Abstract
Potamogeton crispus L. is a cosmopolitan aquatic species and is widely used as a pioneer species for ecological restoration. Many restoration projects that employ P. crispus turions are carried out in turbid water with the result that turions are covered by sediment deposition. Will these turions germinate in the next growing season? Previous studies have focused on the effects of light and temperature on turion sprouting. The purpose of this study is to determine whether sediment deposition influences turion sprouting and early growth of P. crispus. Turions were cultivated at a range of sediment depths (1–5 cm), whereas the control group (0 cm) was not covered by sediment, only inserted into sediment. Turion sprouting, plant height, leaf number, leaf area and plant dry weight were monitored. The results showed that the sprouting time of the turions was delayed with increasing sediment thickness. The maximum sprouting rate and leaf number occurred at 2.0 cm depth; however, the plant height, leaf area and plant dry weight all decreased with increasing deposition thickness. Turion sprouting was delayed by sediment coverage and was affected by anoxic conditions and low temperature without light. It is feasible to employ P. crispus turions in restoration projects in turbid water. These results contribute to a more comprehensive understanding of turion sprouting dynamics of P. crispus and will be useful for restoration programs.
Introduction
Potamogeton crispus is a submerged herbaceous perennial plant. It grows in freshwater lakes, ponds, paddy fields and rivers, and produces large quantities of biomass (Ali et al. Citation2000). It is a native aquatic submerged plant in Europe Asia, Africa, Australia and North America. It has a tolerance for low light and cold water temperatures and, consequently, it can invade both shallow and deep-water areas growing up to 4 m in deep water (Catling & Dobson Citation1985). P. crispus is an important primary producer in freshwater ecosystems providing food for herbivorous fish and waterfowl (Jian et al. Citation2003; Chi Citation2009). As P. crispus consumes large amount of nutrients, it sometimes depletes phosphorus and nitrogen in the water column. It can also decrease chemical oxygen demand and increase water transparency and dissolved oxygen (Blindow et al. Citation1993). In addition, P. crispus can accumulate metals such as Fe, Pb, Ni, Mn and Cu (Ali et al. Citation2000), making it a potential candidate for bioremediation of polluted waters (Ali et al. Citation1999) to remove toxic metals from wastewater (Ali et al. Citation1999, Citation2000; Sivaci et al. Citation2008; Yang et al. Citation2010, Citation2011). Therefore, when present, P. crispus can play an important role in maintaining the balance and health of aquatic ecosystems.
The life history of P. crispus differs from most submerged species (Kunii Citation1982). When the water temperature begins to rise in the spring, it grows rapidly (Stuckey Citation1979; Sastroutomo Citation1981; Rogers & Breen Citation1980). It flowers and produces turions and large number of seeds almost simultaneously when water temperature is higher than 20 ℃ and the photoperiod is longer than 12 h (Sastroutomo Citation1981; Kunii Citation1989). Although seeds might be important in long distance dispersal (Waisel Citation1971), seed germination of P. crispus is extremely low (0.001%, CitationRogers & Breen 1980). In the field, water depth, light intensity, substrate type and predation by fishes probably influence turion sprouting (Jian et al. Citation2003). There are significant negative linear relationships between sprouting rate and water depth in lakes (Jian et al. Citation2003). Turion sprouting is inhibited in the dark, but is little affected by types of substrate (Jian et al. Citation2003). Harada and Ishizawa (Citation2003) found that stem growth in Potamogeton distinctus A. Benn. turions was stimulated in anaerobic conditions. In highly turbid waters, turions may be buried by sediment deposition after sinking to the bed during dormancy. Burial reduces light levels which may inhibit germination and subsequent growth. Therefore, the effects of sediment deposition on turion sprouting were investigated in this study.
