Abstract
Hydraulic fracturing (fracking) poses significant threats to freshwater resources and stream ecosystems. Little research exists to quantify the ecological impact and in Pennsylvania alone over 10,000 wells have been permitted. This study aimed to determine if hydraulic fracturing is having any impacts on stream ecosystem health by measuring stream pH and temperature, macroinvertebrate index of biological integrity (IBI), and the gill morphology of individuals in the Hydropsychidae Diplectrona taxa. Six streams in northwestern Pennsylvania were selected as study sites (three with fracking occurring in their watershed and three without fracking). IBI scores were significantly higher at non-fracked sites and were also correlated with stream pH. Macroinvertebrate gill width did not vary between fracked and non-fracked sites but was correlated with percent hydric soils, suggesting that hydric soils may be a good long-term indicator of stream dissolved oxygen. While our results did not indicate differences in Hydropsychidae Diplectrona gill widths between fracked and non-fracked sites, we did observe that fracked sites had more acidic stream water and lower IBI scores. These results indicate the need for further study to assess the potential impacts of hydraulic fracturing on stream ecosystems.
Introduction
Hydraulic fracturing (fracking) is a relatively new process being employed to extract natural gas in the Marcellus Shale Basin, which is one of the largest and most productive shale formations in the United States. Rapid expansion of the hydraulic fracturing industry has left a gap in scientific knowledge. The hydraulic fracturing technique has recently gained the attention of the scientific community, numerous hazards and risks have been identified, and some recent studies have suggested that the rapid development of this process poses the most significant threat to surface waters or streams, especially in Pennsylvania (Entrekin et al. Citation2011; Grant et al. Citation2015). One pathway of contamination is when fluids used in fracking enter nearby waterways from heavy rain storms or improperly designed holding ponds for the produced flow-back water which contains numerous chemicals such as BTEX (Benzene, Toluene, Ethyl Benzene, and Xylene) compounds, biocide, and salts (Rahm & Riha Citation2012; Peduzzia & Harding Citation2013). Potential impacts from fracking fluid spills are fish kills, contamination of aquifers containing potable water, and increasing salinity levels in nearby streams (Peduzzia & Harding Citation2013). Chemicals from the flow-back fluid spills have the potential to alter in-stream physiochemical conditions for macroinvertebrates. In addition to fracking fluid spills, forest disturbances related to fracking development (e.g., well pads, pipelines, and roads) may increase dissolved organic carbon (Warner et al. Citation2012; Grant et al. Citation2015). Clearing land required for the well pads and infrastructure can increase the chance of run-off into nearby waterways and increase sediment loads (Entrekin et al. Citation2011). Anthropogenically introduced sediment loads have been shown to lower water quality by increasing turbidity and affecting the more sensitive macroinvertebrate taxa (Wood & Armitage Citation1997).
Benthic macroinvertebrates are commonly used as bioindicators because of their high species diversity, variable sensitivity to pollution, and because of their sedentary nature compared to other, more mobile organisms, such as fish (Merritt & Cummins Citation2011; Carew et al. Citation2013). Indices of biological integrity (IBI) are commonly used to evaluate stream macroinvertebrate communities through multiple biodiversity and richness metrics, and pollution tolerance values. The gills of macroinvertebrates are one of the most impacted structures when environmental perturbations occur (Skinner & Bennett Citation2007), such as introduction of chemicals. Many different forms of pollutants can have deleterious effects on gills of aquatic macroinvertebrates. An ideal taxon to observe the gills is the caddisfly family Hydropsychidae since it has tracheal gills on the venter of the abdomen (Vuori & Kukkonen Citation2002), making the gills readily visible and easy to remove.
Herein, we set out to determine if there were differences in macroinvertebrate gill width and biodiversity (through IBI scores) related to hydraulic fracturing development and differences in stream physiochemical values. We anticipated lower dissolved oxygen (DO) levels at fracked sites and larger gill widths to compensate for lower DO levels. We measured tracheal gill widths from 60 Hydropsychidae Diplectrona individuals at 6 streams in various stages of hydraulic fracturing development.
