Abstract
We monitored benthic macroinvertebrates and adult caddisflies along an agricultural stream continuum upstream, within, and downstream of a small forested preserve. The habitat upstream of all sites was >60% disturbed by agricultural activities. The percentage of riparian disturbance was markedly lower adjacent to the sites inside the preserve than those outside. Water physicochemical factors did not exhibit clear changes among sites, except for nitrate concentration, which was highest upstream of the preserve. Biological diversity of adult caddisflies was significantly higher within the preserve. Biological diversity of benthic invertebrates exhibited similar results except for non-significance between the upstream and preserve sites. Pollution tolerance and percentage of filtering collector metrics were unchanged among sites for both assemblages. The percentage of adult caddisflies in the shredder functional group increased significantly within the preserve but remained small relative to that of pollution-tolerant filtering collectors. The small terrestrial preserve promoted a three-fold increase in species diversity, even without corresponding changes in water quality or trophic structure. Such an increase, however, may not be as detectable with traditional benthic biomonitoring techniques due to the difficulties of sampling benthic microhabitats representatively and identifying specimens to the species level.
Introduction
Intensive agriculture has led to stream channelization, draining of wetlands, modification or loss of the surrounding floodplain, and removal of riparian canopy cover with subsequent loss of coarse allochthonous input (Gregory et al. Citation1991; Allan Citation1995; Delong and Brusven Citation1998). Agricultural runoff into aquatic habitats often contains large amounts of sediment and fine organic matter (Zweig and Rabeni Citation2001; Baker and Richards Citation2003; Nord and Lanyon Citation2003). Collectively, these impacts promote the destruction of many stream microhabitats and an increase in secondary production, especially in small and medium streams. Essentially, small streams become ‘homogenized’ and develop the characteristics of larger rivers (Delong and Brusven Citation1992, Citation1993; Pringle et al. Citation1993; Houghton Citation2007). In fact, riparian disturbance with subsequent nutrient and sediment input has been repeatedly found to be the most widespread stressor of streams in the US generally, in the Plains and Lowlands region, and in Michigan (Paulsen et al. Citation2008; Wang et al. Citation2008).
Most studies on aquatic disturbance have tested the effects of increased pollution into relatively undisturbed ecosystems (Mulholland et al. Citation2000; Tank et al. Citation2000; Peterson et al. Citation2001; Wolheim et al. Citation2001; Carlisle and Clements Citation2003; Hall and Tank Citation2003; Chadwick and Huryn Citation2005; Woodcock and Huryn Citation2007). In such studies, tested responses such as nutrient concentrations, biological diversity, or secondary production follow an array of predictable patterns based on the nature of the perturbation. Few studies, however, have addressed the influence of small undisturbed environments on the biology or physicochemistry of an overall agricultural stream.
The distinction is an important one. First, stream systems with high levels of disturbance are fundamentally different than those with low disturbance levels. Most agricultural streams have minimal riparian shade or coarse allochthonous input, have high levels of primary and secondary production, and contain nutrient concentrations 100s of times higher than those of relatively undisturbed streams (Kemp and Dodds Citation2001; Vanni et al. Citation2001; Royer et al. Citation2004; Inwood et al. Citation2005). Such streams may not behave in ways predicted by typical disturbance models (Niyogi et al. Citation2003; Spänoff and Meyer Citation2004; Bernot et al. Citation2006; Gulis et al. Citation2006; Paul et al. Citation2006; McTammany et al. Citation2008).
Studying the importance of small isolated natural habitats is also necessary because it is frequently the real-world situation. Many of the natural ecosystems of the north central US and elsewhere have been fragmented into smaller natural habitats surrounded by an agricultural landscape. Large-scale agriculture, for example, covers more than 85% of the land area of Iowa, Indiana, Illinois, and southern Michigan (USGS Citation2007). Thus, small fragmented habitats are potentially vital for improving stream water quality and protecting aquatic biological diversity.
Several recent studies (Houghton Citation2004a; Rios and Bailey Citation2006; Urban et al. Citation2006) have noted positive correlations between the amount of intact riparian vegetation associated with fragmented terrestrial habitats and the biological diversity in the adjacent streams. This phenomenon appears especially pronounced in watersheds with significant agricultural disturbance. Possible explanations for these observations are the intact riparian corridor is trapping sediment and assimilating nutrients, thereby improving water quality (Carothers Citation1977), or the natural habitat promotes an increase in diversity simply through its presence even without an improvement in water quality.
This study had two basic objectives. First we tested the aforementioned explanations by examining both physicochemical and biological factors down the continuum of a single agricultural stream as it passed through an isolated natural habitat. Second, we analyzed data from both benthic macroinvertebrate and adult caddisfly assemblages collected at the same sites to test if one fauna was more powerful than the other at detecting changes in habitat or water physicochemistry along the lotic continuum.
Materials and methods
Study site and disturbance determination
The east branch of the Saint Joseph River arises in southern Michigan and flows southerly through predominately agricultural land to Ohio. A third order reach of approximately 8 km flows through the Lost Nations State Game Preserve in Hillsdale County, a habitat comprising 10 km2 of natural wetland and secondary deciduous forest. Six sampling sites were selected for this study. Sites 1 and 2 were upstream of the preserve, sites 3 and 4 within it, and sites 5 and 6 downstream of it. Adult caddisflies were sampled from all six sites. Due to logistic constraints, water physicochemical samples were collected from sites 1, 3, and 5. Benthic macroinvertebrates were collected from sites 2, 3, 4, and 5.
The percentage of disturbed habitat upstream of each sampling site was calculated, as was the percentage of disturbed riparian habitat as defined by the 1 km2 area adjacent to the sampling site. Percentages of ‘disturbed’ and ‘undisturbed’ habitat were calculated by determining the relative prevalence of agricultural and forested lands, respectively, using the ‘Tabulate Area’ function of ArcView for Windows® software (ESRI Citation1996) from USGS (Citation2007) data. No other appreciable land use was prevalent within the study area.