Methods
The experimental site was located in an artificial lake of Jiangsu Key Laboratory of Environmental Change and Ecological Construction (32.11° N, 118.91° E). The turions of P. crispus were collected from a pond at Nanjing Normal University on 13 September 2006, rinsed with water and then placed in buckets each of which contained a small amount of silt. Newly formed turions were discriminated from old ones by the color. Newly formed turions, which were green or greenish-brown, were kept and old ones (black) were discarded. The turions of similar sizes were transferred into flowerpots and then covered by the designated thickness of sediment (1, 2, 3, 4 or 5 cm), but the control group (S0) was not covered by sediment. Instead, the turions were sown onto the sediment surface. Each flowerpot contained 100 turions. The six flowerpots were suspended in water and three replicates were used for each treatment. The sediment was taken from the rice paddy soil around the laboratory and conditioned in water for 30 days in a pot (diameter 64 cm × height 72 cm). The sediment was taken out, cut into 1–5 cm thick slabs with a thin string and then used to cover turions that were spread over the sediment surface. Pots were marked as S0, S1, S2, S3, S4 or S5.
All pots were suspended in water at 80 cm depth. The overlying water was tap water that was injected into the experimental pond a year ago. The total nitrogen, total phosphorus content and permanganate index of the overlying water were 0.367, 0.017 and 3.182 mg/L, respectively.
Sunlight is attenuated exponentially in water in accordance with the following formula: I = I0e−K•d, where I0 is the incident light intensity, K is the attenuation coefficient and d is the depth of water. In this study, I0 and K were equivalent to 25.424 and 0.0142, respectively. Therefore, the formula could be rewritten as I = 25.424e − 0.0142d (R2 = 0.9941).
The oxidation--reduction potential (ORP) of the sediment was determined by using FJA-4 redox depolarization (Institute of Soil Science, Chinese Academy of Sciences) at the same depth as the turions for all treatment groups. The ORP of all treatment groups was measured at random, several times. In general, the ORP decreased from S1 to S5 and the anoxic condition was aggravated from S1 to S5. The ORP of S0, S1, S2, S3, S4 and S5 were 147, 100, 65, −42, −68 and −112 mv, respectively.
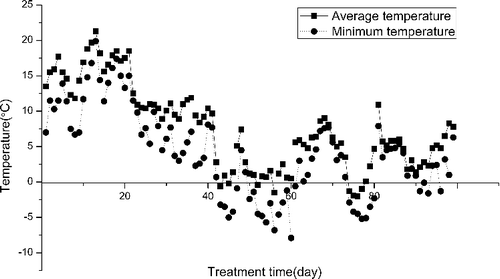
The temperature variation during the experiment was monitored using automated data loggers (). Initially, the water temperature was approximately 15 ℃. After 40 days, the temperature fell to about 5 ℃ and the minimum temperature fell to about 0 ℃.
Several plant growth parameters were measured over the 105 days of the experiment. All variables were determined at intervals of about 7 days and the experiment continued for 105 days for all plants.
Sprouting date: the day the first turion germinated was day 1 and the sprouting time of other turions was recorded successively.
Sprouting rate: the percentage of the turions number that can sprout new seedlings in the total turions number.
Plant height: The average length of 15 plants in each treatment group was determined from the base to the top of the main plant stem using a stainless steel ruler.
Leaf area: The first 3–5 fully expanded leaves from the top of the plants were selected to measure the leaf area. The length of main leaf veins was measured using a ruler and the leaf width was measured at the widest in the leaf. The leaf area of P. crispus was calculated by the following formula:
(Xie et al. Citation2004)
Plant dry weight: Thirty plants in each group were cut from the plant root and washed carefully with tap water and deionized water. The plants were oven dried at 80 ℃ for 48 h to a constant weight and weighted. The dry weight per plant was calculated.
Data from three replicates of all treatments were subjected to analysis of variance (ANOVA) with sediment depth as the main factor using SPSS 16.0 (IBM, Inc. http://www.ibm.com). The comparison of differences among all means was performed using the one-way ANOVA followed by t-tests to determine significant differences between two samples. Statistically significant differences were set at p < 0.05. The effects of the correlated independent variables (sediment thickness and treatment time) were compared and the data-sets were analyzed by multivariate ANOVA (MANOVA). In addition, correlations between dry weight and plant height were analyzed using a Pearson correlation.