Materials and methods
Study area and site selection
Six streams located in the Marcellus basin in northwestern Pennsylvania were sampled in June and July 2014. These streams were a subset of sites from ongoing research by Grant et al. (Citation2015), with three streams having experienced fracking within their watershed prior to sampling (fracked group, ) and three streams having not experienced fracking in their watershed prior to sampling (non-fracked group, ). Multiple Geographic Information Systems (GIS) tools along with land cover data-sets were used in ArcGIS 10.0 to calculate watershed area, percent hydric soils, and percent forest. Representative 100-m reaches of each stream were selected immediately downstream of well pad locations for the fracked sites.
Table 1. List of physiochemical and watershed characteristics by stream which include stream status (fracked or non-fracked), latitude, longitude, stream temperature (ºC), stream pH, stream DO, index of biotic integrity scores, percent hydric soils which is the percent of each watershed that contains hydric soils, percent forest which is the percent forested land within each watershed and watershed area (ha); averages for watershed characteristics are reported according to stream status and Kruskal Wallis p-values are from comparisons of watershed characteristics based on status.
Field methods
Macroinvertebrates were collected from all study sites using standard IBI protocol (Chalfant Citation2012) and an additional 15 Hydropsychidae individuals were collected at each stream site for later gill width measurements. All macroinvertebrates were stored in 70% ethanol until later identification in the lab. DO in stream water was recorded using an Extech Heavy Duty Dissolved Oxygen Meter (0.1 mg/L resolution for DO), and stream temperature and pH (± 0.1 accuracy) were taken with a Eutech Multi-Parameter Testr 35 Series.
Laboratory methods
All macroinvertebrates collected were identified to the genus according to Merritt & Cummins (Citation2011) and the IBI was calculated (Chalfant Citation2012). Gills were removed from 10 Hydropsychidae (Diplectrona genus) individuals according to methods employed by Skinner and Bennett (2011). Gills were mounted with CMPC-9 Macroinvertebrate mounting medium which is a non-resinous, water miscible mounting medium for permanent transparent mounts following a similar methodology to Beckett and Lewis (Citation1982). Slides were then analyzed using dark field microscopy at 40X magnification and pictures were taken using a Scion Corporation camera on a Nikon Phase Contrast 2 light microscope. Pictures were analyzed in ImageJ version 1.46 (Ferreira & Rasband Citation2012) and gill widths were taken to the nearest 0.001 µm across each visible gill lamellae ().
Statistical analyses
All statistical analyses were run in R Studio version 0.98.1079. All data and residuals were checked for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. A one-way analysis of variance (ANOVA) was used to compare overall macroinvertebrate length (from the head to the end of the anal hooks, using forceps to extend the macroinvertebrates to their full length) across streams to ensure that no significant differences existed in macroinvertebrate length between streams, allowing for cross-stream comparisons without length normalization. A nested two-way ANOVA was run to see if macroinvertebrate gill widths were different among sites as well as between fracked and non-fracked streams. Pearson correlations were used to relate gill width to physiochemical stream water measures and watershed characteristics (e.g., percent forest, percent hydric soils, and watershed area). All statistics were considered significant at p < 0.05 unless otherwise stated.
Results and discussion
Macroinvertebrates are known to have varying degrees of sensitivity to pollution, making the more sensitive taxa excellent bioindicators. Macroinvertebrate IBI scores were significantly higher at non-fracked sites compared to fracked sites (Kruskal Wallis χ2 = 3.857, df = 1, p = 0.049; ). This may have been in part due to stream pH, as IBI scores were also highly correlated with pH in this study (r = 0.996, p < 0.001) and streams in the fracked group were found to be more acidic (Kruskal Wallis χ 2 = 3.857, df = 1, p = 0.049; ). Southerland et al. (Citation2007) assessed ways to improve IBI score calculations and stream pH was the main driving factor. Stream pH was not being influenced by wetlands as the amount of wetlands in a watershed did not differ between fracked and non-fracked streams (Kruskal Wallis χ 2 = 0.048, df = 1, p = 0.827). Fracking fluids, such as acids (Kharak et al. Citation2013), used in the extraction of natural gas may be partially responsible for changes in stream pH. Previous work has found that streams that have experienced fracking within their watershed have lower pH (Grant et al. Citation2015), and low stream pH is known to be detrimental to benthic macroinvertebrates (Southerland et al. Citation2007).