Chemical sampling and analysis
Water samples were collected on three dates during May 2007. Nine samples were collected from each site on each date. Samples were collected in polyethylene plastic bottles and stored at room temperature until analysis.
All pH, conductivity, dissolved oxygen, and temperature measurements were made on-site at the time of sample collection. All measurements were made within 2 h of each other to minimize diel fluctuations. Conductivity (Eutech Instruments ECTestr Low) and pH (Fisher AccuMet AP61) were measured electronically. Dissolved oxygen and temperature were measured with a YSI-55 probe (YSI Environmental). Total organic carbon (TOC) measurements were performed on collected samples using a colorimetric TOC reagent set (Hach Method 10129, Hach). Water hardness was measured by EDTA titration in a pH 10 buffer.
Nitrate was detected and measured by high performance liquid chromatography using a Waters IC-Pak Anion HR ion exchange column (4.6 × 75 mm, 6 µm particles, capacity of 30 ± 3 µeq/mL) and two detectors: a Waters 431 conductivity detector and a Perkin Elmer Spectroflow 783 ultraviolet (UV) absorbance detector at a wavelength of 220 nm. A borate/gluconate mobile phase (pH 8.5) was prepared and used according to the Waters IC Pak Column Care and Use Manual 091064TP rev. 0, section 3.3 (Waters). The mobile phase was filtered and de-gassed through a HAWP 0.45 µm filter (Millipore) prior to use. Purified water for all solution preparation was obtained from a Milli-Q system (Millipore). River samples were injected through Fisherbrand 0.45 µm syringe filters (13 mm) in order to eliminate particulate matter.
Biological sampling
Larval insects were sampled in May 2007 using Hess samplers with a 0.3 m2 area (Barbour et al. Citation1999). To mitigate the natural variation associated with benthic sampling, three samples were collected from each of the three riffles within a sampling site, and combined into a single sample for analysis. All collected specimens were identified to the lowest identifiable taxon, typically genus, after Hilsenhoff (Citation1995).
Adult caddisflies were sampled during late June 2007 with UV light traps. Each trap consisted of an 8-watt UV light placed over a white pan filled with 70% EtOH. A trap was placed within 2 m of a stream at dusk and retrieved approximately 2 h later. Three samples were collected at each of the six sites. To standardize weather conditions, samples were collected only if the peak daytime temperature was >22°C, dusk temperature was >13°C, and there was no noticeable wind or precipitation at dusk. For an in-depth discussion of this technique, see Houghton (Citation2004a).
Analyses
The following biotic metrics were calculated from adult caddisfly data: taxa richness at the genus and species level, percentage of filtering collectors, percentage of shredders, and pollution tolerance. The first three metrics have been shown to be the most accurate in reflecting upstream disturbance levels in small and medium streams in Minnesota (Houghton Citation2006). The pollution-tolerant metric combined specimen abundance with predetermined tolerance to organic pollution values for each taxon to produce a single tolerance value for each site. Tolerance values were determined from Hilsenhoff (Citation1987). Taxa without predetermined tolerance values were not included in the analyses. Taxa richness, percentage of filtering collectors, percentage of shredders, and pollution tolerance metrics were also calculated for larval insects. Trophic functional groups were determined at the generic level for both assemblages using Merritt et al. (Citation2008).
A one-way analysis of variance (ANOVA) with post hoc Tukey test was run on each of the calculated metrics and physiochemical measurements using JMP for Windows® Software (JMP Citation2002) to assess differences in mean values between sampling sites. Percentages were transformed through an ArcSine function before analysis (Zar Citation2007). Linear regression models were calculated using the freeware program Arc for Windows® (http://www.wiley.com/mathematics) (Cook and Weisberg Citation1999). All biological metrics and physiochemical measurements were tested individually for their ability to predict the known values of percentage of disturbed riparian habitat. A model was considered significant if its slope was significantly different from zero (Cook and Weisberg Citation1999).
Results
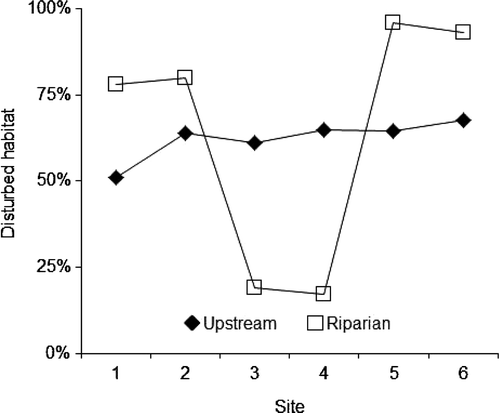
The relative percentages of habitat disturbance upstream of each sampling site exhibited a slight increase from the upstream sites to the downstream sites, with no appreciable difference associated with the sites within the preserve. The percentage of riparian disturbance, however, was markedly lower (<25%) adjacent to the sites within the preserve than those outside (>75%) ().
Figure 1. The percentage of disturbed habitat upstream of each of the six respective sampling sites and the percentage of disturbed riparian habitat within a defined 1 km2 area adjacent to each sampling site (USGS Citation2007).
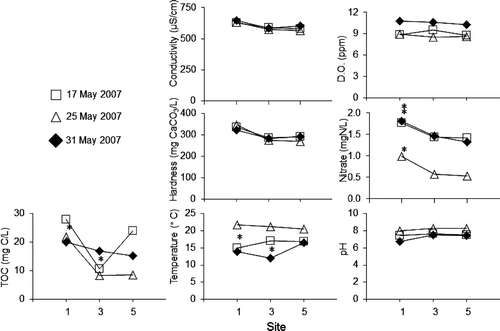
The temperature was significantly lower upstream of the preserve on 25 May, and significantly lower within the preserve on 31 May. TOC concentration was significantly lower within the preserve on 17 May and significantly higher upstream of the preserve on 25 May. The only consistent result was for nitrate concentration, which was significantly higher upstream of the preserve for all three sampling dates; overall concentrations were lower on 25 May than the other dates. There was no significant difference in conductivity, dissolved oxygen, hardness, or pH between any of the three sampling locations for any of the three sampling dates ().