Results
The average temperature during the course of the experiment decreased from day 1 to day 40 (), but the temperature was the same among all treatments. The sprouting time of the turions was delayed with increasing sediment thickness. At 14 days after sowing (DAS), only the turions of S0 and S1 had germinated. By 33 DAS, all treatments had germinated except for S5. At 40 DAS, the turions of all groups began to germinate. It is likely that the sediment depth led to the delayed sprouting time. The delay effect became more obvious with increasing sediment thickness.
Table 1. The initial sprouting date of Potamogeton crispus turions for all treatment groups. Freshly collected turions were sown in a silty sediment in flower pots on day 1.
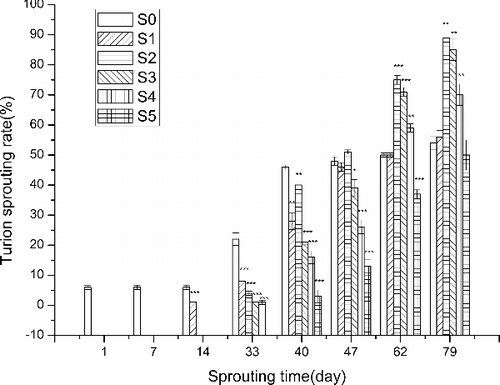
The sprouting rate decreased from S0 to S5 (). At 47 DAS, the sprouting rate of S2 was the greatest and S0 was the next greatest. From 62 to 79 DAS, the sprouting rate of S2 was fastest and S3 was second. At 79 DAS, the sprouting rate of all treatment groups was more than 50%. The sprouting rate of S0, S1, S2, S3, S4 and S5 at 79 DAS was greater by 17.4%, 100.0%, 122.5%, 304.8%, 337.5% and 1566.7%, respectively, compared to that at 40 DAS.
Figure 2. Effect of sediment thickness on sprouting rate of turions. The error bars indicate the standard deviation; the asterisks indicate significant difference between the treatment groups (S1–S5) and the control group (S0) at p < 0.05(*), p < 0.01(**) and p < 0.001 (***).
At 40 DAS, there was little difference in plant height of the seedlings among treatments; the height of all plants varied from 2 to 4 cm ((a)). However, from 62 to 105 DAS, the height of the seedlings showed a significant negative correlation with sediment thickness. The height decreased from S0 to S5 in sequence with the increase of sediment thickness. At 62 DAS, the height of all plants increased compared to that at 40 DAS. At 105 DAS, the height of S0 and S5 was 94.7 and 51.0 cm, respectively, and the difference was significant.
Figure 3. Effect of sediment thickness on (a) plant height, (b) leaf number and (c) plant dry weight. The error bars indicate the standard deviation and the asterisks indicate significant difference between the control group (S0) and the treatment groups (S1–S5) at p < 0.05(*), p < 0.01(**) and p < 0.001 (***).
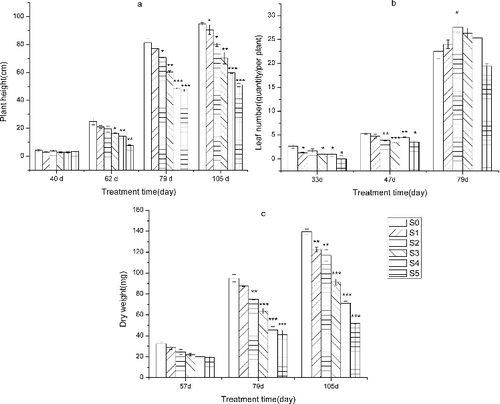
At 33 DAS, there were no significant differences in leaf number between S0 and S2. However, significant differences were observed between S0 and each of S1, S3, S4 and S5 ((b)). At 47 DAS, the leaf number of all treatment groups increased compared to that at 33 DAS. There were no significant differences in leaf number between S0 and S1, but significant differences between S0 and each of S2, S3, S4 and S5 at 47 DAS were observed. At 79 DAS, there was a significant positive correlation between leaf number and sprouting rate, and the correlation coefficient (r) was 0.904. The leaf number and sprouting rate for S2 and S5 was the greatest and least, respectively. The leaf number increased from S0 to S2 and decreased from S2 to S5.