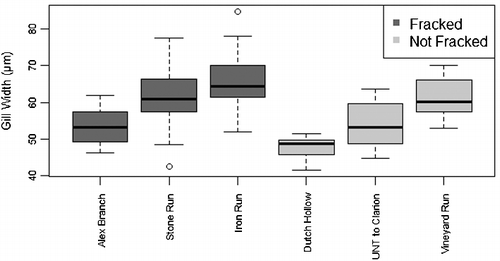
Others have shown that DO can largely drive differences in macroinvertebrate gill widths, although significant differences can exist even with similar DO environments (Crispo & Chapman Citation2010). While minimal variation in gill width was noted within streams and no differences in DO or gill widths were observed between fracked and non-fracked streams (F4,59 = 8.868, p = 0.315), significant variation was observed among streams (). This variation may be due to long-term unobserved differences in stream DO. Percent hydric soils were found to be correlated with gill width at a 90% confidence level (r = 0.73, p = 0.099). Hydric soils are known to create anaerobic conditions (similar to those found in wetlands) in the upper layers of the soils (Vepraskas et al. Citation1999), presumably lowering in-stream DO levels. We believe our findings indicate that gill widths were driven by DO, but the amount of hydric soils was a better long-term indicator of stream DO levels as seasonal fluctuations of DO can be considerable (Jamshidi & Bakar Citation2011). While no significant differences in DO were observed between fracked and non-fracked groups, we did observe a significant negative trend between the number of wells within a watershed and DO (r = –0.9981, p = 0.0395), which may suggest that the variation (of macroinvertebrate gill widths) within our fracked group may be due to differences in degree of fracking development. However, this trend was for a small sample size (n = 3) and needs to be verified with a more robust data-set.
Figure 2. Length normalized gill widths by stream, categorized by stream fracking status, open circles represent outliers and whiskers indicate variability outside the upper and lower quartiles represented by the top and bottom of the boxes. The black bars in the boxes represent the median. Nested ANOVA: there was significant variation among streams but not between groups (status F1,59 = 1.316, stream F4,59 = 8.868, status p = 0.315, stream p < 0.001).
The results of this study are particularly needed with the recent increase and rapid expansion of fracking in Pennsylvania and across the Northeast. In this first study of its kind, differences in macroinvertebrate biodiversity and gill widths were observed between streams and appear to be largely driven by stream pH as well as hydric soils, which are potentially a better long-term indicator of stream DO levels. Decreased IBI scores at fracked streams appear to be caused by acidic stream pH. Number of wells within a watershed may be impacting nearby stream DO, although further work is needed to verify this finding. Future work in this area is needed to determine pathways of morphological effect. More specifically, future work should aim to increase the number of streams across varying degree of impact of fracking (i.e., number of wells in a watershed) and the inclusion of several additional taxonomic groups.
Acknowledgements
The authors thank Devin Beck, Jon Dubensky, Jennifer Graves, Alyssa Grube, Nicole Marks, Jacob Oster, and Nikea Ulrich who are Juniata College research students for the help with field collection and Dr John Matter who helped with gill mounting. The authors would also like to thank Taylor Johnston, a Juniata College student, who reviewed the manuscript before submission. The authors would also like to thank Mike Keating, Genna Kasun, Leslie Leckvarcik, and Karla Wiser for their help with procuring and administering the grant to support this student-lead research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Beckett DC, Lewis PA. 1982. An efficient procedure for slide mounting of larval chironomids. Trans Am Microsc Soc. 101:96–99.
