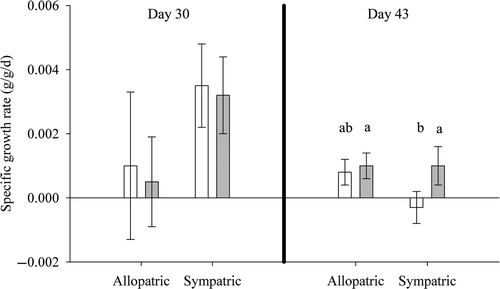
WAE in sympatry with SMB lost some weight but not significantly so (t = −0.61, p = 0.55); there was no difference in WAE growth between the two treatments (t = 1.79, p = 0.08). Sympatric SMB gained weight (t = 2.51, p = 0.02), and there was no difference in SMB specific growth rate between the two treatments (t = −0.57, p = 0.57). Specific growth rate differed between sympatric WAE and SMB (t = 2.24, p = 0.03), as SMB gained weight while WAE lost weight.
Growth experiments using juvenile WAE and SMB fed during midday were run for 15 days. WAE in allopatry gained weight overall, but the specific growth rate was not significantly different from zero (; t = 1.16, p = 0.25). In contrast, allopatric SMB lost weight (t = −2.88, p < 0.01). The difference in specific growth rate between the two species was significant (t = −2.86, p < 0.01).
Figure 2. Specific growth rates of juvenile WAE (white bars) and SMB (gray bars) in allopatry and sympatry fed during daytime for 15 days. Error bars represent one standard deviation. Means with the same letter are not significantly different at α = 0.05.
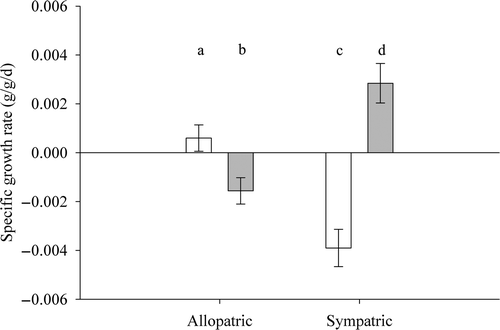
Juvenile WAE sympatric with juvenile SMB lost weight (; t = −5.06, p < 0.0001); differences in WAE specific growth rates were significantly different between treatments (t = 4.80, p < 0.0001). Sympatric SMB gained weight (t = 3.51, p < 0.01); the difference in SMB specific growth rates between treatments was significant (t = −4.52, p < 0.0001). Sympatric WAE and SMB specific growth rates were significantly different (t = 6.03, p < 0.0001), as SMB increased in weight while WAE lost weight.
Growth experiments using juvenile WAE and SMB fed near dusk were run for 12 days. WAE in allopatry lost some weight but the specific growth rate was not significantly different from zero (; t = −0.32, p = 0.75). SMB in allopatry lost significant weight (t = −3.00, p < 0.01). However, the difference in specific growth rate between the two species in allopatry was not significant (t = −1.47, p = 0.15).
Figure 3. Specific growth rates of juvenile WAE (white bars) and SMB (gray bars) in allopatry and sympatry fed within 30 min prior to dusk for 15 days. Error bars represent one standard deviation. Means with the same letter are not significantly different at α = 0.05.
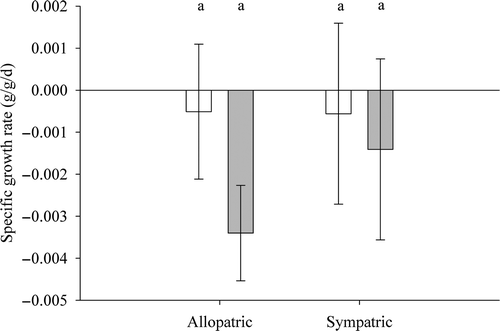
Both WAE and SMB in sympatry lost weight overall, but specific growth rate for both species was not significantly different from zero (; WAE: t = −0.26, p = 0.80; SMB: t = −0.65, p = 0.52). There was no difference in specific growth rate between sympatric WAE or SMB (t = −0.28, p = 0.78) nor between treatments for either species (WAE: t = 0.02, p = 0.99; SMB: t = −0.82, p = 0.42).