The plant dry weight of all treatment groups increased with increasing plant height ((c)). At 79 and 105 DAS, there was a significant positive correlation between plant dry weight and plant height and the correlation coefficient (r) was 0.997 and 0.989, respectively. The plant weight increased with the increase of plant height from 79 to 105 DAS. The plant dry weight decreased gradually with the increase of sediment thickness during the whole experiment period. At 57 and 105 DAS, the plant dry weight for S0 was significantly higher than that of S3–S5. The difference in dry weight between treatments was growing at the end of the experiment.
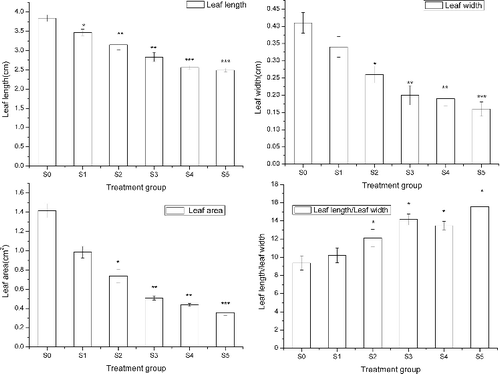
At 80 DAS, the leaf morphology of all treatment groups was measured. The leaf length, leaf width and leaf area all decreased gradually with the increase of sediment thickness (). The leaf length, leaf width and leaf area of S0 were 54.22%, 156.2% and 295.2%, respectively, higher than that of S5. The ratio of leaf length:leaf width increased significantly with increasing sediment thickness, resulting in more slender leaves for the treatment groups compared to the control group.
Discussion
Sastroutomo (Citation1981) and Jian et al. (Citation2003) suggested that the rate and percentage of turion sprouting of P. crispus were higher under natural light conditions than under dark conditions, indicating that light is required for sprouting and sprout growth. There was a significant negative linear relationship between sprouting rate and water depth; turion sprouting was inhibited in the dark (Jian et al. Citation2003). Kadono (Citation1982) also found that light was necessary for turion sprouting. However, the present study showed that turion sprouting was delayed under coverage by sediment deposition. Natural light conditions can induce turion sprouting early, but sediment deposition did not reduce the turion sprouting rate in dark conditions. Sediment thickness was an important factor for turion sprouting and sprout growth; in fact, sediment deposition increased the turion sprouting rate compared to the treatment group of no sediment deposition. CitationChen et al. (2006) also indicated that the turion sprouting rate increased in the dark, which was consistent with the results in this study.
Besides light intensity, anoxic sediment is also a major determinant of turion sprouting and sprout growth (Short Citation1987; Lauridsen et al. Citation1993; Clarke & Wharton Citation2001; Haluna et al. Citation2002). This study indicated that anoxic sediment, rather than light, is a key determinant of turion sprouting. The sediment became more anoxic with increasing sediment thickness. For example, at sediment depths of 2–3 cm, the degree of hypoxia did not affect sprouting during the sprouting period. However, when the sediment depth was 4–5 cm, the ORP reached the maximum and began to inhibit turion sprouting. The sediment deposition changed the oxygen condition around the turions and, therefore, affected the final sprouting rate.
