Abstract
We investigated the effects of turion size and water depth on the emergence and size of the submerged macrophyte Myriophyllum oguraense Miki subsp. yangtzense in an outdoor experiment in order to determine the underlying factors affecting its persistence at early life-history stages. Turions were sampled and sorted into three groups (small, medium, and large) and buried in plastic pots with unsterilized lake sediment under four different water depth treatments. Percent emergence, total plant biomass and plant height were strongly affected by turion size, increasing with increasing turion size. Emergence time was significantly affected by both turion size and size × water depth interaction and was delayed with decreasing turion size. Plant emergence of both small and large turions was significantly delayed with increasing water depth. The results indicate that large turions and shallow water might favor the early establishment and survivorship of the immature plant. Allocation to sprouting appears to enhance the ability of immature plants to survive unfavorable conditions. We propose that turion size and water depth play an important role in influencing the abundance and distribution of this endemic population.
Introduction
Many aquatic macrophytes prefer to enhance natural regeneration by propagules such as plant fragments, winter buds, tubers, and turions (Sculthorpe Citation1967; Hutchinson Citation1975; Grace Citation1993). Turions are specialized, overwintering, detachable buds of tightly arranged leaves that may enable plants to survive unfavorable conditions (Jacobs Citation1947; Sculthorpe Citation1967) and to promote the spread of plants to an unoccupied area (Spencer and Rejmánek Citation1989). Therefore, germination and survival of turions in the early life-history stages are events that could be critical for predicting the abundance, spread and distribution of aquatic macrophytes.
Water depth can have a profound influence on early growth and survivorship of seedlings (Clevering and Hundscheid Citation1998; Hussner et al. Citation2009). Previous studies indicated that water depth has a significant impact on biomass production of establishing plants (Stanley Citation1994; Clevering and Hundscheid Citation1998; Poorter and Nagel Citation2000), such that as the water depth increases, more resources are allocated to root production (Barko et al. Citation1986). The type and size of propagule also affects early growth and survivorship of submerged aquatic macrophytes (Spencer Citation1987; Spencer and Rejmánek Citation1989; Spencer and Ksander Citation2001). Compared to small ones, large propagules are thought to enable fast growth of immature plants and, thus, increase subsequent plant survival due to their greater energy reserves and resulting higher emergence rates and biomass (Barko et al. Citation1986; Spencer Citation1987). While the influence of water depth and the intrinsic properties of both wetland and aquatic plant species have been frequently reported for seed germination, seedling survivorship, and seedling regenerative strategies (Sacchi and Price Citation1992; Barrat-Segretain and Bornette Citation2000; Combroux and Bornette Citation2004; Elsey-Quirk et al. Citation2009), the intrinsic property of turion size and the abiotic factor of water depth on turion performance of submerged macrophytes are poorly understood.
Myriophyllum oguraense subsp. yangtzense is a submerged macrophyte endemic to China and restricted to the lower Yangtze River valley, which relies more heavily on turions for regeneration than on seeds (Wang and Yu Citation2007). Our field observations suggested that plants produced turions of varying lengths which often strand on damp mud in late autumn and early winter. Subsequent plants grew in a range of water depths of less than 1.5 m the following spring in the littoral zone of shallow lakes. The probability of turion sprouting in varying water depths may be quite significant for predicting population dynamics, raising the question of the effects of turion size and water depth on a turion's performance and establishment of immature plants. Herein, we proposed two questions. First, does turion size and water depth affect the emergence of the species studied? Second, how do the trade-offs in biomass allocation affect plant establishment?
Methods
Plant material and treatments
In December 2008, more than 1000 turions of M. oguraense subsp. yangtzense were collected from damp mud habitats (about 30 × 50 m) of Liangzi Lake in Hubei Province, China (N 30°12′, E 114°30′). When collected, all turions were already detached from their parent plants. They were washed in lake water to remove attached sediment and brought into the laboratory. Turions with similar thickness (2–3 mm in diameter) were measured singly and sorted into three size-classes based on turion length (mean ± SE): large (2.99 ± 0.0168 cm, n = 200), medium (2.01 ± 0.0045 cm, n = 200) and small (1.11 ± 0.0091 cm, n = 200). Turions were then stored in a refrigerator at 4°C until used.