Figure 2. Mean values for seven water physicochemical measurements taken from three sites. TOC: total organic carbon, DO: dissolved oxygen. Error bars omitted for clarity. Asterisks represent statistically distinct groups (One-way ANOVA with post hoc Tukey test, p < 0.01 for all). Tests on the other data were not significant.
Over 30,000 adult caddisfly specimens and nearly 9000 benthic specimens were identified during this study. Adult caddisfly species richness was significantly higher within the preserve (). A total of 33 caddisfly species was collected within the preserve, compared to 13 outside ( and ). Of the 20 species found only in the preserve, eight were shredders, five were predators, four were gathering collectors, two were scrapers, and one was a filtering collector ( and ). All species found outside the preserve were also found inside the preserve. Benthic macroinvertebrate taxa richness exhibited a similar pattern, although the difference between the upstream site and the preserve sites was not significant (). All larval caddisfly genera collected in benthic samples were also found as adults except for Neophylax and Pycnopsyche. Eleven caddisfly genera were found as adults and not found as larvae. Outside the preserve, 78% of the known genera were collected as larvae, whereas only 52% were found as larvae within the preserve.
Figure 3. Mean (+1 SE) values for four water quality metrics calculated using adult caddisfly data (a) and benthic macroinvertebrate data (b) obtained from our six sampling sites. Asterisks denote statistically distinct groups (One-way ANOVA with post hoc Tukey test, p < 0.01 for all). Tests on the other data were not significant.
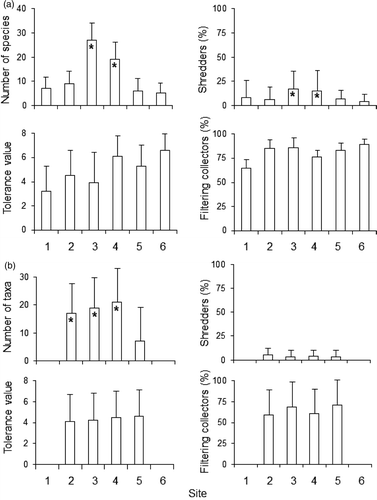
Table 1. The 13 species found in the Saint Joseph River outside of the Lost Nations Game Preserve showing functional group, tolerance value, and if its genus was located during benthic sampling.
Table 2. The 33 species found in the Saint Joseph River inside the Lost Nations Game Preserve showing functional group, tolerance value, and if its genus was located during benthic sampling.
Fauna both inside and outside of the preserve was dominated by pollution-tolerant gathering collectors and filtering collectors, the latter group composed 60–70% of the benthic fauna and 60–90% of the adult caddisfly fauna. Shredders, conversely, comprised <20% of the adult caddisflies and <5% of the benthic invertebrates. Neither the pollution tolerance metric nor the percentage of filtering collectors exhibited clear trends among sites for either fauna (). The percentage of shredders was low for both benthic invertebrates and adult caddisflies, although shredders exhibited significant increase in the preserve sites for the adults.
Linear regression models produced using adult caddisfly species richness, adult genus richness, larval taxa richness, and percentage of adult caddisfly shredders all explained significant amounts of variation in the percentage of protected riparian habitat. All other metrics explained <50% of variation with insignificant slopes ().
Table 3. Models generated with linear regression analysis of biological metrics and physiochemical variables individually on the percentage of disturbed adjacent riparian habitat at six sampling sites on the Saint Joseph River.
Discussion
It is unclear if the natural habitat of the Lost Nations Game Preserve improves the water quality of the Saint Joseph River. Nitrate concentrations, while consistent with Michigan agricultural streams throughout the entire sampled continuum (Castillo et al. Citation2000; Bernot et al. Citation2006; Arango et al. Citation2007), do show a small decrease as the stream interacts with the protected riparian zone. Conversely, no consistent differences between sites were seen in the other parameters tested. Overall, the conductivity, pH, hardness, and dissolved oxygen values were also consistent with those of southern Michigan agricultural streams (Castillo et al. Citation2000; Bernot et al. Citation2006; Arango et al. Citation2007). TOC values were higher than expected, especially upstream of the preserve; however, the high variability in our data precludes definite conclusions. Likewise, the inconsistencies seen in our temperature data also preclude definite conclusions and may be due to naturally occurring differences between stream microhabitats.
Despite our unclear physicochemical data, biological assemblages in the river suggest minimal differences in water quality between sites. The Saint Joseph River is a third-order woodland stream (Strahler Citation1952). Under natural conditions in the river continuum, a fairly high abundance of shredders and low abundance of filtering collectors is expected (Vannote et al. Citation1980). For example, the adult caddisfly fauna in third-order woodland streams under natural conditions in Minnesota averaged almost 50% shredders and <25% filtering collectors (Houghton Citation2007), as opposed to the <20% shredders and >70% filtering collectors found at all sites of the Saint Joseph River. A high abundance of filtering collectors and a low abundance of shredders in small and medium streams is a reliable indicator of ‘stream homogenization’ – small streams taking on the characteristics of larger rivers due to high levels of agricultural input (Delong and Brusven Citation1998; Houghton Citation2004b; Houghton Citation2006). This phenomenon appears to be occurring at all study sites and is further reinforced by the pollution tolerance metric, which likewise did not exhibit a clear trend between sites for either fauna.