Observations of exploitative feeding behaviors
Mean attack rate of WAE was the same whether in the presence of another WAE or a SMB (; t = −0.88, p = 0.88). SMB did not appear to attack prey as frequently in the presence of another SMB as when WAE were present; however, the difference was not significant among the two treatments (t = −0.36, p = 0.36). Mean attack rate of WAE and SMB in sympatry did not differ overall (t = 0.04, p = 0.97).
Figure 4. Mean attack rates and capture efficiencies of WAE (white circles) and SMB (black circles) under allopatry and sympatry. Error bars represent one standard deviation. Means with the same letter are not significantly different at α = 0.05.
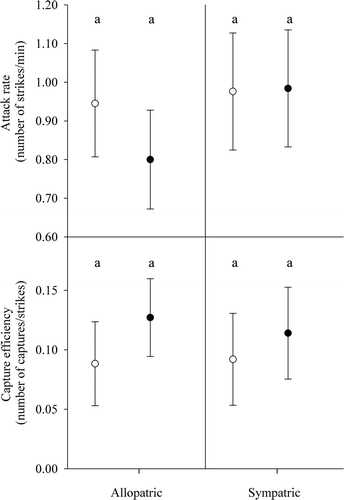
Mean capture efficiency of WAE did not change with another WAE versus a SMB (; t = −0.49, p = 0.63). SMB capture efficiency did decline slightly when in the presence of another SMB as compared to when SMB were with WAE, but the difference was not significant between treatments (t = 0.69, p = 0.50). Mean capture efficiency of WAE and SMB in sympatry did not differ overall (t = 0.81, p = 0.43).
Prey switching experiments and observations of interference behaviors
When alone, WAE positively selected for fathead minnow, and no crayfish were consumed (). After exposure to intraspecific competition, WAE still positively selected for fathead minnow, but one WAE consumed a single crayfish. However, mean selectivity for crayfish was still negative among all WAE in intraspecific competition.
Figure 5. Mean Chesson's alpha values for WAE (white bars) and SMB (gray bars) across treatments and prey types (fathead minnow = open bars; crayfish = hatched bars), before (top panel) and after (bottom panel) exposure to competition. Error bars represent one standard error. Dashed lines indicate random feeding. Mean values at, above, or below the dashed lines indicate neutral, positive, or negative selection, respectively.
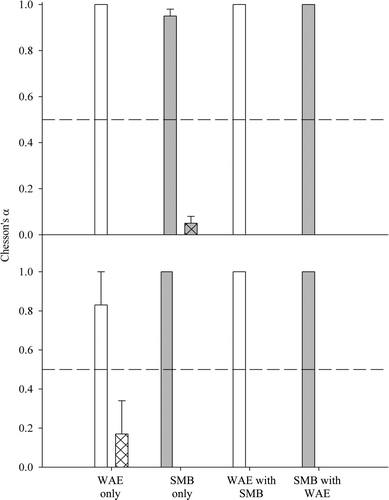
Patterns differed somewhat for SMB under intraspecific competitive conditions. When alone, SMB positively selected for fathead minnow (), but two SMB each consumed a single crayfish. Overall selectivity for crayfish was negative for SMB prior to exposure to another SMB. After exposure, no crayfish were consumed, and selection for fathead minnow remained positive.
In the sympatric treatments, WAE did not consume any crayfish pre- or post-exposure to SMB (). Selection for fathead minnow was positive, pre- and post-exposure to SMB, but fewer WAE consumed any minnows when SMB were present. SMB did not consume any crayfish, regardless of whether alone or when WAE were present; SMB positively selected for fathead minnows, whether alone or in competition with WAE.
WAE seldom displayed agonistic behaviors when in the presence of an intra- or interspecific competitor (). Wig-wag displays were most often used against another WAE, but ramming was most often used against SMB. In contrast, SMB displayed numerous and varied agonistic behaviors against intra- and interspecific competitors. SMB most often bit other SMB as a means to display dominance. However, SMB would most often nip, ram, or chase WAE.
Table 1. Summary of agonistic behaviors [mean number per 3 h observation period (standard deviation)] displayed toward an intra- or interspecific competitors for WAE and SMB by treatment.