On December 22, 2008, the experiment was initiated in the South-Lake Campus of Central China Normal University (N 30°30′, E 114°21′). A completely randomized 3 × 4 factorial design with three replicates was used. Treatments consist of three size-classes of turions (large, medium, and small) under four levels of water depth (0, 20, 40, and 60 cm), providing 12 treatments. Three outdoor ponds (300 cm long × 250 cm wide × 80 cm deep) were employed for each water depth and each pond corresponded to one replicate. In total, 12 ponds were used. There were eight pots per turion size treatment in each pond. All pots were filled with unsterilized sediments collected from Liangzi Lake and then were randomly placed on the bottom surface of ponds. One turion was planted horizontally at 1 cm depths in each plastic pot (16 cm diameter, 12.5 cm depth). Pots in each pond were situated in the same orientation towards the sun. The water depths were manipulated every 2 days and tap water was manually supplied in drought or drained away on rainy days to maintain stable water depths. The water depth was measured from the top surface of the pots to the water surface. For the 0 cm water depth, the ponds were filled with tap water to the height of the pot, making the sediment waterlogged. During the experiment, seedlings of other aquatic plants that emerged from pots were removed by hand every few days.
Air-dried sediment was sampled for analysis of total nitrogen and total phosphorus using colorimetry (Novozamsky et al. Citation1984). Organic matter content was estimated as weight loss after ignition at 430°C for 24 h (Shi Citation1994). All chemical analyses were based on dry weight and carried out in three replicates. The concentration (mean ± SE) of total nitrogen, total phosphorus, and organic matter were 1.97 ± 0.03, 0.110 ± 0.005, and 106 ± 1.8 mgg−1. Water temperature and irradiance 5 cm under the water surface in each pond were measured using Waterproof ECTestr (Eutech Instruments, Thermo Fisher Scientific Inc.) and underwater irradiance sensors (LI-193SA; LI-COR Inc., Lincoln, NE), respectively, at 8:00, 13:00, and 18:00 on three cloudless days (three times every month). From shallow to deep water depths, the light intensity (photosynthetic photon flux density, mean ± SE) was 1263 ± 11, 1197 ± 16, 1132 ± 14, and 1039 ± 19 µmol m−2 s−1 and the water temperature (mean ± SE) was 10.8 ± 0.2, 10.3 ± 0.1, 10.2 ± 0.1, and 10.0 ± 0.2°C.
Data collection
Emerged plants were counted every 2 days. Immature plants started to emerge in late February 2009. Plant emergence was defined as the first appearance of plant at the sediment surface. The experiment was terminated in early May 2009. All plants were measured for their height above the sediment surface and then harvested. Since the plants at 0 cm water depth produced several ramets, the highest stem was measured as the final plant height. At harvest, plants were dug out by hand with care to collect as many roots belonging to plants as possible and cleaned with tap water. Plants were separated into three parts: stems, leaves, and roots. After drying at 60°C for 48 h to constant weight, each fraction was weighed to measure biomass production. Relative root mass = root mass/total biomass, relative stem mass = stem mass/total biomass, and relative leaf mass = leaf mass/total biomass.
Statistical analysis
The effects of turion size and water depth on the mean number of days to emergence, percent emergence, plant biomass, plant height, and biomass allocation (relative root mass, relative stem mass, and relative leaf mass) were analyzed using two-way ANOVA. Duncan's multiple comparison tests were used to analyze discrepancies in each parameter among turion size-classes within different water depths. To meet assumptions of normality prior to the ANOVA, plant biomass and height data were log10 transformed, and homogeneity was tested using Levene's test. The statistical package SPSS 16.0 was utilized for all analyses.