Although the natural vegetation of the preserve may be assimilating some nutrients and blocking some sediment, it is probably not doing enough of either to fundamentally change the water quality of the adjacent stream. Midwestern streams with >50% of their watershed in agriculture often have phosphate and nitrate levels that are at or approaching saturation (Bernot et al. Citation2006). Thus, a very substantial amount of nutrients must be assimilated by riparian vegetation to have any noticeable effect on levels within the stream. Such a phenomenon is unlikely to be occurring in the Saint Joseph River, due to its high levels (50–70%) of disturbed habitat within the watersheds of all sites (). Thus, the relatively small riparian zone within the preserve is likely too small to significantly affect the water quality of our study sites (Bernot et al. Citation2006).
Even without a correspondingly clear change in water quality, the Saint Joseph River clearly does support greater biological diversity when it flows through the preserve than when surrounded by the prevailing agroecosystem of the watershed. Almost triple the number of adult caddisfly species were found in the preserve as outside. Results obtained using benthic invertebrates were less clear than with adult caddisflies, but both assemblages exhibited the same trend.
This observed increase was almost certainly due to the presence of natural riparian canopy cover and habitat in the sites adjacent to the preserve. Natural riparian vegetation is one of the most important factors influencing biological diversity and stream processing, even in disturbed agricultural streams (Rios and Bailey Citation2006; McTammany et al. Citation2007). Increased canopy cover can mitigate the effects of warm upstream temperatures by shading and cooling the stream, thus allowing the re-colonization of cold stenothermic invertebrates such as Rhyacophila or Glossosoma (Allan Citation2004). Both genera were found exclusively at the preserve sites in our study, although our inconsistent temperature data preclude definite conclusions. More importantly, the coarse allochthonous input from riparian vegetation is crucial for normal stream processing (Wallace et al. Citation1997; Gregory et al. Citation2003; Li and Dudgeon Citation2008). Shredders in particular are dependent on allochthonous input; thus, an increase in such input promotes higher shredder diversity (Hieber and Gessner Citation2002; Houghton Citation2007; Moline and Poff Citation2008). Adult caddisfly shredders in our study rose from one species outside the preserve to eight within and provided the single best model to predict levels of disturbed riparian habitat.
It appears from the results of this study and elsewhere that the effects of pollution and the effects of habitat loss are two separate mechanisms disrupting normal stream functioning. An increase in nutrients and sediment will increase stream autotrophy, decrease populations of pollution intolerant taxa, and increase the abundance of tolerant taxa, especially the filtering collectors that can utilize fine (<0.25 mm) particulate organic matter as a food resource (Barbour et al. Citation1999; Allan Citation2004; Houghton Citation2004b; Houghton Citation2007). The confounding water chemistry values, high abundance of filtering collectors, and high pollution tolerance values seen at all sites of our study appears to indicate such a phenomenon occurring in the Saint Joseph River. It also appears, however, that agricultural streams like the Saint Joseph River can still support small populations of intolerant taxa simply due to the presence of natural habitat and allochthonous input. Such populations, however, will likely remain too small to have a noticeable effect on water quality metrics such as pollution tolerance value or percentage of filtering collectors. High levels of inorganic sediment input, for example, decrease the ability of colonizers and shredders to process allochthonous debris even when such material is present (McTammany et al. Citation2008). Such decreases will lower the populations of shredders as well as those of other woodland stream taxa (Li and Dudgeon Citation2008).
Many studies have pointed out problems inherent with using benthic macroinvertebrates for biomonitoring. Adult caddisflies have been alternatively proposed as a potentially ideal taxon for single-order adult biomonitoring due to their high species richness, ecological diversity, varying susceptibilities to different types of human disturbance, ease of collection, and abundance in virtually all types of freshwater ecosystems (Mackay and Wiggins Citation1979; Resh Citation1993; Rosenberg and Resh Citation1993; Barbour et al. Citation1999; Dohet Citation2002; Houghton Citation2004a; Houghton Citation2006). While it was not the objective of this study to provide the final answer on the fauna to use in biomonitoring, our results do yield some insights.
Adult caddisflies can be readily identified to the species level, whereas most larvae cannot. A lack of taxonomic resolution often leads to a loss of information and sensitivity in detecting changes between sites (Hawkins et al. Citation2000; Lenat and Resh Citation2001; Houghton Citation2006; Stribling et al. Citation2008). This phenomenon appeared to be occurring in our study as well. Adult caddisflies more accurately predicted determined differences in riparian habitat disturbance than were benthic taxa, although both faunas had value in doing so. Likewise, adult caddisflies identified to the species level had greater predictive power than those identified to the genus level, the same taxonomic resolution as most benthic larvae. Species-level resolution appears especially important when differences between sampling sites are subtle (Houghton Citation2006; Chessman et al. Citation2007); this was likely the situation along our sampled continuum.
Taxonomic resolution does not entirely explain the greater ability of adult caddisflies to detect changes in riparian disturbance, however. A more important issue may be the inherent difficulty of obtaining representative samples of the benthic environment. Aquatic habitats contain microhabitats that are nearly impossible to sample representatively or consistently; the choice of sampled microhabitat can have a significant impact on the results obtained (Carter and Resh Citation2001; Roy et al. Citation2003; Gerth and Herily Citation2006; Chessman et al. Citation2007). In contrast, adult caddisflies have left the natal habitat, rendering microhabitat sampling irrelevant. Further, several studies have shown that dispersal of adult caddisflies between habitats is of negligible importance (Sode and Wiberg-Larson Citation1993; Petersen et al. Citation1999; Houghton Citation2004a).
We sampled benthic larvae only from shallow riffles due to the ease of doing so. This strategy, obviously, left out many other habitats, such as runs, stream edges, deep pools, or woody debris. Such habitats often contain disparate faunas and can be difficult to sample with Hess samplers or other devices (Pridmore and Roper Citation1985; Gerth and Herily Citation2006; Chessman et al. Citation2007). The fact that 11 caddisfly genera, including large and conspicuous specimens such as Hesperophylax, Agrypnia, and Phryganea, were found as adults but not larvae strongly suggests that biological diversity was missed with our benthic sampling. The two caddisfly genera collected from the benthic community but not found as adults, Neophylax and Pycnopsyche, commonly emerge during autumn and were not present during adult sampling. Of the 11 genera missed in benthic samples, seven were shredders, specimens likely found in slow-moving water on woody debris (Wiggins Citation2008). The difficulty of finding larval shredders also explains why the percentage of adult caddisfly shredders found in sites of the preserve was more than 10 times that of larval shredders.