Discussion
To our knowledge, this is the first study that has examined competition for prey between WAE and SMB in a controlled setting. Previous field studies have indicated some or complete diet overlap between the two species (Fedoruk Citation1966; Johnson and Hale Citation1977; Frey et al. Citation2003; Fayram et al. Citation2005; Bacula Citation2009; Wuellner et al. Citation2010), but none indicated that prey resources were limiting, which would be a necessary criterion for competition (Crowder Citation1990). Controlling prey availability allowed us to examine both inter- and intraspecific competitions between WAE and SMB at two life stages and under different light conditions and prey resources while also examining feeding behaviors to help elucidate competitive mechanisms; this provided for a more complete understanding of potential interspecific competition that may occur in nature.
When fed during the daytime, specific growth rates of adult and juvenile SMB were greater than those for WAE, indicating that SMB was the better competitor. Both intra- and interspecific competitive interactions appeared to be greater for juvenile SMB compared to adults. Specific growth rate was relatively higher for juvenile SMB sympatric with WAE than for adult SMB in the same treatment. Further, allopatric juvenile SMB lost weight, but allopatric adult SMB gained. Stronger competitive interactions among younger fish compared to older fish is common, as younger individuals are often establishing territories and hierarchies within populations and communities through agonistic behaviors; these types of hierarchies are already established among adults (Fausch and White Citation1986; Cushing and Li Citation1991; Gerking Citation1994; Keddy Citation2001). Complex agonistic behaviors have been observed among juvenile SMB in streams as early as 50 days post-hatch (Sabo et al. Citation1996). Perhaps these behaviors allow SMB to garner prey resources when fed during the daytime.
Contrastingly, results from nighttime feeding experiments between juveniles of the two species showed no competitive advantage for WAE or SMB. WAE is known as a crepuscular feeder (Mathias and Li Citation1982) and has specialized sensory organs that may help confer an advantage over SMB when feeding at low light levels. Similar patterns have been observed for ruffe (which also has a specialized tapidum lucidum) and Eurasian perch (a visually oriented predator; Schlueter and Eckmann Citation2006). However, we observed that not all prey was consumed by WAE in the first night. Further, some sympatric SMB did gain weight, indicating that SMB still fed either the first night or the following day. Given these results and observations, it is possible that WAE and SMB could partition resources based on a temporal scale (Pianka Citation1973) if prey resources are available throughout a 24 h period. Temporal separation of feeding activities may allow the WAE and SMB to co-exist with no negative effects to fitness or survival of either species.
Surprisingly, providing diverse prey did not mitigate competitive interactions between WAE and SMB as prey selectivity for SMB did not change whether they were alone or in the presence of WAE. In fact, crayfish were selected against in all treatments for both species. Negative selection may have occurred for several reasons, including a higher relative energy density of fathead minnow compared to crayfish [4100 J/g (Chipps et al. Citation2000) versus 3063 J/g (Eggleton and Schramm Citation2002), respectively] and a lower energetic cost of capturing and digesting fathead minnows compared to crayfish. However, SMB is considered a generalist in its food habits (Winemiller and Taylor Citation1987), and consumption of crayfish by SMB is common (Clady Citation1974; Hubert Citation1977; Johnson and Hale Citation1977; Lott Citation1996; Gangl et al. Citation1997; Frey et al. Citation2003; Bacula Citation2009). Further, low levels of diet overlap have been observed between WAE and SMB when prey diversity is greatest (Frey et al. Citation2003; Bacula Citation2009; Wuellner et al. Citation2010). Thus, the generalist nature of SMB coupled with high prey diversity may mitigate competitive interactions between WAE and SMB in nature.
Our feeding behavior experiments did not clearly identify a single mechanism of competition. Several lines of evidence indicate that both interference and exploitation competition work in concert to allow SMB a competitive advantage at higher light levels. We commonly observed that dominant SMB, whether with another SMB or a WAE, appeared to attack prey first and quickly. For example, two SMB in our prey diversity experiments consumed all of the fathead minnows within 20 min of trial start time, disallowing two subordinate SMB to feed on this resource (i.e., exploitative competition). However, agonistic behaviors were often observed between a dominant and subordinate SMB or a SMB and a WAE. Such intimidation tactics may prevent feeding by the lesser competitor (i.e., interference competition). Schoener (Citation1983) recognized the difficulty in teasing interference and exploitative competition from one another as both mechanisms likely operate in concert to some degree. Indeed, both interference and exploitative competition were observed between yellow perch (Perca flavescens) and ruffe in laboratory experiments (Savino and Kolar Citation1996).