Results
Plant emergence in response to turion size and water depth
The mean number of days for the first plant emergence was significantly affected by both turion size and water depth (F = 63.56, df = 2, p < 0.001; F = 10.77, df = 3, p < 0.001, respectively) and was also affected by a significant turion size × water depth interaction (F = 6.33, df = 6, p < 0.001). Plant emergence occurred earliest for the large turions from the 0 cm water depth and was delayed with the decreasing turion size at each water depth (). Small and large turion responses differed significantly at each water depth except for 40 cm. The plant emergence of both small and large turions was significantly delayed by the increasing water depth of the shallower water treatments (0 and 20 cm).
Figure 1. Mean (±SE) number of days to the first emergence (a), percent emergence (b), plant biomass (c), and plant height (d) of immature plants at different water depths (0, 20, 40, and 60 cm) for small (S), medium (M) and large (L) sized turions of M. oguraense subsp. yangtzense. Different superscript letters on each bar denote when significant differences were observed at p < 0.05 according to Duncan's multiple comparison test after ANOVA (n = 3).
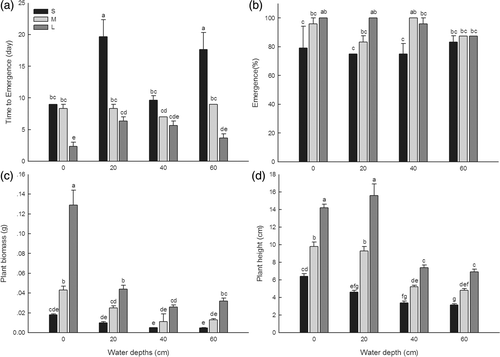
Percent emergence was significantly affected by turion size (F = 11.85, df = 2, p < 0.001) but not by water depth (F = 0.85, df = 3, p = 0.480). Plant emergence was highest at 0 cm water depth and increased with increasing turion size at each water depth except for 40 cm (). A significant difference occurred between small and large turions in 0 and 20 cm water depth.
Effects of turion size and water depth on biomass allocation
Plant biomass and height were significantly affected by both turion size (F = 431.72, df = 2, p < 0.001; F = 326.97, df = 2, p < 0.001, respectively) and water depth (F = 181.75, df = 3, p < 0.001; F = 161.11, df = 3, p < 0.001, respectively; ). Both plant biomass and height were greatest at 0 cm water depth and decreased with increasing water depth at each turion size (). However, for plant height, a slight difference was observed in large turions at 20 cm water depth which were affected by a significant turion size × water depth interaction (F = 4.04, df = 6, p = 0.001). Plant biomass and height were greatest for large turions and least for small turions at each water depth and there were significant differences between the three size classes.
Table 1. F-values and significance of two-way ANOVA of the effects of turion size and water depth on plant height, biomass, and biomass allocation ratio of M. oguraense subsp. yangtzense.
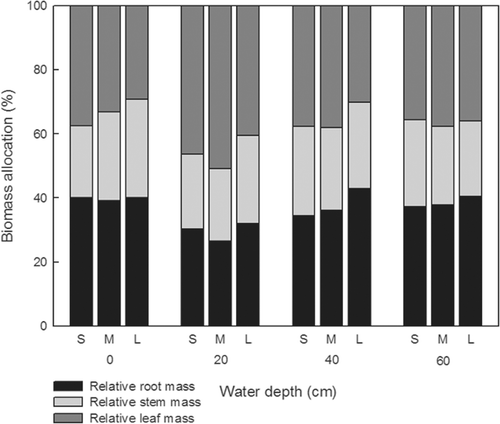
Both relative root mass and relative leaf mass were significantly affected by turion size (F = 4.13, df = 2, p = 0.017; F = 11.86, df = 2, p < 0.001, respectively) and water depth (F = 14.05, df = 3, p < 0.001; F = 23.77, df = 3, p < 0.001, respectively; ). Relative root mass was least at 20 cm water depth for each size class and increased with increasing turion size in deeper water (40 and 60 cm; ). Relative leaf mass was greatest at 20 cm water depth for each turion size class and a significant difference occurred between 20 cm and the other three water depths at each size class. For relative stem mass, there was a significant turion size × water depth interaction (F = 3.35, df = 6, p = 0.003).