- Carew ME, Pettigrove VJ, Metzeling L, Hoffmann AA. 2013. Environmental monitoring using next generation sequencing: rapid identification of macroinvertebrate bioindicator species. Front Zool. [Internet]. [cited 2015 Feb 28]; 10. Available from: http://www.frontiersinzoology.com/content/10/1/45
- Chalfant B. 2012. Benthic macroinvertebrate index of biotic integrity from wadeable freestone riffle-run streams in Pennsylvania. Harrisburg (PA): Pennsylvania Department of Environmental Protection, Division of Water Quality Standards.
- Crispo E, Chapman LJ. 2010. Geographic variation in phenotypic plasticity in response to dissolved oxygen in an African cichlid fish. J Evol Biol. 23:2091–2103.
- Entrekin S, Evans-White M, Johnson B, Hagenbuch E. 2011. Rapid expansion of natural gas development poses a threat to surface waters. Front Ecol Environ. 9:503–511.
- Ferreira T, Rasband W. 2012. Image J version 1.46. Bethesda (MD): National Institutes of Health.
- Grant CJ, Weimer AB, Marks NK, Perow ES, Oster JM, Brubaker KM, Trexler RV, Solomon CM, Lamendella R. 2015. Marcellus and mercury: assessing potential impacts of unconventional natural gas extraction on aquatic ecosystems in northwestern Pennsylvania. J Environ Sci Health, Part A. 50:1–20.
- Jamshidi S, Bakar NBA. 2011. Variability of dissolved oxygen and active reaction in deep water of the southern Caspian Sea, near the Iranian coast. Pol J Environ Stud. 20:1167–1180.
- Kharak YK, Thordsen JJ, Conaway CH, Thomas RB. 2013. The energy-water nexus: potential groundwater-quality degradation associated with production of shale gas. Procedia Earth Planet Sci. 7:417–422.
- Merritt RW, Cummins KW. 2011. An introduction to the aquatic insects of North America. Dubuque (IA): Kendall/Hunt.
- Peduzzia P, Harding R. 2013. Gas fracking: can we safely squeeze the rocks? Environ Dev. [Internet]. [cited 2015 Feb 28]; 6. Available from: http://archive-ouverte.unige.ch/unige:32185
- Rahm BG, Riha SJ. 2012. Toward strategic management of shale gas development: regional, collective impacts on water resources. Environ Sci Policy. 17:12–23.
- Skinner KM, Bennett JD. 2007. Altered gill morphology in benthic macroinvertebrates from mercury enriched streams in the Neversink Reservoir Watershed, New York. Ecotoxicology. [Internet]. [cited 2015 Feb 10]; 16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17253158
- Southerland MT, Rogers GM, Kline MJ, Morgan RP, Boward DM, Kazyak PF, Klauda RJ, Stranko SA. 2007. Improving biological indicators to better assess the condition of streams. Ecol Indicators. 7:751–767.
- Vepraskas MJ, Richardson JL, Tandarich JP, Teets SJ. 1999. Dynamics of hydric soil formation across the edge of a created deep marsh. Wetlands. 19:78–89.
- Vuori KM, Kukkonen JVK. 2002. Hydropsychid (Trichoptera, Hydropsychidae) gill abnormalities as morphological biomarkers of stream pollution. Freshwater Biol. 47:1297–1306.
- Warner NR, Jackson RB, Darrah TH, Osborn SG, Down A, Zhao K, White A, Vengosh A. 2012. From the cover: geochemical evidence for possible natural migration of Marcellus Formation brine to shallow aquifers in Pennsylvania. Proc Natl Acad Sci USA. 109:11961–11966.
- Wood P, Armitage P. 1997. Biological effects of fine sediment in the lotic environment. Environ Manage. [Internet]. [cited 2015 Mar 29]; 21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9008071