While our laboratory experiments provided insight on interspecific competition between WAE and SMB, caution should be exercised when applying these results to the field. Prey rations had to be reduced in order for competitive interactions to occur. Certainly, our initial rations may have been too high due to common errors in bioenergetics models (Chipps and Wahl Citation2008). However, our reduced rations were likely unrealistic as we know of no case where prey are that limiting. In our feeding behaviors experiments, we used both starvation periods and low food rations in order to induce competitive interactions. Hunger coupled with a novel environment such as a tank may increase aggression among competitors (Cutts et al. Citation2001, Citation2002), so observations of aggressive acts among WAE and SMB may be artificially high.
Despite these limitations, this study provides information regarding interspecific interactions between WAE and SMB and how those interactions are influenced by life stage, light level, and feeding behavior. The results suggest that WAE and SMB may co-exist by partitioning resources along a temporal scale and that interactions decrease as fish age. Further, the apparent aggressive nature of SMB likely enhances its competitive ability.
Acknowledgements
We thank T. St. Sauver, D. Lucchesi, and B. Johnson at South Dakota Department of Game, Fish, and Parks and W. Gibbons at South Dakota State University for providing assistance with collecting fish for experiments. Invaluable laboratory and field assistance was provided by J. Dagel, J. Grote, S. Heidebrink, N. Lorenz, J. McAllister, J. Seibert, G. Soupir, and E. Weeman. Primary funding for this research was provided by the Federal Aid in Sport Fish Restoration program, Project F-15-R, Study 1505, Job 1, administered through the South Dakota Department of Game, Fish and Parks. Funding for the purchase of observation tanks was provided by the Jesse W. West Fisheries Research Endowment supported by Pond Boss members and administered through the Department of Wildlife and Fisheries Sciences at South Dakota State University. We appreciate the comments of P. Bettoli (Tennessee Cooperative Fishery Research Unit) and K. Pope (Nebraska Cooperative Fish and Wildlife Research Unit) on previous drafts of this manuscript. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Bacula , TD . 2009. Smallmouth bass seasonal dynamics in northeastern South Dakota glacial lakes [MS thesis]. [Brookings (SD)]: South Dakota State University
- Berejikian , BA , Tezak , EP , Park , L , LaHood , E , Schroder , SL and Beall , E . 2001 . Male competition and breeding success in captively reared and wild coho salmon (Oncorhynchus kisutch) . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 804 – 810 .
- Chesson , J . 1983 . The estimation and analysis of preferences and its relationship to foraging models . Ecology , 70 : 1227 – 1235 .
- Chipps , SR , Einfalt , LM and Wahl , DH . 2000 . Growth and food consumption by tiger muskellunge: effects of temperature and ration level on bioenergetic model predictions . Transactions of the American Fisheries Society , 129 : 186 – 193 .
- Chipps , SR and Wahl , DH . 2008 . Bioenergetics modeling in the 21st century: reviewing new insights and revising old constraints . Transactions of the American Fisheries Society , 137 : 298 – 313 .
- Clady , MD . 1974 . Food habits of yellow perch, smallmouth bass, and largemouth bass in two unproductive lakes in northern Michigan . The American Midland Naturalist , 91 : 453 – 459 .
- Crowder , LB . 1990 . “ Community ecology ” . In Methods for fish biology , Edited by: Schreck , CB and Moyle , PB . 609 – 632 . Bethesda, MD : American Fisheries Society .
- Cummings , TR . 1987. Brook trout competition with greenback cutthroat trout in Hidden Valley, Colorado [MS thesis]. [Fort Collins (CO)]: Colorado State University
- Cushing , JM and Li , J . 1991 . Juvenile versus adult competition . Journal of Mathematical Biology , 29 : 457 – 473 .
- Cutts , CJ , Adams , CE and Campbell , A . 2001 . Stability of physiological and behavioural determinants of performance in Arctic char (Salvelinus alpines) . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 961 – 968 .
- Cutts , CJ , Metcalfe , NB and Taylor , AC . 2002 . Fish may fight rather than feed in a novel environment: metabolic rate and feeding motivation in juvenile Atlantic salmon . Journal of Fish Biology , 61 : 1540 – 1548 .
- Eggleton , MA and Schramm Jr , HL . 2002 . Caloric densities of selected fish prey organisms from the lower Mississippi River . Journal of Freshwater Ecology , 17 : 409 – 414 .