Even within a constant habitat type, such as shallow riffles, benthic samples may be variable due to the inherent heterogeneity of macroinvertebrate distributions and the difficulty of consistent sampling (Downes et al. Citation1993; Palmer et al. Citation1997; Barbour et al. Citation1999), although we did attempt to account for this problem by combining benthic samples. Some taxa, such as Oecetis, Triaenodes, and most hydroptilids, are simply difficult to find in the stream relative to their abundance regardless of sampling device (Wiggins Citation2008 and our observations). All of these taxa were found as adults but not larvae in our study. Other genera, such as Cheumatopsyche, Chimarra, Hydropsyche, and Pycnopsyche, are conspicuous and nearly always abundant in samples. Microhabitat selection may lead to missing taxa that are conspicuous but rare, such as Glossosoma in our study. Other taxa, such as Lepidostoma, Anabolia, many hydroptilds, and many leptocerids, exhibit an aggregated dispersion; thus, samples may yield either none or many specimens (Karl and Hilsenhoff Citation1979; Martin and Barton Citation1987; Hoffmann Citation1997, and our observations). These phenomena make it difficult to representatively sample benthic habitats and determine the biological diversity of a site. They also explain why the standard errors (SEs) of our benthic metrics were higher than that of adult metrics.
This study supports two basic conclusions and raises some interesting questions. First, a small terrestrial preserve does appear to increase biological diversity in the adjacent stream simply due to the presence of natural riparian habitat, even without an obvious corresponding improvement in water quality. Second, while this increase is detectable using traditional benthic biomonitoring techniques, information is lost due to the problems of taxonomic resolution and microhabitat selection. What is less clear is how biomonitoring techniques and metrics should be applied to agricultural streams.
A fundamental assumption inherent in water quality biomonitoring for the last 30+ years is: ‘the valley rules the stream’ (Hynes Citation1975). That is, the conditions at a stream site reflect the watershed conditions upstream of that site, and changes in watershed conditions will be accompanied by corresponding changes in stream physicochemistry and invertebrate composition. Many recent studies have supported this assumption (Snelder and Biggs Citation2002; Townsend et al. Citation2003; Houghton Citation2007; Norris et al. Citation2007; Paulsen et al. Citation2008). The bases for this assumption, however, are the predictable responses of natural stream conditions to a disturbance, not the responses of a disturbed stream to a small natural habitat. For example, a decrease in the taxonomic richness of diverse groups such as Trichoptera is one of the most well-established responses to stream disturbance (Kerans and Karr Citation1994; Karr and Chu Citation1999; Berlin and Thiele Citation2002; Houghton Citation2006; Houghton Citation2007). A common method of quantifying stream disturbance is measuring the percentage of disturbed habitat upstream of a sampling site. In our study, however, taxonomic richness of neither adult caddisflies nor benthic invertebrates corresponded with this measurement. Both assemblages, as well as the percentage of adult caddisfly shredders, correlated instead with percentage of disturbed riparian habitat. Most water physicochemical factors and percentage of filtering collectors did not correspond to the percentage of disturbed riparian habitat, reflecting instead the overall agricultural nature of the watershed and stream.
If the goal of aquatic biomonitoring is to assess the ‘health’ (Karr and Chu Citation1999) of a stream and, ultimately, the health of the surrounding landscape, then water quality metrics must be chosen carefully to accurately evaluate an agricultural stream. The specific definition of stream health is important to ascertain as well. For example, based on their level of upstream habitat disturbance, the Lost Nations Game Preserve sites of the Saint Joseph River have nearly double the number of adult caddisfly species than do deciduous woodland streams of southern Minnesota at similar upstream disturbance levels (UMSP Citation2009). Conversely, the percentages of filtering collectors, normally one of the most reliable indicators of stream homogenization (Houghton Citation2006, Citation2007), are comparable to these same streams. Thus, if the priority is on the protection of biological diversity, then the Lost Nations Game Preserve sites could be considered ‘healthier’ than the disturbed Minnesota streams (and the rest of the Saint Joseph River) due to their preserved riparian habitat. If the primacy is on watershed and water quality protection, then they might not.
A clearer conclusion is that biological diversity of caddisfly species nearly tripled within the preserve. This result reflects merely one aquatic insect order in a single small terrestrial preserve. If our results can be extrapolated to other taxa and other agroecosystems of the north central US, then it is likely that a substantial component of the remaining aquatic biological diversity is concentrated in preserves that are very small relative to the surrounding agricultural landscape. The continuing fragmentation of the landscape into these small ‘refuge’ habitats necessitates greater study and, more importantly, the preservation of such refuges.
Acknowledgements
The authors thank M.J. Jordan, E.L. Julianus, and A.M. Zebell for laboratory and field assistance. Research costs were paid by Laboratory for Advanced Undergraduate Research Education Adapted for Talented and Extraordinary Students (LAUREATES) grants to AG and JT and by Hillsdale College biology department funds to DCH and chemistry funds to MAN. The valuable comments of Y. Cao and two anonymous reviewers improved earlier versions of this article.
References
- Allan , JD . 1995 . Stream ecology: structure and function of running waters , London, , UK : Chapman and Hall .
- Allan , JD . 2004 . Landscapes and riverscapes: the influence of land use on stream ecosystems . Annual Review of Ecology and Evolutionary Systematics , 35 : 257 – 284 .
- Arango , CP , Tank , JL , Schaller , JL , Royer , TV , Bernot , MJ and David , MB . 2007 . Benthic organic carbon influences denitrification in streams with high nitrate concentration . Freshwater Biology , 52 : 1210 – 1222 .