Discussion
Plant emergence in response to turion size and water depth
In natural conditions, plant survival might depend on life-history strategies adopted by turions of different sizes (Jacobs Citation1947; Schaffer and Gadgil Citation1975). In this study, large turions had a greater plant emergence and height compared to small ones at different water depths, implying that the large turion size was advantageous for plant establishment. This is consistent with previous reports of effects of both tuber size on aquatic plant species (Spencer Citation1987) and seed size on terrestrial plant species (Hendrix and Trapp Citation1992; Seiwa Citation2000). Water depth is also of great importance in influencing plant establishment of aquatic macrophytes (Coops et al. Citation1996). Most previous studies revealed that plant emergence was negatively related to water depth (Clevering and Hundscheid Citation1998; Casanova and Brock Citation2000). In this study, higher plant emergence and less emergence time were detected for large turions at each water depth and emergence time was significantly affected by water depth and interaction of turion size and water depth. However, no significant effect of water depth on emergence percentage was detected. These findings imply that the early growth of this species depends more on turion size, which is an indication of the amount of carbohydrate stored, rather than on external factors like water depth, although it has been demonstrated that early growth of other aquatic macrophytes were influenced by interaction between biotic and abiotic factors (Spencer Citation1986, Citation1987).
Effects of turion size and water depth on allocation strategies
Biomass allocation in the growth response reflects the way a species interacts with its environment (Antonovics Citation1980; Poorter and Nagel Citation2000). In this study, plants from large turions had a higher biomass compared to small ones at different water depths. Plant biomass from large turions was remarkably higher at 0 cm water depth than in other treatments, caused by the number of ramets the plants produced (1–2, 2–4, and 3–5 ramets in small, medium, and large size treatments, respectively). Since the species stranded on damp mud, where the plants seldom produce flowers, generating more ramets could enhance survival. Myriophyllum oguraense subsp. yangtzense plants may have the ability to temporarily tolerate dry conditions and develop a terrestrial form like its congeneric species, such as Myriophyllum spicatum and M. verticillatum (Yu et al. Citation2002). However, in deeper water treatments (40 and 60 cm), plants allocated more resources to the root system compared to the stem with increasing turion size. This may lead to a reduction in plant height (Spencer Citation1987; Casanova and Brock Citation2000). Deeper water may also decrease light available for plant growth, which in turn would reduce the plant height.
Biomass allocation of the studied species was affected by turion size and water depth. Relative root mass increased with increasing turion size, and relative root mass was least at 20 cm water depth for each size of turions. This indicates that the allocation strategies could be altered by the intrinsic property of turion size of the studied species and the abiotic factor of water depth. Considering sprouting and early growth performance, we conclude that both turion length of approximately 2.99 cm and water depths of 0–20 cm may favor immature plant growth and survival of this endemic species, which further influence its abundance and distribution.
Acknowledgments
We are sincerely grateful to the anonymous reviewers for their critical and helpful comments of the manuscript. We thank Dr. Michele Funston for correcting the English. This work was supported by the Natural Science Foundation of China (30870151), the key project of Chinese Ministry of Education (105110), and self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (CCNU09B01002).
References
- Antonovics , J . 1980 . “ Concepts of resource allocation and partitioning in plants ” . In Limits to Action: the allocation of individual behaviour , Edited by: Staddon , JER . 135 New York , NY : Academic Press .
- Barko , JW , Adams , MS and Clesceri , NL . 1986 . Environmental factors and their consideration in the management of submersed aquatic vegetation: a review . Journal of Aquatic Plant Management , 24 : 1 – 10 .
- Barrat-Segretain , M-H and Bornette , G . 2000 . Regeneration and colonization abilities of aquatic plant fragments: effect of disturbance seasonality . Hydrobiologia , 421 : 31 – 39 .
- Casanova , MT and Brock , MA . 2000 . How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? . Plant Ecology , 147 : 237 – 250 .
- Clevering , OA and Hundscheid , MPJ . 1998 . Plastic and non-plastic variation in growth of newly established clones of Scripus (Bolboschoenus) maritimus L. grown at different water depths . Aquatic Botany , 62 : 1 – 17 .