- Fausch , KD and White , RJ . 1986 . Competition among juveniles of coho salmon, brook trout, and brown trout in a laboratory stream, and implications for Great Lakes tributaries . Transactions of the American Fisheries Society , 115 : 363 – 381 .
- Fayram , AH , Hansen , MJ and Ehlinger , TJ . 2005 . Interactions between walleyes and four fish species with implications for walleye stocking . North American Journal of Fisheries Management , 25 : 1321 – 1330 .
- Fedoruk , AN . 1966 . Feeding relationship of walleye and smallmouth bass . Journal of the Fisheries Research Board of Canada , 23 : 941 – 943 .
- Frey , AP , Bozek , MA , Edwards , CJ and Newman , SP . 2003 . Diet overlap and predation between smallmouth bass and walleye in a north temperate lake . Journal of Freshwater Ecology , 18 : 43 – 54 .
- Gangl , RS , Pope , KL and Willis , DW . 1997 . Seasonal trends in food habits and growth of smallmouth bass in Lake Poinsett, South Dakota , South Dakota : Department of Game, Fish and Parks, Fisheries Division. . Report 97-5. p. 17
- Gerking , SD . 1994 . The feeding ecology of fish , 416 San Diego, CA : Academic Press .
- Hanson , PC , Johnson , TB , Schindler , DE and Kitchell , JF . 1997 . Fish bioenergetics 3.0 for windows , Madison : University of Wisconsin Sea Grant .
- Hanson , SD and Curry , RA . 2005 . Effects of river herring management in the Saint John River, New Brunswick on trophic interactions with age-0 smallmouth bass . Transactions of the American Fisheries Society , 134 : 356 – 368 .
- Hubert , WA . 1977 . Comparative food habits of smallmouth and largemouth basses in Pickwick Reservoir . Journal of the Alabama Academy of Science , 48 : 167 – 178 .
- Jackson , DA . 2002 . “ Ecological impacts of Micropterus introductions: the dark side of black bass ” . In Black bass: ecology, conservation and management , Edited by: Phillip , DP and Ridgway , MS . 221 – 234 . Bethesda, MD : American Fisheries Society .
- Johnson , FH and Hale , JG . 1977 . Interrelations between walleye (Stizostedion vitreum vitreum) and smallmouth bass (Micropterus dolomieui) in four northeastern Minnesota lakes, 1948–69 . Journal of the Fisheries Research Board of Canada , 34 : 1626 – 1632 .
- Keddy , PA . 2001 . Competition, , 2nd , 576 Dordrecht, , The Netherlands : Kluwer Academic Publishers .
- Knight , RL , Margraf , FJ and Carline , RF . 1984 . Piscivory by walleyes and yellow perch in western Lake Erie . Transactions of the American Fisheries Society , 113 : 677 – 693 .
- Kwak , TJ , Wiley , MJ , Osborne , LL and Larimore , RW . 1992 . Application of diel feeding chronology to habitat suitability analysis of warmwater stream fishes . Canadian Journal of Fisheries and Aquatic Sciences , 49 : 1417 – 1430 .
- Lott , JP . 1996 . Relationships between smallmouth bass feeding ecology and population structure and dynamics in lower Lake Oahe, South Dakota , South Dakota : Department of Game, Fish and Parks, Fisheries Division. . Report 96-3. p. 52
- Lyons , J . 1987 . Prey choice among piscivorous juvenile walleyes (Stizostedion vitreum) . Canadian Journal of Fisheries and Aquatic Sciences , 44 : 758 – 764 .
- Mathias , JA and Li , S . 1982 . Feeding habits of walleye larvae and juveniles: comparative laboratory and field studies . Transactions of the American Fisheries Society , 124 : 886 – 897 .
- Mougi , A and Nishimura , K . 2005 . Coexistence of competitive species with a stage-structured life cycle . Ecological Research , 20 : 581 – 589 .
- Moyle , PB , Li , HW and Argon , BA . 1986 . “ The Frankenstein effect: impact of introduced fishes on native fishes in North America ” . In Fish culture in fisheries management , Edited by: Stroud , RH . Bethesda, MD : American Fisheries Society, Fish Culture Section and Fisheries Management Section .