- Baker , DB and Richards , RP . 2003 . Phosphorous budgets and riverine phosphorous export in northwestern Ohio watersheds . Journal of Environmental Quality , 31 : 96 – 108 .
- Barbour , MT , Gerritsen , J , Snyder , BD and Stribling , JB . 1999 . Rapid bioassessment protocols for use in streams and rivers: periphyton, benthic macroinvertebrates, and fish, 2nd ed , Washington, DC : Office of Water, US Environmental Protection Agency .
- Berlin , A and Thiele , V . 2002 . Trichoptera in assessment and classification of streams in the lowlands of northeastern Germany , Mey W, editor. . Proceedings of the 10th International Symposium on Trichoptera, 30 July–05 August, Potsdam, Germany. Nova Supplementa Entomologica, Keltern, Germany. p. 481–490
- Bernot , MJ , Tank , JL , Royer , TV and David , MB . 2006 . Nutrient uptake in rivers draining agricultural catchments of the Midwestern United States . Freshwater Biology , 51 : 499 – 509 .
- Carlisle , DM and Clements , WH . 2003 . Growth and secondary production of aquatic insects along a gradient of Zn contamination in Rocky Mountain streams . Journal of the North American Benthological Society , 22 : 582 – 597 .
- Carothers , SW . 1977 . Importance, preservation, and management of riparian habitat: an overview , 2 – 4 . Fort Collins, Colorado : Rocky Mountain Forest and Range Experiment Station, USDA Forest Service General Technical Report RM 43, Importance, Preservation, and Management of Riparian Habitat .
- Carter , JL and Resh , VH . 2001 . After site selection and before data anylsis: sampling, sorting, and laboratory procedures used in stream benthic macroinvertebrate monitoring programs by USA state agencies . Journal of the North American Benthological Society , 20 : 658 – 682 .
- Castillo , MM , Allan , JD and Brunzell , S . 2000 . Nutrient concentrations and discharges in a Midwestern agricultural catchment . Journal of Environmental Quality , 29 : 1142 – 1151 .
- Chadwick , MA and Huryn , AD . 2005 . Response of macroinvertebrate production to atmospheric nitrogen deposition and channel drying . Limnology and Oceanography , 50 : 228 – 236 .
- Chessman , B , Williams , S and Besley , C . 2007 . Bioassessment of streams with macroinvertebrates: effect of sampled habitat and taxonomic resolution . Journal of the North American Benthological Society , 26 : 546 – 565 .
- Cook , RD and Weisberg , S . 1999 . Applied regression including computing and graphics , New York : John Wiley and Sons, Inc .
- Delong , MD and Brusven , MA . 1992 . Patterns of chlorophyll a in an agricultural non-point source impacted stream . Water Resources Bulletin , 28 : 731 – 741 .
- Delong , MD and Brusven , MA . 1993 . Storage and decomposition of particulate organic matter along the longitudinal gradient of an agriculturally-impacted stream . Hydrobiologia , 262 : 77 – 88 .
- Delong , MD and Brusven , MA . 1998 . Macroinvertebrate community structure along the longitudinal gradient of an agriculturally impacted stream . Environmental Management , 22 : 445 – 457 .
- Dohet , A . 2002 . Are caddisflies an ideal group for the assessment of water quality in streams? In: Mey W, editor . Proceedings of the 10th International Symposium on Trichoptera . 30 July–05 August 2002 , Potsdam. Germany. Nova Supplementa Entomologica, Keltern, Germany. p. 507–520
- Downes , BJ , Lake , PS and Schreiber , ESG . 1993 . Spatial variation in the distribution of stream invertebrates: implications of patchiness for models of community organization . Freshwater Biology , 30 : 119 – 132 .
- ESRI (Environmental Systems Research Institute). 1996. Using ArcView GIS, owner's manual. Redlands, California: ESRI
- Gerth , W and Herily , AT . 2006 . Effect of sampling different habitat types in regional macroinvertebrate bioassessment surveys . Journal of the North American Benthological Society , 25 : 501 – 512 .
- Gregory , SV , Boyer , KL and Gurnell , AM . 2003 . The ecology and management of wood in world rivers , Bethesda, , Maryland : American Fisheries Society, American Fisheries Society Symposium .
- Gregory , SV , Swanson , FJ , McKee , WA and Cummins , KW . 1991 . An ecosystem perspective of riparian zones . Bioscience , 41 : 540 – 551 .
- Gulis , V , Ferreira , V and Graca , MAS . 2006 . Stimulation of leaf litter decomposition and associated fungi and invertebrates by modern eutrophication: implications for stream assessment . Freshwater Biology , 51 : 1655 – 1669 .
- Hall , RO and Tank , JL . 2003 . Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming . Limnology and Oceanography , 48 : 1120 – 1128 .
- Hawkins , CP , Norris , RH , Gerritsen , J , Hughes , RM , Jackson , SK , Johnson , RK and Stevenson , RJ . 2000 . Evaluation of the use of landscape classifications for the prediction of freshwater biota: synthesis and recommendations . Journal of the North American Benthological Society , 19 : 541 – 556 .
- Hieber , M and Gessner , MO . 2002 . Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates . Ecology , 83 : 1026 – 1038 .
- Hilsenhoff , WL . 1987 . An improved biotic index of organic stream pollution . Great Lakes Entomologist , 20 : 31 – 39 .
- Hilsenhoff , WL . 1995 . Aquatic insects of Wisconsin: generic keys and notes on biology, ecology and distribution , Technical bulletin, Madison, Wisconsin : Wisconsin Dept. . of Natural Resources, No. 89
- Hoffmann , A . 1997. To settle or not to settle? The aggregation behavior of Lasiocephala basalis (Kol.) (Trichoptera: Lepidostomatidae) larvae prior to pupation. In: Holzenthal RW, Flint OS, editors. Proceedings of the 8th International Symposium on Trichoptera. Ohio Biological Survey, Columbus. p. 151–156
- Houghton , DC . 2004a . Minnesota caddisfly biodiversity (Insecta: Trichoptera): delineation and characterization of regions . Environmental Monitoring and Assessment , 95 : 153 – 181 .