Sastroutomo (Citation1981) found that the sprouting of non-dormant green turions of P. crispus was affected by water temperature, but not by light intensity. Dormancy was fully broken by a cold treatment (5 °C for one week), a warm treatment (30 °C) or a heat treatment (35 °C) for two weeks, but not by an almost freezing temperature (−1 to 2 °C). There was significant interaction between the duration of heat treatment and light or dark conditions. In this study, the temperature declined with the extending of the treatment time. At the beginning, the temperature was approximately 15 ℃. At 40 DAS, the mean temperature fell to about 5 ℃ and the minimum temperature fell to about 0 ℃. The turions' dormancy could be broken by such low temperature conditions trigger sprouting. We considered that the turion sprouting of S0 was affected by light because it had no sediment coverage. The turion sprouting of S1–S5 was affected by anoxic conditions and low temperature rather than light. Harada and Ishizawa (Citation2003) suggested that P. crispus turions could grow in the absence of oxygen. Such a type of anaerobic tolerance should be supported by an efficient operation of carbohydrate metabolism to produce energy in anoxia. In the case of plants storing starch as a carbon source, the regulation of starch degradation is the first important step for a supply of substrate to energy metabolism. Their study on starch degradation and sucrose metabolism in pondweed turions under anaerobic conditions indicates that regulation of the breakdown of native starch granules in amyloplasts is important for anaerobic tolerance. Their research may partly explain why an increase in sediment depth increased the turion sprouting rate compared to no sediment deposition.
Clonal plants can break through the limitation of resource distribution in a certain range and respond to varied environments by adjusting their morphological and physiological characteristics to match current conditions (Gao et al. Citation2012). For example, plant height and leaf area of P. crispus are altered in order to adapt to environmental change under different nutrient conditions as a clonal plant (Xie et al. Citation2004; Wu et al. Citation2009; Wang et al. Citation2013). In the present experiment, the plant height, leaf number and leaf area of P. crispus were all adjusted to match deposition variation. The sprouting time of the turions at different depths exhibited obvious differences; the sprouting time was delayed with increasing sediment thickness. Sprouting time was the dominant factor influencing the final sprouting rate, plant height, leaf number and so on, according to ANOVA (). The plant height decreased with the increase of sediment thickness. The leaf length, width and leaf area also decreased with the increase of sediment thickness. The plant dry weight decreased with the variation of plant height and leaf area. Wu et al. (2009) suggested that the growth of newly formed sprouts was also significantly inhibited by sediment anoxia, and photosynthesis and shoot biomass were reduced under sediment anoxia. Because the anoxic condition was related to sediment thickness, the growth of newly formed sprouts may be inhibited. Therefore, the plant height, leaf length, width and leaf area of P. crispus decreased with increasing sediment thickness. Leaves are photosynthetic organs and are more sensitive to environmental change. The nutrients provided to seedling growth increased with the increase of deposition thickness. Wu et al. (2009) also suggested that root biomass of P. crispus was highest in the low anoxic condition treatment. Thus, in this study, the root biomass of P. crispus may be increased by sediment coverage and the nutrient from sediment can be absorbed for improving leaf number and maintaining sprout growth. Therefore, the leaf number of S1–S4 were all greater than S0 and only S5 was less than S0 because the growth time was far short compared to S0 at 79 DAS.
Table 2. Results of ANOVA of sediment thickness and date on sprouting rate of P. crispus turions.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41105113 and 41361017). The constructive comments of anonymous reviewers are greatly appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ali MB, Tripathi RD, Rai UN, Pal A, Singh SP. 1999. Physico-chemical characteristics and pollution level of Lake Nainital (U.P., India): role of macrophytes and phytoplankton in biomonitoring and phytoremediation of toxic metal ions. Chemosphere. 39:2171–2182.
- Ali MB, Vajpayee P, Tripathi RD, Rai UN, Kumar A, Singh N, Behl HM, Singh SP. 2000. Mercury bioaccumulation induces oxidative stress and toxicity to submerged macrophyte Potamogeton crispus L. Bull Environ Contam Toxicol. 65:573–582.
- Blindow I, Andersson G, Hargeby A, Johansson S. 1993. Long-term pattern of alternative stable states in two shallow eutrophic lakes. Freshw Biol. 30:159–167.
- Catling PM, Dobson I. 1985. The biology of Canadian weeds. 69. Potamogeton crispus L. Can J Plant Sci. 65:655–668.
- Chen XF, Chen KN, Xiao Y, Zhang SD, Wang QY. 2006. Effects of light and matrix on turion germination, seedling growth and leaf photosynthesis efficiency of Potamogeton crispus. Chin J Appl Ecol. 17:1413–1418.