- Noakes , DLG . 1980 . “ Social behavior in young charrs ” . In Charrs: salmonid fishes of the genus Salvelinus , Edited by: Balon , EK . The Hague, , The Netherlands : Dr W. Junk Publishers .
- Novinger , DC . 2000. Reversals in competitive ability: do cutthroat trout have a thermal refuge from competition with brook trout? [PhD dissertation]. [Laramie (WY)]: University of Wyoming
- Persson , L . 1983 . Effects of intra- and interspecific competition on dynamics and size structure of a perch Perca fluviatilis and roach Rutilus rutilus population . Oikos , 41 : 126 – 132 .
- Persson , L . 1987 . Competition-induced diet shifts in young-of-the-year perch (Perca fluviatilis) . Environmental Biology of Fishes , 19 : 235 – 239 .
- Pianka , ER . 1973 . The structure of lizard communities . Annual Review of Ecology and Systematics , 4 : 53 – 74 .
- Rottiers , DV and Lemm , CA . 1985 . Movement of underyearling walleyes in response to odor and visual cues . The Progressive Fish-Culturist , 47 : 34 – 41 .
- Sabo , MJ , Pert , EJ and Winemiller , KO . 1996 . Agonistic behavior of juvenile largemouth bass and smallmouth bass . Journal of Freshwater Ecology , 11 : 115 – 118 .
- Savino , JF and Kolar , CS . 1996 . Competition between nonindigenous ruffe and native yellow perch in laboratory studies . Transactions of the American Fisheries Society , 125 : 562 – 571 .
- Savino , JF and Kostich , MJ . 2000 . Aggressive and foraging behavioral interactions among ruffe . Environmental Biology of Fishes , 57 : 337 – 345 .
- Schleuter , D and Eckmann , R . 2006 . Competition between perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus): the advantage of turning night into day . Freshwater Biology , 51 : 287 – 297 .
- Schlick , RO . 1978 . Management for walleye or sauger, south basin, Lake Winnipeg , 11 America. Bethesda, MD : American Fisheries Society, Special Publication .
- Schoener , TW . 1983 . Field experiments on interspecific competition . The American Naturalist , 122 : 240 – 285 .
- Schulze , T , Baade , U , Dörner , H , Eckmann , R , Haertel-Borer , SS , Hölker , F and Mehner , T . 2006 . Interactions of residential piscivores with an introduced new predator type in a mesotrophic lake . Canadian Journal of Fisheries and Aquatic Sciences , 63 : 2202 – 2212 .
- Stewart , JI . 1978. Daily feeding chronology of young smallmouth bass in the Snake River, Minnesota [MS thesis]. [St. Paul (MN)]: University of Minnesota
- Thomas , HM . 1996. Competitive interactions between a native and exotic trout species in high mountain streams [MS thesis]. [Logan (UT)]: Utah State University
- Tilman , D . 1987 . On the meaning of competition and the mechanisms of competitive superiority . Functional Ecology , 1 : 304 – 315 .
- Vander Zanden , MJ , Wilson , KA , Casselman , JM and Yan , ND . 2004 . “ Species introductions and their impacts in North American shield lakes ” . In Boreal shield watersheds: lake trout ecosystems in a changing environment , Edited by: Gunn , JM , Ryder , RA and Steedman , RJ . 239 – 263 . Boca Raton, FL : CRC Press .
- Warren Jr , ML . 2009 . “ Centrarchid identification and natural history ” . In Centrarchid fishes: diversity, biology and conservation , Edited by: Cooke , SJ and Philipp , DP . 375 – 481 . New York : Blackwell Publishing .
- Werner , EE and Gilliam , JF . 1984 . The ontogenetic niche and species interactions in size-structured populations . Annual Review of Ecology and Systematics , 15 : 1042 – 1052 .
- Winemiller , KO and Taylor , DH . 1987 . Predatory behavior and competition among laboratory-housed largemouth and smallmouth bass . The American Midland Naturalist , 117 : 148 – 166 .
- Wuellner , MR . 2009. Determining whether competitive interactions exist between walleye and smallmouth bass in South Dakota waters [PhD dissertation]. [Brookings (SD)]: South Dakota State University
- Wuellner , MR , Chipps , SR , Willis , DW and Adams Jr , WE . 2010 . Interactions between walleyes and smallmouth bass in a Missouri River reservoir with consideration of the influence of temperature and prey . North American Journal of Fisheries Management , 30 : 445 – 463 .