- Houghton , DC . 2004b . Utility of caddisflies (Insecta: Trichoptera) as indicators of habitat disturbance in Minnesota . Journal of Freshwater Ecology , 19 : 97 – 108 .
- Houghton , DC . 2006 . The ability of common water quality metrics to predict habitat disturbance when biomonitoring with adult caddisflies (Insecta: Trichoptera) . Journal of Freshwater Ecology , 21 : 705 – 716 .
- Houghton , DC . 2007 . The effects of landscape-level disturbance on the composition of Minnesota caddisflies (Insecta: Trichoptera) trophic functional groups: evidence for ecosystem homogenization . Environmental Monitoring and Assessment , 135 : 253 – 264 .
- Hynes , HBN . 1975 . The stream and its valley . Edgardo Baldi Memorial Lecture. Verhandlungen der Internationelen Vereinigung für Theoretische und Angewandte Limnologie , 19 : 1 – 15 .
- Inwood , SE , Tank , JL and Bernot , MJ . 2005 . Patterns of denitrification associated with land use in 9 midwestern headwater streams . Journal of the North American Benthological Society , 24 : 227 – 245 .
- JMP 2002 . JMP, The statistical discovery software. User's manual, Version 5. Cary, North Carolina: SAS Institute
- Karl , TS and Hilsenhoff , WL . 1979 . The caddisflies (Trichoptera) of Parfrey's Glen Creek, Wisconsin . Transactions of the Wisconsin Academy of Science, Arts, and Letters , 67 : 31 – 42 .
- Karr , JR and Chu , EW . 1999 . Restoring life to running waters: better biological monitoring , Washington, DC : Island Press .
- Kemp , MJ and Dodds , WK . 2001 . Spatial and temporal patterns of nitrogen in pristine and agriculturally-influenced streams . Biogeochemistry , 53 : 125 – 141 .
- Kerans , BL and Karr , JR . 1994 . A benthic index of biotic integrity (B-IBI) for rivers of the Tennessee Valley . Ecological Applications , 4 : 768 – 785 .
- Lenat , DR and Resh , VH . 2001 . Taxonomy and stream ecology—the benefits of genus- and species-level identifications . Journal of the North American Benthological Society , 20 : 287 – 298 .
- Li , AOY and Dudgeon , D . 2008 . Food resources of shredders and other benthic macroinvertebrates in relation to shading conditions in tropical Hong Kong streams . Freshwater Biology , 53 : 2011 – 2025 .
- Mackay , RJ and Wiggins , GB . 1979 . Ecological diversity in Trichoptera . Annual Review of Entomology , 24 : 185 – 208 .
- Martin , ID and Barton , DR . 1987 . The formation of diapause aggregations by larvae of Neophylax fuscus Banks (Trichoptera: Limnephilidae) and their influence on mortality and development . Canadian Journal of Zoology , 65 : 2612 – 2618 .
- McTammany , ME , Benfield , EF and Webster , JR . 2007 . Recovery of stream ecosystem metabolism from historic agriculture . Journal of the North American Benthological Society , 26 : 532 – 545 .
- McTammany , ME , Benfield , EF and Webster , JR . 2008 . Effects of agriculture on wood breakdown and microbial biofilm respiration in southern Appalachian streams . Freshwater Biology , 53 : 842 – 854 .
- Merritt , RW , Cummins , KW and Berg , MB . 2008 . An introduction to the aquatic insects of North America, , 4th , Dubuque, Iowa : Kendall/Hunt .
- Moline , AB and Poff , NL . 2008 . Growth of an invertebrate shredder on native (Populus) and non-native (Tamarix, Elaeagnus) leaf litter . Freshwater Biology , 53 : 1012 – 1020 .
- Mulholland , PJ , Tank , JL , Sanzone , DM , Wolheim , WM , Peterson , BJ , Webster , JR and Meyer , JL . 2000 . Nitrogen processing in a deciduous forest stream: results from a tracer 15N experiment in Walker Branch, Tennessee . Ecological Monographs , 70 : 471 – 493 .
- Niyogi , DK , Simon , KS and Townsend , CR . 2003 . Breakdown of tussock grass in streams along a gradient of agricultural development in New Zealand . Freshwater Biology , 48 : 1698 – 1708 .
- Nord , EA and Lanyon , LE . 2003 . Managing material transfer and nutrient flow in an agricultural watershed . Journal of Environmental Quality , 32 : 562 – 570 .
- Norris , RH , Linke , S , Prosser , I , Young , WJ , Liston , P , Bauer , N , Sloane , N , Dyer , F and Thoms , M . 2007 . Very-broad-scale assessment of human impacts on river condition . Freshwater Biology , 52 : 959 – 976 .
- Palmer , MA , Hakenkamp , CC and Nelson-Baker , K . 1997 . Ecological heterogeneity in streams: why variance matters . Journal of the North American Benthological Society , 16 : 189 – 202 .
- Paul , MJ , Meyer , JL and Couch , CA . 2006 . Leaf breakdown in streams differing in catchment land use . Freshwater Biology , 51 : 1684 – 1695 .
- Paulsen , SG , Mayio , A , Peck , DV , Stoddard , JL , Tarquinio , E , Holdsworth , SM , Van Sickle , J , Yuan , LL , Hawkins , CP Herlihy , AT . 2008 . Condition of stream ecosystems in the U.S.: an overview of the first national assessment . Journal of the North American Benthological Society , 27 : 812 – 821 .