- Chi J. 2009. Phthalate acid esters in Potamogeton crispus L. from Haihe River, China. Chemosphere. 77:48–52.
- Clarke SJ, Wharton G. 2001. Sediment nutrient characteristics and aquatic macrophytes in lowland English rivers. Sci Total Environ. 266:103–112.
- Gao Y, Xing F, Jin YJ, Nie DD, Wang Y. 2012. Foraging responses of clonal plants to multi-patch environmental heterogeneity: spatial preference and temporal reversibility. Plant Soil. 358:1–2.
- Haluna Z, Terrados J, Borum J, Kamp-Nielsen L, Duarte CM, Fortes MD. 2002. Experimental evaluation of the effects of siltation-derived changes in sediment conditions on the Philippine seagrass Cymodocea rotundata. J Exp Mar Biol Ecol. 279:73–87.
- Harada T, Ishizawa K. 2003. Starch degradation and sucrose metabolism during anaerobic growth of pondweed (Potamogeton distinctus A. Benn.) turions. Plant Soil. 253:125–135.
- Jian YX, Li B, Wang JB, Chen JK. 2003. Control of turion germination in Potamogeton crispus. Aquat Bot. 75:59–69.
- Kadono Y. 1982. Germination of the turion of Potamogeton crispus L. Physiol Ecol Jpn. 19:1–5.
- Kunii H. 1982. Life cycle and growth of Potamogeton crispus L. in a shallow pond, ojaga-ike. Bot Mag (Tokyo). 95:109–124.
- Kunii H. 1989. Continuous growth and clump maintenance of Potamogeton crispus L. in Narutoh river, Japan. Aquat Bot. 33:13–26.
- Lauridsen TL, Jeppesen E, Andersen FØ. 1993. Colonization of submerged macrophytes in shallow fish manipulated Lake Væng: impact of sediment composition and waterfowl grazing. Aquat Bot. 46:1–15.
- Rogers KH, Breen CM. 1980. Growth and reproduction of Potamogeton crispus L. in a South Africa lake. J Ecol. 68:561–571.
- Sastroutomo SS. 1981. Turion formation, dormancy and germination of curly pondweed, Potamogeton crispus L. Aquat Bot. 10:161–173.
- Short FT. 1987. Effects of sediment nutrients on seagrasses: literature review and mesocosm experiment. Aquat Bot. 27:41–57.
- Sivaci A, Elmas E, Gümüs F, Sivaci ER. 2008. Removal of cadmium by Myriophyllum heterophyllum Michx. and Potamogeton crispus L. and its effect on pigments and total phenolic compounds. Arch Environ Contam Toxicol. 54:612–618.
- Stuckey RL. 1979. Distributional history of Potamogeton crispus (curly pondweed) in North America. Bartonia. 46:22–42.
- Waisel Y. 1971. Seasonal activity and reproductive behavior of some submerged hydrophytes in Israel. Hydrobiologia. 12:219–227.
- Wang L, Yang TW, Zhu DW, Hamilton D, Nie ZN, Liu LQ, Wan XQ, Zhu CM. 2013. Growth and turion formation of Potamogeton crispus in response to different phosphorus concentrations in water. Aquat Ecol. 47:87–97.
- Wu J, Cheng SP, Liang W, He F, Wu ZB. 2009. Effects of sediment anoxia and light on turion germination and early growth of Potamogeton crispus. Hydrobiologia. 628:111–119.
- Xie YH, Yu D, Geng XH, Yang YQ, Huang YM. 2004. Phenotypic plasticity in the submersed macrophyte Potamogeton crispus as a response to elevated [CO2]. J Freshw Ecol. 19:701–708.
- Yang HY, Shi GX, Wang HX, Xu QS. 2010. Involvement of polyamines in adaptation of Potamogeton crispus L. to cadmium stress. Aquat Toxicol. 100:282–288.
- Yang HY, Shi GX, Xu QS, Wang HX. 2011. Cadmium effects on mineral nutrition and stress in Potamogeton crispus. Russ J Plant Physiol. 58:253–260.