- Combroux , I and Bornette , G . 2004 . Propagule banks and regenerative strategies of aquatic plants . Journal of Vegetation Science , 15 : 13 – 20 .
- Coops , H , van den Brink , FWB and van der Velde , G . 1996 . Growth and morphological response of four helophyte species in an experimental water-depth gradient . Aquatic Botany , 54 : 11 – 24 .
- Elsey-Quirk , T , Middleton , BA and Proffitt , CE . 2009 . Seed flotation and germination of salt marsh plants: the effects of stratification, salinity, and/or inundation regime . Aquatic Botany , 91 : 40 – 46 .
- Grace , JB . 1993 . The adaptive significance of clonal reproduction in angiosperms: an aquatic perspective . Aquatic Botany , 44 : 159 – 180 .
- Hendrix , SD and Trapp , EJ . 1992 . Population demography of Pastinaca sativa (Apiaceae): effects of seed mass on emergence, survival, and recruitment . American Journal of Botany , 79 : 365 – 375 .
- Hussner , A , Meyer , C and Busch , J . 2009 . The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum . Weed Research , 49 : 73 – 80 .
- Hutchinson , GE . 1975 . A treatise on limnology: limnological botany , Vol. 3 , 660 New York , NY : John Wiley and Sons .
- Jacobs , DL . 1947 . An ecological life-history of Spirodela polyrhiza (Greater duckweed) with emphasis on the turion phase . Ecological Monographs , 17 : 437 – 469 .
- Novozamsky , I , Houba , VJG , Temminghoff , E and van der Lee , JJ . 1984 . Determination of ‘total’ N and ‘total’ P in a single soil digest . Netherlands Journal of Agricultural Science , 32 : 322 – 324 .
- Poorter , H and Nagel , O . 2000 . The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review . Australian Journal of Plant Physiology , 27 : 595 – 607 .
- Sacchi , CF and Price , PW . 1992 . The relative roles of abiotic and biotic factors in seedling demography of arroyo willow (Salix lasiolepis: Salicaceae) . American Jounral of Botany , 79 : 395 – 405 .
- Schaffer , WM and Gadgil , MD . 1975 . “ Selection for optimal life histories in plants ” . In Ecology and evolution of communities , Edited by: Cody , ML and Diamond , JM . 142 – 157 . Cambridge : Harvard Universality Press .
- Sculthorpe , CD . 1967 . The biology of aquatic vascular plants , 610 London : Arnold Ltd .
- Seiwa , K . 2000 . Effects of seed size and emergence time on tree seedling establishment importance of development constraints . Oecologia , 123 : 208 – 215 .
- Shi , RH . 1994 . Agriculture and chemical analysis for soil , Beijing : Chinese Agriculture Press .
- Spencer , DF . 1986 . Early growth of Potamogeton pectinatus L. in response to temperature and irradiance: morphology and pigment composition . Aquatic Botany , 26 : 1 – 9 .
- Spencer , DF . 1987 . Tuber size and planting depth influence growth of Potamogeton pectinatus L . American Midland Naturalist , 118 : 77 – 84 .
- Spencer , DF and Ksander , GG . 2001 . Field evaluation of degree-day based equations for predicting sprouting of Hydrilla (Hydrilla verticillata) turions and tubers . Journal of Freshwater Ecology , 16 ( 3 ) : 479 – 486 .
- Spencer , DF and Rejmánek , M . 1989 . Propagule type influences competition between two submersed aquatic macrophytes . Oecologia , 81 : 132 – 137 .
- Stanley , AN . 1994 . Factors influencing the distribution of Eurasian watermifoil (Myriophyllum spciatum L.) biomass in Lake Wingra, Wisconsin . Journal of Freshwater Ecology , 9 ( 2 ) : 145 – 151 .
- Wang , D and Yu , D . 2007 . A new subspecies of Myriophyllum oguraense (Haloragaceae) from China . Annales Botanici Fennici , 44 : 227 – 230 .
- Yu , D , Wang , D , Li , ZY and Funston , AM . 2002 . Taxonomic revision of the genus Myriophyllum (Haloragaceae) in China . Rhodora , 104 : 396 – 421 .