- Petersen , I , Winterbottom , JH , Orton , S , Friberg , N , Hildrew , AG , Spiers , DC and Gurney , WSC . 1999 . Emergence and lateral dispersal of adult Plecoptera and Trichoptera from Broadstone stream, U.K . Freshwater Biology , 42 : 401 – 416 .
- Peterson , BJ , Wolheim , WM and Mulholland , PJ . 2001 . Control of nitrogen export from watersheds by headwater streams . Science , 292 : 86 – 90 .
- Pridmore , RD and Roper , DS . 1985 . Comparison of the macroinvertebrate faunas of runs and riffles in three New Zealand streams . New Zealand Journal of Marine and Freshwater Research , 19 : 283 – 291 .
- Pringle , C , Vellidis , G , Heliotis , F , Bandacu , D and Cristofor , S . 1993 . Environmental problems for the Danube delta . American Scientist , 81 : 350 – 361 .
- Resh , VH . 1993 . Recent trends in the use of Trichoptera in water quality monitoring , Otto C, editor. . Proceedings of the 7th International Symposium on Trichoptera, 14–21 August, Umea, Sweden. Leiden, The Netherlands: Blakhyus Publishers. p. 285–291
- Rios , SL and Bailey , RC . 2006 . Relationship between Riparian vegetation and stream benthic communities at three spatial scales . Hydrobiologia , 553 : 153 – 160 .
- Rosenberg , DM and Resh , VH . 1993 . Freshwater biomonitoring and benthic macroinvertebrates , New York : Chapman and Hall .
- Roy , AH , Rosemund , AD , Paul , MJ and Wallace , JB . 2003 . Habitat-specific responses of stream insects to land cover disturbance: biological consequences and monitoring applications . Journal of the North American Benthological Society , 22 : 292 – 307 .
- Royer , TV , Tank , JL and David , MB . 2004 . Transport and fate of nitrate in headwater agricultural streams in Illinois . Journal of Environmental Quality , 33 : 1296 – 1304 .
- Snelder , TH and Biggs , BJ . 2002 . Multiscale river environment classification for water resources . Journal of the American Water Resources Association , 38 : 1225 – 1239 .
- Sode , A and Wiberg-Larson , P . 1993 . Dispersal of adult Trichoptera at a Danish forest brook . Freshwater Biology , 30 : 439 – 446 .
- Spänoff , B and Meyer , EI . 2004 . Breakdown rates of wood in streams . Journal of the North American Benthological Society , 23 : 189 – 197 .
- Strahler , AN . 1952 . Hypsometric (area altitude) analysis of erosional topology . Geological Society of America Bulletin , 63 : 1117 – 1142 .
- Stribling , JB , Pavlik , KL , Holdsworth , SM and Leppo , EW . 2008 . Data quality, performance, and uncertainty in taxonomic identification for biological assessment . Journal of the North American Benthological Society , 27 : 906 – 919 .
- Tank , JL , Meyer , JL , Sanzone , DM , Wolheim , WM , Peterson , BJ , Webster , JR and Meyer , JL . 2000 . Analysis of nitrogen cycling in a forest stream during autumn using a 15N tracer addition . Limnology and Oceanography , 45 : 1013 – 1029 .
- Townsend , CR , Dolédec , S , Norris , R , Peacock , K and Arbuckle , C . 2003 . The influence of scale and geography on relationships between stream community composition and landscape variables: description and prediction . Freshwater Biology , 48 : 768 – 785 .
- UMSP (University of Minnesota Insect Museum). 2009. UMSP BIOTA online database [cited 2009 Feb 24]. Available from: http://www.entomology.umn.edu/museum/databases/
- Urban , MC , Skelly , DK , Burchsted , D , Price , W and Lowry , S . 2006 . Stream communities across a rural–urban landscape gradient . Diversity and Distributions , 12 : 337 – 350 .
- USGS (United States Geological Survey). 2007. Water resources of the United States [Cited 2007 Oct 14]. Available from: http://water.usgs.gov/
- Vanni , MJ , Renwick , WH , Headworth , JL , Auch , JD and Schaus , MH . 2001 . Dissolved and particulate nutrient flux from three adjacent agricultural watersheds: a five-year study . Biogeochemistry , 54 : 85 – 114 .
- Vannote , RL , Minshall , GW , Cummins , KW , Sedell , JR and Cushing , CE . 1980 . The river continuum concept . Canadian Journal of Fisheries and Aquatic Sciences , 37 : 130 – 137 .
- Wallace , JB , Eggert , SL , Meyer , JL and Webster , JR . 1997 . Multiple trophic levels of a forest stream linked to terrestrial leaf litter . Science , 277 : 102 – 104 .
- Wang , L , Brenden , T , Seelbach , P , Cooper , A , Allan , D , Clark , R and Wiley , M . 2008 . Landscape based identification of human disturbance gradients and reference conditions for Michigan streams . Environmental Monitoring and Assessment , 141 : 1 – 17 .
- Wiggins , GB . 2008 . “ Trichoptera families ” . In An introduction to the aquatic insects of North America, , 4th , Edited by: Merrit , RW , Cummins , KW and Berg , MB . 439 – 480 . Dubuque, Iowa : Kendall/Hunt .
- Wolheim , WM , Peterson , BJ , Deegan , LA , Hobbie , JE , Hooker , B , Bowden , W , Edwardson , KJ , Arscott , DB , Hershey , AE and Finlay , J . 2001 . Influence of stream size on ammonium and suspended particulate nitrogen processing . Limnology and Oceanography , 46 : 1 – 13 .
- Woodcock , TS and Huryn , AD . 2007 . The response of macroinvertebrate production to a pollution gradient in a headwater stream . Freshwater Biology , 52 : 177 – 196 .
- Zar , JH . 2007 . Biostatistical analysis, , 5th , Englewood Cliffs, NJ : Prentice-Hall .
- Zweig , LD and Rabeni , CF . 2001 . Biomonitoring effects of deposited sediment in streams . Journal of the North American Benthological Society , 20 : 643 – 657 .